Abstract
Yeast cytokinesis entails a rejuvenation process by which the aged mother cell generates daughter cells enjoying full replicative potential. Here we show that this process includes a precipitous reduction in the levels of reactive oxygen species in the progeny immediately after completion of cytokinesis. The reduction in hydrogen peroxide is the result of a Sir2p and actin cytoskeleton-dependent segregation of the cytosolic catalase Ctt1p such that the daughter cell receives a higher load of undamaged and active Ctt1p than the progenitor cell. Such spatial quality control provides the daughter cells with a superior capacity to combat external oxidative stress and delays self-inflicted oxidative damage to their cellular proteins.
Keywords: aging, catalase, damage segregation, rejuvenation
The “stochastic” as opposed to “programmed” view of aging states that aging results from random deleterious events and oxidative damage has been suggested to be one contributor to such stochastic degeneration of cells, tissues, and organisms. Indeed, the levels of oxidatively damaged proteins increase with age in a large variety of species, including mammals, birds, bats, nematodes, and flies (1). The increase in protein oxidation, measured as protein carbonyls, during aging in animals is quite substantial (2), eventually reaching a level of one of every third protein molecule carrying this modification (3). In view of the fact that carbonylation reduces, or totally abrogates, the catalytic functions of the targeted proteins (4) and may trigger formation of high molecular, potentially cytotoxic aggregates (5), this modification is likely to play havoc on cellular/tissue functions in the aging organism. One causal factor behind age-dependent carbonylation may be a gradual decline in the antioxidant defense systems (6–8). For example, catalase activity has been demonstrated to decrease with age in specific tissues, and it has been suggested that protein oxidation levels increase as a direct consequence of a diminished activity of the primary antioxidant defense systems (8, 9). The role of these primary defenses is underscored also by the fact the ectopic overproduction of superoxide dismutases (SODs) and catalase can prolong the lifespan of specific species, e.g., Drosophila (10, 11) and mice (12), at least in some genetic backgrounds.
Similar to senescence in mammals, mother-cell-specific aging in yeast is characterized by a progressive accumulation of oxidatively damaged proteins and a diminished ability to cope with oxidative stress (4, 13, 14). With progressive divisions, the mother cell becomes increasingly deteriorated and will eventually lose its ability to produce daughter cells. Thus, yeast age as a function of generations rather than time. Yet aged mother cells generate offspring exhibiting a full replicative potential (15) and a renewed capacity to withstand external oxidants. Thus, the acquired aging phenotype is obliterated in the progeny during the process of asymmetric division (13, 16). This rejuvenation process entails retention of both extra chromosomal rRNA-encoding DNA circles (ERCs) (17) and oxidatively damaged proteins (13) in the mother cell during cytokinesis. Both the generation of ERCs and the segregation of damaged proteins are under the control of Sir2p, a conserved NAD-dependent histone deacetylase, acting as a key regulator of aging in a variety of organisms, including yeast, worms, and flies (18–21). The failure of the sir2Δ mutant mother cells to retain damage appears to act independently of ERC accumulation, because preventing the propagation of ERCs in a sir2 mutant by deleting the replication fork block gene FOB1 does not rescue the loss of damage asymmetry caused by deleting SIR2 (unpublished data). In addition, the failure in damage segregation is not due to aging as such because the sir2Δfob1Δ double mutant does not age prematurely.
In this work, we show that Sir2p is also involved in an actin cytoskeleton-dependent, daughter-cell-specific reduction in reactive oxygen species (ROS) levels after completion of cytokinesis. Thus, Sir2p protects the progeny both by preventing the daughter from inheriting oxidative damage accumulated in the aged progenitor cell and reducing the levels of ROS in the daughter after separation from the mother.
Results
During the course of our analysis of damage distribution during cytokinesis, we observed that the asymmetry in protein carbonylation did not correspond to an asymmetrical distribution of mitochondria or ROS. In fact, as long as mother and daughter cells were attached to each other (and presumably shared a cytoplasm), the levels of both superoxide and hydrogen peroxide were nearly identical in the two compartments (Fig. 1 A–D). In contrast, once the daughter cell detached from its progenitor, its ROS levels dropped precipitately (Fig. 1 B–D). This daughter-cell-specific reduction in ROS levels depended on Sir2p; the purging of superoxide was totally abolished in sir2Δ daughter cells, whereas scavenging of hydrogen peroxide was largely impaired (Fig. 1 C and D). Note that damage (carbonyls) asymmetry was established before the reduction in intracellular ROS (Fig. 1E).
Fig. 1.
ROS levels are reduced in daughter cells in a Sir2p-dependent way after completion of cytokinesis. (A and B) The images show 10- to 12-generation-old WT mother cells stained with dihydrorhodamine, an indicator of hydrogen peroxide, before (A) and after (B) completing cytokinesis; the arrowhead indicates the daughter cell. (C and D) Quantification of superoxide (C) and hydrogen peroxide (D) levels in the mother (M) and daughter (D) while they are still attached (left) and after separation (right). WT (empty bars) and sir2Δ (filled bars) cells were analyzed. (E) Carbonyl levels determined by in situ staining of samples described in C. Values were normalized relative to the WT mother cell during cytokinesis, which was arbitrarily set to 1. Error bars represent the SD of the mean. (Scale bar, 5 μm.)
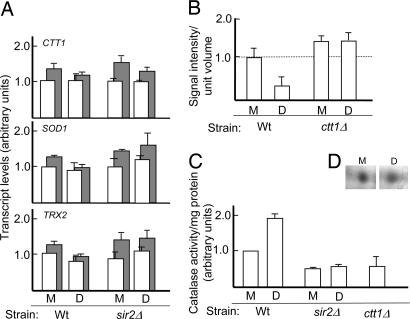
The reduction in superoxide and hydrogen peroxide levels in newborn daughter cells is not a result of a programmed, elevated expression of antioxidant genes. Analysis of transcript levels, by using RT-PCR, in isolated mother and daughter cells showed that the levels of antioxidant transcripts, e.g., CTT1, SOD1, and TRX2, were similar in mother and daughter cells of both the WT and sir2Δ (Fig. 2A). However, a deletion of ctt1, encoding the major cytoplasmic catalase of yeast, demonstrated that the ability of daughter cells to reduce peroxide levels after completion of cytokinesis required the presence of this enzyme (Fig. 2B). Measurements of the activity and abundance of the enzyme revealed that, after detachment, the progeny benefited from a 2-fold higher cytoplasmic catalase activity compared with their mothers (Fig. 2C), rather than inheriting higher levels of the enzyme (Fig. 2D). The observed elevated activity could be attributed, to a large extent, to Ctt1p, as seen by the reduced hydrogen peroxide detoxification in a ctt1Δ mutant (Fig. 2C). The fact that some peroxide conversion was still detected even in the absence of Ctt1p can be ascribed to the activity of other enzymes, such as cellular peroxidases. Catalase activity was markedly lower in cells lacking Sir2p, and sir2Δ daughters failed to elevate the enzyme activity upon completion of cytokinesis (Fig. 2C), which is consistent with the notion that Sir2p is required for daughter cells to reduce peroxide levels (Fig. 1D). This spatial Sir2p-dependent control of enzyme activity explains the drop in ROS levels in the progeny cells upon completion of cytokinesis, after which the oxidants can no longer diffuse between the mother/daughter compartments.
Fig. 2.
A Sir2p- and Ctt1p-dependent process ensures asymmetrical hydrogen peroxide scavenging. (A) Levels of CTT1, SOD1, and TRX2 transcripts, determined by real-time PCR, in WT (Wt) and sir2Δ mother (M) and daughter (D) cells after completion of cytokinesis. Levels are shown normalized to both IPP1 (empty bars) and ACT1 (filled bars). (B) Hydrogen peroxide levels in WT and ctt1Δ mother and daughter cells after separation. (C) Cytoplasmic catalase activity in WT and sir2Δ mother and daughter cells after separation; a ctt1Δ mutant is shown for a comparison. (D) Ctt1p protein levels in WT mother and daughter cells visualized by silver staining. Values in A–C were normalized relative to the WT mother cell, which was arbitrarily set to 1. Error bars refer to the SD of the mean (A) or the SE (B and C).
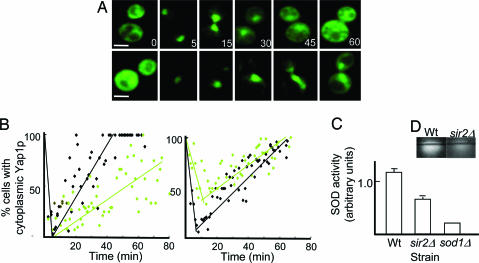
To approach the question of whether other antioxidant systems than the catalase display differential activity in mother and daughter cells, we measured the localization of Yap1p upon tert-butylhydroperoxide (tBOOH) exposure of isolated mother and daughter cells of the WT and sir2Δ strains. The detoxification of tBOOH relies on alkyl hydroperoxide reductase rather than catalase (22, 23). Yap1p is a transcriptional regulator, which upon peroxide exposure becomes oxidized and, as a result, localizes to the nucleus, where it induces an appropriate transcriptional response (24). The time it takes for cells to relocalize Yap1p to the cytoplasm after exogenous addition of tBOOH is proportional to their capacity to scavenge the oxidant and repair damage (e.g., illegitimate disulfide bonds) (Fig. 3A). We found that WT daughter cells were more efficient in this respect than their sir2Δ counterparts (Fig. 3B Left), which was consistent with a diminished segregation of functional enzymes in the mutant. In addition, WT daughter cells recovered faster than their mothers; almost 100% of daughters had relocalized Yap1p to the cytoplasm in 35 min (Fig. 3B Left), whereas mother cells required double that time (Fig. 3B Right). Notably, a large fraction (40%) of sir2Δ mother cells failed to respond to the oxidant by localizing Yap1p into the nucleus (Fig. 3B Right), suggesting that Sir2p-deficient cells are impaired, both in reacting properly to the oxidant and in efficiently repairing damage. The data support the notion that antioxidant defenses other than catalase are negatively affected by the absence of Sir2p. Accordingly, daughter cells of the sir2Δ mutant were also found to possess lower SOD activity (Fig. 3 C and D), which explains the failure of the sir2Δ cells to detoxify superoxide upon completion of cytokinesis and the generally increased levels of this oxidant in the mutant (Fig. 1C). It should be noted that, as for Ctt1p, the diminished SOD activity in the sir2Δ mutant is not matched by lower SOD protein levels (data not shown).
Fig. 3.
Daughter cells display a Sir2p-dependent superior capacity to respond to external oxidants. (A) Representative pictures of Yap1p–GFP localization in daughter cells of the WT (top row) and sir2Δ mutant (bottom row) upon exposure to tBOOH. The numbers denote 0, 5, 15, 30, 45, and 60 min after addition of the oxidant. (B) Recovery from tBOOH stress, measured as relocalization of Yap1p to the cytoplasm, in WT (black diamonds) and the sir2Δ (green diamonds) mother (Right) and daughter (Left) cells. (C and D) SOD activity in WT, sir2Δ, and sod1Δ (for a comparison) daughter cells upon completion of cytokinesis (C); corresponding images of in-gel SOD activity (D). Error bars correspond to the SE. (Scale bar, 5 μm.)
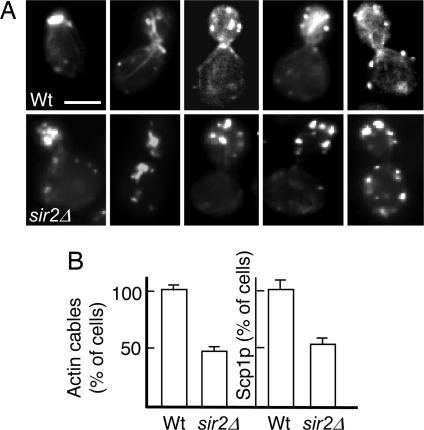
The ratio of damaged (carbonylated) to undamaged proteins is markedly lower in virgin daughter cells compared with their progenitors, and it applies to most yeast proteins (unpublished data), including the Ctt1p catalase (4). This asymmetry can be abolished by either a sir2 deletion or treatment with latrunculin A, a compound that binds to F-actin monomers, preventing their polymerization. This observation raises the possibility that differential catalase activity and ROS scavenging in mother and daughter cells may depend on a functional actin cytoskeleton. Indeed, transient depolymerization of actin, followed by subsequent removal of latrunculin A and completion of cytokinesis, resulted in a total breakdown of asymmetry with respect to catalase activity (Fig. 4A) and hydrogen peroxide levels (Fig. 4B). The data demonstrate that segregation of damaged and undamaged (functional) catalase and the purging of peroxide in yeast daughter cells is driven by a Sir2p and actin cytoskeleton mediated mechanism. Whether these two components act in parallel or whether Sir2p has a direct effect on actin cytoskeleton formation and function remains to be determined; however, we have noticed that nearly 50% of sir2Δ cells display aberrant actin cable formation and a reduced actin cable-dependent localization of proteins such as the transgelin homologue Scp1p (Fig. 5).
Fig. 4.
The actin cytoskeleton is required for elevated catalase activity and the purging of peroxide in the yeast progeny. (A) Catalase activity in mother (M) and daughter (D) cells upon transient depolymerization of F-actin during cytokinesis [with latrunculin A (LatA +)] or under normal conditions [without latrunculin A (LatA −)]. (B) Hydrogen peroxide levels in mother and daughter cells treated as in A. Values are normalized relative to WT mother cells, which were arbitrarily set to 1. Error bars refer to the SD of the mean.
Fig. 5.
Lack of Sir2p results in loss of actin cables and cable-dependent Scp1p localization. (A) Representative images of phalloidin-stained actin cables and patches in WT (top row) and sir2Δ (bottom row) cells at an early, intermediate, or late stage of budding. (B) Percentage of 12-generation-old WT or sir2Δ cells with detectable actin cables or Scp1p-GFP patches. Numbers represent average values normalized to the WT, which was arbitrarily set to 1. Error bars refer to the SD of the mean. (Scale bar, 5 μm.)
Discussion
The task of identifying the causal factors behind age-dependent increased oxidation, e.g., carbonylation, to target proteins has proven difficult. Several possibilities have been proposed, including an age-dependent decline in the antioxidant defense system, an increased production of ROS, a diminished capacity for removal of oxidized proteins, or an increased susceptibility of aged and aberrant proteins to oxidative attack (25). These possibilities are not mutually exclusive, and evidence both for and against all of these possibilities can be found in the literature, depending on the cells, tissues, or organisms studied (6–8, 26–28). However, it is clear that the primary enzymes involved in ROS scavenging/detoxification, i.e., SODs, catalases, and peroxidases, are key members of the cellular defense against protein carbonylation (25, 26).
One might wonder why evolution has failed to provide the soma with more proficient primary oxidant scavenging systems that more efficiently combat oxidative damage throughout the lifespan of an organism. This problem is adequately explained by the evolutionary theory of aging, which states that there is no need for such a defense: aging is argued to be a consequence of the fact that natural selection's power to influence the fate of genes gradually wanes as the age at which those genes have their effects increases. In line with this concept, the carbonyl content increases at a drastically accelerated rate in the last third of the lifespan of an animal (2), i.e., after termination of the reproductive period. In other words, the carbonylation load of young individuals is sufficiently low not to affect the fitness of the offspring, and the increased load of damage of the soma in late life is impervious to natural selection. However, these arguments are only applicable to organisms, e.g., animals, whose life history entails early reproduction. If a high carbonyl load is a genuine hazard to fitness, organisms producing reproductive organs at the closing stages of their development should have evolved systems that can clear out damage before reproduction. Interestingly, it appears as the plant Arabidopsis thaliana has evolved defense systems to do just that (29). In addition, unicellular microbes such as the asymmetrically dividing yeast Saccharomyces cerevisiae, has evolved systems that retain carbonylated proteins in the mother cell compartment during mitotic cytokinesis (13). This segregation process directly limits the oxidative damage inherited by the progeny and, as shown here, simultaneously provides the daughter cell with a superior capacity to scavenge ROS, which would otherwise give rise to protein oxidation. This process depends on a functional Sir2p and actin cytoskeleton. Thus, the cytoskeleton has a dual function in ensuring that daughters inherit a full complement of genomic material and active organelles while preventing inheritance of old and damaged macromolecules.
Yeast longevity and ROS production have previously been linked to the performance of the actin cytoskeleton. Specifically, genetic alterations that result in increased actin dynamics prolong replicative lifespan, whereas mutations reducing actin dynamics have the opposite effect (30). This report and the article by Gourlay et al. (30) point to two important functions of the cytoskeleton in regulating ROS levels in the progeny. First, the establishment of a functional actin cytoskeleton is required for damage retention, which results in a preferential distribution of undamaged and functional antioxidant enzymes into the daughter cells. Secondly, the dynamic properties of the actin filaments formed affect ROS levels by a mechanism suggested to involve the propensity of the mitochondria to produce superoxide (30). The retention of dysfunctional mitochondria in mother cells (31, 32) has previously been argued to be a key feature in maintaining age asymmetry, and this may, like damaged proteins, rely on the proper formation and dynamics of the actin cytoskeleton. However, it appears that unequal ROS scavenging, rather than mitochondrial ROS production, is more important in establishing hydrogen peroxide asymmetry because the absence of the cytosolic Ctt1p abolished the ability of daughter cells to diminish peroxide levels after completion of cytokinesis.
The effect of Sir2p on cytoskeleton functions raises the question of how a nuclear localized deacetylase affects actin functions in the cytoplasm. Support for sirtuins having additional roles apart from nuclear silencing comes from recent evidence showing that histone (protein) deacetylases exhibit novel roles linked to cytoskeleton function in mammalian cells (33, 34). Possibly, deletion of SIR2 might affect the abundance and/or activity of cytoplasmic proteins involved in cytoskeleton functions. For example, the lack of SIR2 has been reported to cause a redistribution of the cytosolic Sir2p homolog, Hst2p, to the nucleus (35), and this may limit the deacetylation of possible downstream targets, i.e., elements of the actin cytoskeleton, in the cytosol.
The work presented here highlights the physiological importance of damage segregation and how this allows generation of a rejuvenated and superior performing progeny from old and damaged ancestor cells. Future analysis may clarify whether such segregation is restricted to asymmetrically dividing microbes or whether this phenomenon is also a common feature of, e.g., mammalian development, generation of the germ cell line, and tissue turnover. It has been shown that carbonylated proteins are asymmetrically distributed in the developing blastocysts and that differentiation of embryonic stem cells encompasses a clearance of damaged proteins (36). Possibly, oxidatively damaged proteins and ROS defenses may be asymmetrically distributed during the apparent symmetrical division of mammalian tissue cells, and such segregation may continue during subsequent divisions. Such a mechanism could improve the “vigor” of an organ during the lifespan of the animal by segregating damage to “waste-disposal cells” directed toward apoptosis. The role of the sirtuins in such a mechanism would have an obvious impact on the preservation of the soma.
Experimental Procedures
Strains.
All S. cerevisiae strains used in this study were derivatives of BY4741 (MATa; his3Δ1; leu2Δ; met15Δ; ura3Δ), here referred to as WT. The sir2Δ, sod1Δ, and ctt1Δ mutants had the SIR2 and CTT1 genes replaced by a KanMX4 cassette (European Saccharomyces Cerevisiae Archive for Functional Analysis, Frankfurt, Germany). The tandem affinity purification–tagged SOD1 and SOD2 strains and the GFP-tagged SCP1 were from Invitrogen (Carlsbad, CA) (37); the corresponding sir2Δ SCP1–GFP mutant was constructed by replacing the SIR2 gene with the URA3 marker and verified thereafter by PCR. YAP1–GFP WT and sir2Δ strains were generated by transforming the cells with a pRS316 plasmid harboring a C-terminal GFP fusion to YAP1 (kind gift from Scott Moye-Rowley, Univ. of Iowa, Iowa City, IA). All strains were grown at 30°C on YPD (1% yeast extract/2% peptone/2% glucose).
Separation of Mother and Daughter Cells.
Old mother cells were obtained by centrifugal elutriation (38) by using a J-20 XP centrifuge equipped with a JE 5.0 rotor (Beckman Coulter, Fullerton, CA). To enrich the culture for old cells, two successive rounds of elutriation were performed, allowing for overnight growth in between. Cells were subsequently resuspended in PBS and loaded in a 40-ml separation chamber at 32 ml/min and 312–385 × g; old cells were elutriated at 90 ml/min and 35–62 × g. The cells thus obtained were grown again for one generation and subjected to a last round of elutriation at the same settings to separate the mothers from their daughters. Separation of young mother and daughter cells was achieved by loading exponentially growing cultures in a 5-ml separation chamber and by using a cutoff of 10 ml/min at 728 × g (daughters) or 20 ml/min at 385 × g (mothers). The efficiency of every sorting was confirmed by Calcofluor White (Sigma, St. Louis, MO) staining and bud scar counting.
ROS Imaging and Quantification.
Cells were incubated in the presence of either 14 μM dihydrorhodamine for 2 h or 0.8 mM dihydroethidium for 30 min (both from Fluka, Buchs, Switzerland), which are indicators of hydrogen peroxide and superoxide, respectively. The signal was detected by using a DMRXA fluorescence microscope (Leica, Wetzlar, Germany) equipped with a C4742 CCD camera (Hamamatsu, Hamamatsu City, Japan) and Cy3 and FITC filter sets and quantified with NIH Image J software (National Institutes of Health, Bethesda, MD). Experiments were repeated independently at least twice.
Antioxidant Enzyme Analysis.
CTT1, SOD1, and TRX2 transcript levels were measured by real-time PCR at the TATAA Biocenter (Göteborg, Sweden) according to standard protocols. The following sets of forward and reverse primers were used: CTCATCACCCATACGCTTCTC and TCCAGTCTACAACCACCACCT (CTT1); AAAGACGGACGAAAATGGTG and AACGGAGGTAGGACCGATAAG (SOD1); and CAGATGTTGCTCAAAAAGCTGA and GCACCGACGACTCTGGTAA (TRX2). Transcript levels were normalized both to ACT1 and IPP1.
Catalase activity was determined after H2O2 decomposition at 240 nm (39). Briefly, mother and daughter cells obtained by elutriation were washed three times with cold 50 mM phosphate buffer (pH 7.4), resuspended in 750 μl of the same buffer, and then broken with an equal amount of glass beads, five times for 30 s with cooling on ice in between. One hundred microliters (≈20 μg) of crude protein extract were then added to 567 μl of the same buffer and 333 μl of 54 mM H2O2 in phosphate buffer (pH 7.0). Decrease in absorbance at 240 nm was followed every 10 s for up to 3 min by using an Ultrospec 2000 spectrophotometer (Amersham Pharmacia Biotech, Uppsala, Sweden). Specific catalase activity (units per milligram of total protein) was calculated as ΔA240 per milligram of protein. Reference curves were obtained by using catalase C30 from bovine liver (Sigma), and all experiments were carried out in triplicate. Ctt1p levels were determined by two-dimensional PAGE (40) of whole-protein extracts, followed by silver staining or blotting on PVDF membranes and probing them with anti-catalase antibodies (Sigma). Quantification was performed by using NIH Image J software (National Institutes of Health).
SOD activity was determined by an in-gel assay (41). Briefly, protein samples were prepared as for the catalase activity assay and run on a 10% native gel. The gel was incubated for 20 min in 100 mM phosphate buffer (pH 7.8) containing 2 mg/ml nitroblue tetrazolium, followed by another 20-min incubation in the same buffer containing 20 μg/ml riboflavin and 0.1% TEMED (N,N,N′,N′-tetramethylethylenediamine). The gel was then exposed on a light table allowing for the color to develop and scanned and quantified as was done for Ctt1p. Each sample was assayed independently at least twice. Sod1p and Sod2p levels were determined by tandem affinity purification according to standard procedures.
Yap1–GFP Localization.
Old cells of different ages and their daughters were separated by centrifugal elutriation and resuspended in YPD, followed by addition of tBOOH to a final 5 mM concentration. An aliquot was placed on a microscope slide and Yap1p–GFP localization was monitored by using a DMRXA microscope (Leica). Images were recorded before addition of tBOOH and thereafter every 2 min for up to 80 min. The fraction of cells exhibiting cytoplasmic Yap1p–GFP localization was determined for 60–104 cells at every time point. Experiments were performed in triplicates, and controls were carried out by adding water only.
Protein Damage Analysis.
In situ detection of protein carbonyls was performed and quantified as described previously (13). Cells treated with latrunculin A were incubated with a final 200 μM concentration of the drug, and depolymerization of actin cables was assessed by staining with 0.05 units/μl fluorescein–phalloidin (Molecular Probes, Carlsbad, CA). The same concentration of fluorescein–phalloidin also was used when visualizing actin distribution in WT and sir2Δ cells.
Acknowledgments
This work was supported by grants from the Swedish Natural Research Council and an award from the Göran Gustafsson Foundation for Scientific Research in Molecular Biology. T.N. was supported in part by European Commission Contract 512020 (MiMage).
Abbreviations
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- tBOOH
tert-butylhydroperoxide.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Stadtman ER. Science. 1992;257:1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- 2.Levine RL. Free Radic Biol Med. 2002;32:790–796. doi: 10.1016/s0891-5849(02)00765-7. [DOI] [PubMed] [Google Scholar]
- 3.Stadtman ER, Levine RL. Ann NY Acad Sci. 2000;899:191–208. doi: 10.1111/j.1749-6632.2000.tb06187.x. [DOI] [PubMed] [Google Scholar]
- 4.Reverter-Branchat G, Cabiscol E, Tamarit J, Ros J. J Biol Chem. 2004;279:31983–31989. doi: 10.1074/jbc.M404849200. [DOI] [PubMed] [Google Scholar]
- 5.Grune T, Jung T, Merker K, Davies KJA. Int J Biochem Cell Biol. 2004;36:2519–2530. doi: 10.1016/j.biocel.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 6.Sohal RS. Free Radic Biol Med. 2002;33:37–44. doi: 10.1016/s0891-5849(02)00856-0. [DOI] [PubMed] [Google Scholar]
- 7.Sohal RS, Weindruch R. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ji LL, Dillon D, Wu E. Am J Physiol. 1990;258:R918–R923. doi: 10.1152/ajpregu.1990.258.4.R918. [DOI] [PubMed] [Google Scholar]
- 9.Sohal RS, Agarwal A, Agarwal S, Orr WC. J Biol Chem. 1995;270:15671–15674. doi: 10.1074/jbc.270.26.15671. [DOI] [PubMed] [Google Scholar]
- 10.Orr WC, Sohal RS. Science. 1994;263:1128–1130. doi: 10.1126/science.8108730. [DOI] [PubMed] [Google Scholar]
- 11.Parkes TL, Elia AJ, Dickinson D, Hilliker AJ, Phillips JP, Boulianne GL. Nat Genet. 1998;19:171–174. doi: 10.1038/534. [DOI] [PubMed] [Google Scholar]
- 12.Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, et al. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 13.Aguilaniu H, Gustafsson L, Rigoulet M, Nyström T. Science. 2003;299:1751–1753. doi: 10.1126/science.1080418. [DOI] [PubMed] [Google Scholar]
- 14.Jazwinski SM. FEMS Yeast Res. 2004;5:119–125. doi: 10.1016/j.femsyr.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy BK, Austriaco NR, Jr, Guarente L. J Cell Biol. 1994;127:1985–1993. doi: 10.1083/jcb.127.6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egilmez NK, Jazwinski SM. J Bacteriol. 1989;171:37–42. doi: 10.1128/jb.171.1.37-42.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sinclair DA, Guarente L. Cell. 1997;91:1033–1042. doi: 10.1016/s0092-8674(00)80493-6. [DOI] [PubMed] [Google Scholar]
- 18.Guarente L. Genes Dev. 2000;14:1021–1026. [PubMed] [Google Scholar]
- 19.Kaeberlein M, McVey M, Guarente L. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tissenbaum HA, Guarente L. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- 21.Rogina B, Helfand SL. Proc Natl Acad Sci USA. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galiazzo F, Schiesser A, Rotillo G. Biochem Biophys Res Comm. 1987;147:1200–1205. doi: 10.1016/s0006-291x(87)80197-3. [DOI] [PubMed] [Google Scholar]
- 23.Chae HZ, Chung SJ, Rhee SG. J Biol Chem. 1994;269:27670–27678. [PubMed] [Google Scholar]
- 24.Delaunay A, Isnard A-D, Toledano M. EMBO J. 2000;19:5157–5166. doi: 10.1093/emboj/19.19.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nyström T. Mol Microbiol. 2003;48:17–23. doi: 10.1046/j.1365-2958.2003.03385.x. [DOI] [PubMed] [Google Scholar]
- 26.Dukan S, Nyström T. Genes Dev. 1998;12:3431–3441. doi: 10.1101/gad.12.21.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dukan S, Nyström T. J Biol Chem. 1999;274:26027–26032. doi: 10.1074/jbc.274.37.26027. [DOI] [PubMed] [Google Scholar]
- 28.Dukan S, Farewell A, Ballesteros M, Taddei F, Radman M, Nyström T. Proc Natl Acad Sci USA. 2000;97:5746–5749. doi: 10.1073/pnas.100422497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johansson E, Olsson O, Nyström T. J Biol Chem. 2004;279:22204–22208. doi: 10.1074/jbc.M402652200. [DOI] [PubMed] [Google Scholar]
- 30.Gourlay CW, Carpp LN, Timpson P, Winder SJ, Ayscough KR. J Cell Biol. 2004;164:803–809. doi: 10.1083/jcb.200310148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simon VR, Karmon SL, Pon LA. Cell Motil Cytoskel. 1997;37:199–210. doi: 10.1002/(SICI)1097-0169(1997)37:3<199::AID-CM2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 32.Lai C-Y, Jaruga E, Borghouts C, Jazwinski SM. Genetics. 2002;162:73–87. doi: 10.1093/genetics/162.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. Mol Cell. 2003;11:437–444. doi: 10.1016/s1097-2765(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 34.Kawaguchi Y, Kovacs JJ, McLaurin A, Vance JM, Ito A, Yao T-P. Cell. 2003;115:727–738. doi: 10.1016/s0092-8674(03)00939-5. [DOI] [PubMed] [Google Scholar]
- 35.Perrod S, Cockell MM, Laroche T, Renauld H, Ducrest A-L, Bonnard C, Gasser SM. EMBO J. 2001;20:197–209. doi: 10.1093/emboj/20.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hernebring M, Brolen G, Aguilaniu H, Semb H, Nyström T. Proc Natl Acad Sci USA. 2006;103:7700–7705. doi: 10.1073/pnas.0510944103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huh W-K, Falvo JV, Gerke LC, Carrol AS, Howson RW, Weissman JS, O'Shea EK. Nature. 2003;425:686–691. doi: 10.1038/nature02026. [DOI] [PubMed] [Google Scholar]
- 38.Woldringh CL, Fluiter K, Huls PG. Yeast. 1995;11:361–369. doi: 10.1002/yea.320110409. [DOI] [PubMed] [Google Scholar]
- 39.Jakubowski W, Bilinski T, Bartosz G. Free Radic Biol Med. 2000;28:659–664. doi: 10.1016/s0891-5849(99)00266-x. [DOI] [PubMed] [Google Scholar]
- 40.O'Farrell PH. J Biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- 41.Flohe L, Ötting F. Methods Enzymol. 1984;105:93–104. doi: 10.1016/s0076-6879(84)05013-8. [DOI] [PubMed] [Google Scholar]