Abstract
Consumer and resource control of diversity in plant communities have long been treated as alternative hypotheses. However, experimental and theoretical evidence suggests that herbivores and nutrient resources interactively regulate the number and relative abundance of coexisting plant species. Experiments have yielded divergent and often contradictory responses within and among ecosystems, and no effort has to date reconciled this empirical variation within a general framework. Using data from 274 experiments from marine, freshwater, and terrestrial ecosystems, we present a cross-system analysis of producer diversity responses to local manipulations of resource supply and/or herbivory. Effects of herbivory and fertilization on producer richness differed substantially between systems: (i) herbivores reduced species richness in freshwater but tended to increase richness in terrestrial systems; (ii) fertilization increased richness in freshwater systems but reduced richness on land. Fertilization consistently reduced evenness, whereas herbivores increased evenness only in marine and terrestrial ecosystems. Producer community evenness and ecosystem productivity mediated fertilization and herbivore effects on diversity across ecosystems. Herbivores increased producer richness in more productive habitats and in producer assemblages with low evenness. These same assemblages also showed the strongest reduction in richness with fertilization, whereas fertilization increased (and herbivory decreased) richness in producer assemblages with high evenness. Our study indicates that system productivity and producer evenness determine the direction and magnitude of top-down and bottom-up control of diversity and may reconcile divergent empirical results within and among ecosystems.
Keywords: fertilization, herbivory, evenness, species richness, metaanalysis
Human activities have altered nutrient availability and the numbers and kinds of species in virtually all habitats on earth (1–3). These changes, in turn, strongly affect the diversity of primary producer communities. Local species richness is determined in part by the supply of new species via speciation and dispersal (4). The fate of these colonists, and therefore the diversity of a community, depends on differential species responses to intrinsic local-scale processes. Two local factors are often especially important: the ability to exploit or tolerate spatial variability in the supply of abiotic resources (bottom-up processes) and the ability to withstand consumption by herbivores (top-down processes) (5). These two forms of local control affect rates of extinction and colonization (and thus species richness) as well as the relative abundance, or evenness, of species within the community.
The response of producer diversity to alterations of resource and consumer abundance has been inconsistent within and among producer communities and ecosystem types. Resource enrichment and herbivore manipulations can have positive or negative effects on producer diversity (6–8); no consensus has been reached as to what determines the direction or magnitude of these effects, although ecosystem productivity is thought to strongly influence community responses (9). Resource enrichment in unproductive environments may enhance diversity by allowing rare species to use new resources (10, 11) or persist in larger local populations that are less susceptible to stochastic extinction (12). In productive environments, however, resource enrichment may depress diversity by favoring competitively dominant species and thereby reducing evenness (13, 14). Likewise, the effects of consumers on plant diversity can also be positive or negative, depending on the balance between increased mortality and weakened competitive interactions due to herbivory (9, 15, 16). Consumers may promote stable coexistence if competitively dominant species suffer increased mortality (e.g., keystone predation) (17, 18), or may decrease richness when they are unselective or select rare species (19). Thus, the effects of consumers on coexistence depend on their diet specificity, their biomass removal efficiency, and the dominance structure of the prey assemblage (15).
Here, we present the results of a metaanalysis testing the generality of fertilization and herbivore effects on producer diversity across marine, freshwater, and terrestrial ecosystems. We expected systematic differences in the regulation of diversity by consumers and resources, because the importance of herbivory and the response of producers to resource supply vary among ecosystems. Herbivores in aquatic ecosystems remove a 3-fold greater fraction of primary production than those on land, and aquatic producers typically have shorter generation times and higher nutrient-uptake rates (20, 21). Moreover, other constraints on herbivory, such as body size ratios of herbivores to their prey (22) and producer food quality (20, 23), differ substantially between aquatic and terrestrial systems.
Interactions among herbivores, producers, and abiotic resources are central to understanding differences in trophic structure and composition between aquatic and terrestrial systems (21). Metaanalyses of trophic cascades across ecosystems found that systematic differences in the strength of top-down control of producer biomass occurred largely at the plant-herbivore link (24–26). We lack similar information on the importance and interdependency of top-down and bottom-up factors mediating producer diversity across systems. Two previous reviews (27, 28) on the interactive effects of resource availability and consumers on diversity corroborated models (29, 30) predicting that consumer effects on prey diversity switch from negative to positive along a gradient of increasing productivity. However, these analyses were either specific to aquatic communities (28) or did not apply quantitative metaanalysis statistics (27).
In our analysis, we used richness and community evenness as separate response variables, because fertilization and herbivory can differentially affect species coexistence (richness) and species relative abundance (evenness). We calculated effect sizes as log response ratios (31) in 75 experiments manipulating resource supply (nutrients or light, hereafter fertilization), 157 experiments manipulating herbivore presence, and 42 experiments factorially manipulating fertilization and herbivory. This global data set was used to (i) obtain global average effect sizes of fertilization and consumer presence on producer diversity, (ii) test for significant differences between ecosystem types, and (iii) analyze the importance of factors potentially mediating effect sizes across ecosystems. These factors include characteristics of the environment (ecosystem productivity, producer community structure and biomass responses to herbivory and fertilization), and experimental design (site, size, and duration). Based on our analysis, we propose a conceptual model that synthesizes the seemingly disparate findings across ecosystem types, and we show that diversity responses vary consistently with basal ecosystem productivity and community evenness.
Results
Overall Effects and Differences Between Ecosystems.
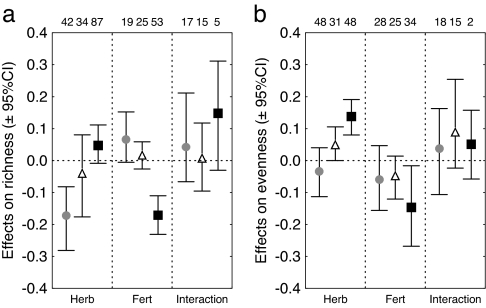
Across all ecosystems, fertilization significantly reduced the number of producer species [grand mean effect on richness (95% confidence interval): −0.076 (−0.124 to −0.032)], whereas the average effect of herbivores on producer richness was negative but not different from zero [−0.027 (−0.080 to 0.027)]. Herbivory [metaanalysis (MA) between ecosystems, P = 0.001] and fertilization (P < 0.001) effects on producer richness differed between ecosystems. In freshwater systems, richness was reduced by herbivores and enhanced by fertilization (Fig. 1a). The opposite was observed in terrestrial systems, where herbivores increased richness and fertilization depressed richness. The signs of the responses in marine habitats were the same as in freshwater (positive for fertilization and negative for herbivory), but average effects did not differ significantly from zero (Fig. 1a).
Fig. 1.
Average effect sizes (±95% confidence intervals) of herbivory and fertilization and their interaction on producer species richness (a) and evenness (b) in freshwater (gray circles), marine (triangles), and terrestrial (black squares) systems. Numbers represent numbers of studies used for each average. Herb, Herbivory; Fert, Fertilization.
Across ecosystems, producer evenness was consistently enhanced by the presence of herbivores [0.052 (0.012 to 0.092)], and it decreased with fertilization [−0.090 (−0.156 to −0.027)]. Herbivores significantly increased evenness in marine and terrestrial systems but not in freshwater (MA on ecosystem differences, P = 0.002; Fig. 1b). Producer evenness tended to decline with fertilization in all three ecosystems (MA, P > 0.1; Fig. 1b).
Interaction effect sizes for factorial manipulations of herbivory and fertilization were, on average, positive, but not different from zero, for both evenness [0.061 (−0.031 to 0.153)] and richness [0.043 (−0.030 to 0.138)]. For evenness, a positive interaction indicates more positive effects of herbivores in enriched treatments. Interaction effect sizes did not vary significantly between ecosystems (MA, P > 0.1) (Fig. 1 a and b).
Environmental Mediation of Fertilization and Herbivory Effects on Producer Diversity.
To understand the variation in effect sizes across ecosystems, we analyzed the importance of environmental and experimental context in mediating the effects of fertilization and herbivory. Because studies differed strongly in the number of covariates they reported alongside the experimental results, we used two methods. First, we used linear MA regressions to analyze the bivariate relationships of continuous covariates with effect sizes. Second, we identified the most parsimonious general linear model (hereafter GLM) using all continuous covariates and ecosystem type as predictor variables. Thus, we compared the bivariate MA approach using the full data set (but lacking the ability to account for covariation among the predictor variables) with the GLM approach, which accounted for colinearity among predictor variables (but used a reduced data set because studies with missing information on one or more variables could not be included). In the following, we focus on predictor variables that showed both a significant bivariate trend in the MA and were retained in the most-parsimonious GLM. Full information on all predictor variables can be obtained from supporting information (SI) Figs. 4–9.
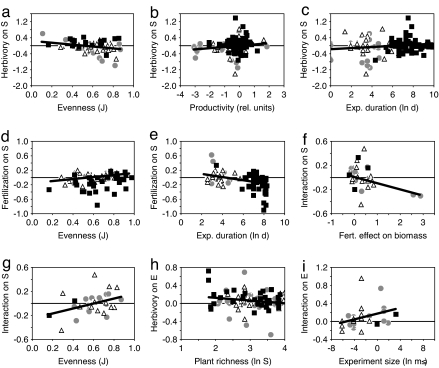
Herbivore effects on producer species richness were mainly associated with system productivity, producer evenness, and experimental duration (Table 1 and Fig. 2 a–c). Herbivores enhanced species richness in producer communities with low evenness but reduced richness in communities with high evenness (Fig. 2a). Herbivores reduced producer richness at low ecosystem productivity (measured as integrated ambient resource availability, see Methods), but tended to enhance richness at high productivity (Fig. 2b). Experimental duration affected herbivore effect sizes in both the MA and the GLM but in different directions (Table 1). Increasing herbivore effects with longer experiments are seen in the MA mainly because terrestrial studies, which tended to have positive herbivore impacts (Fig. 1a), ran for longer periods of time (Fig. 2c). However, in the final GLM, which retained system type as a significant variable (Table 1), the negative relationship between duration and herbivore effects suggests that longer exposure of producers to consumption increased net extinctions in the community.
Table 1.
Results of bivariate MA and GLM testing for significant impact of environmental variables on effect sizes of herbivory and fertilization and their interactive effect on richness and evenness of the producer community
| Factor | Effects on species richness |
Effects on evenness |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Herbivory | Fertilization | Interaction | Herbivory | Fertilization | Interaction | |||||||
| Model | MA | GLM | MA | GLM | MA | GLM | MA | GLM | MA | GLM | MA | GLM |
| System† | T > F** | T > M > F** | F > T*** | / | NS | / | T > F*** | / | NS | F = M > T+ | NS | / |
| Productivity (rel. units) | 0.062* | 0.043+ | −0.038* | / | NS | 0.081+ | NS | / | NS | 0.086** | NS | / |
| Herbivore effect biomass | 0.080** | / | — | — | NS | / | NS | / | — | — | NS | −0.081* |
| Fertilization effect biomass | — | — | NS | / | −0.119** | −0.217* | — | — | NS | / | NS | 0.182*** |
| Pielou's evenness (J) | −0.471** | −0.445** | 0.267** | 0.334** | 0.414* | 0.435* | — | — | — | — | — | — |
| Species richness (ln S) | — | — | — | — | — | — | −0.061* | −0.148*** | NS | / | −0.144* | / |
| Experiment duration (ln d) | 0.028** | −0.126*** | −0.059** | −0.051*** | NS | / | NS | / | NS | / | NS | / |
| Experiment size (ln m2) | NS | / | −0.036** | / | NS | / | NS | / | −0.034* | / | 0.036* | 0.027* |
| Latitude,° N or S | NS | / | NS | / | / | −0.004* | / | NS | / | NS | / | |
| F ratio (d.f.) | — | 7.46 (5;45) | — | 16.68 (2;63) | — | 3.53 (3;22) | — | 11.92 (1;92) | — | 2.30 (3;81) | — | 9.23 (3;22) |
| P | — | <0.0001 | — | 0.0024 | — | 0.0316 | — | 0.0008 | — | 0.0838 | — | 0.0004 |
| R2 | — | 0.453 | — | 0.346 | — | 0.325 | — | 0.115 | — | NS | — | 0.557 |
For each response variable, the significant parameter estimates (NS, not significant; /, not included in the final model, –, not applicable) and their significance levels (∗∗∗, P < 0.001; ∗∗, P < 0.01; ∗, P < 0.5; +, P < 0.1; NS, P > 0.1) are given as well as overall model statistics (F ratio with degrees of freedom, significance level, and R2). rel., relative; ln, natural log.
†Significant differences between terrestrial (T), freshwater (F), and marine (M) ecosystems are shown.
Fig. 2.
Effect sizes of herbivory (a–c and h) and fertilization (d and e) and their interaction (f, g, and i) on producer species richness (a–g) and producer community evenness (h and i). Effect sizes are significantly related to producer community evenness (a, d, and g), ecosystem productivity (b), experiment duration (c and e), fertilization effects on producer biomass (f), producer community richness (h), and experiment size (i). Symbols represent freshwater (gray circles), marine (triangles), and terrestrial (black squares) systems. Horizontal lines indicate neutral effects, and thick lines indicate significant regressions from bivariate metaanalysis (see Table 1 for statistical results). rel., relative; Exp., Experiment; Fert., Fertilization; ln, natural log.
Fertilization reduced richness in producer communities with low evenness (i.e., assemblages that were heavily dominated by a few species), suggesting that enrichment favored those species that were already at a competitive advantage. However, fertilization had a neutral-to-positive effect on richness in more even communities (Table 1 and Fig. 2d). In addition, fertilization effect sizes became more negative the longer the experiment lasted (Table 1 and Fig. 2e), a result driven by the longer experimental duration and more negative fertilization effects in terrestrial relative to aquatic experiments (Fig. 1a).
The combined interaction effect size of herbivory and fertilization on producer species richness was positive when producer evenness was high (Table 1 and Fig. 2g); however, this positive interaction between herbivory and fertilization was restricted to communities showing weak to moderate biomass responses to fertilization (Fig. 2f; please note that this regression was nonsignificant when the two extreme points were omitted).
Effect sizes on evenness were only weakly affected by environmental variables relative to the strong environmental mediation of fertilization and herbivory effects on producer richness. Fertilization effects on evenness were consistent across all ecosystems, resulting in a nonsignificant final GLM (Table 1). Herbivores enhanced producer evenness mainly in communities with low species richness, but had near-neutral effects on evenness in speciose communities (Table 1 and Fig. 2h). As experiment size (i.e., the size of a single experimental unit) increased, herbivory and fertilization together tended to increase producer evenness (Table 1), suggesting that positive herbivore effects outweighed negative fertilization effects, especially in larger experiments (Fig. 2i).
Discussion
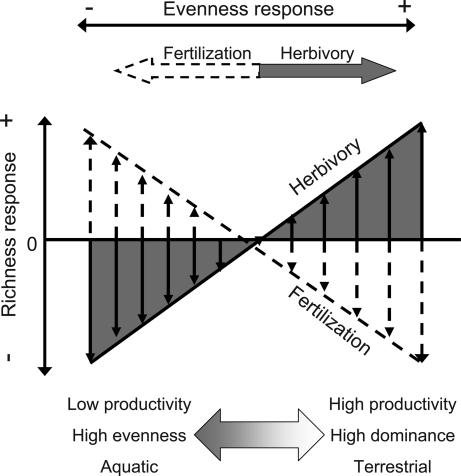
Few generalities have been previously identified regarding the role of consumers and resource availability in controlling producer diversity. In fact, negative and positive effects of both consumers and resources are commonly reported. Our synthesis provides a general framework for reconciling these divergent outcomes across ecosystems into a conceptual model (Fig. 3). This model is based on three primary results of our analysis. First, consumers and fertilization produced differing effects on producer richness and evenness, two distinct aspects of community diversity. Second, fertilization and herbivores affected producer evenness consistently across ecosystems, whereas effects on richness differed among marine, freshwater, and terrestrial systems. Third, effects of fertilization and herbivores on producer richness were significantly related to producer community evenness and ecosystem productivity. Herbivores increase and fertilization decreases producer community evenness with different consequences for the intrinsic control of species richness (Fig. 3). By moving the community toward greater evenness, herbivores increase coexistence where productivity and dominance are high. Fertilization drives the community toward lower evenness, thereby reducing richness in communities already dominated by few species (Fig. 3).
Fig. 3.
Conceptual diagram of local control of producer diversity. The effects of herbivores and fertilization on producer richness differ across gradients of ecosystem productivity and producer community structure and between ecosystem types. Herbivore effects are denoted by solid arrows and lines, fertilization effects by broken lines. The effects of herbivores and fertilization on producer community evenness are consistent across gradients and systems.
Our results indicate that fertilization increased competitive dominance within producer assemblages across all ecosystem types but had different effects on richness in different ecosystems. In terrestrial ecosystems, fertilization reduced both species richness and evenness, consistent with studies showing increased dominance and competitive exclusion with high nutrient deposition (13, 32). This increased dominance by a few species is attributable in part to increased intensity of size-asymmetric competition for light because of increased average producer height (8). In aquatic habitats, fertilization also reduced evenness but enhanced species richness, which may be due in part to the low ability of producer species to monopolize light use in well mixed plankton communities (33–35). Alternatively, larger algal species in periphyton communities, often favored by fertilization, can provide habitat structure for additional life forms such as epiphytes (7).
Herbivory enhanced producer evenness across ecosystems but promoted richness only in terrestrial, but not aquatic, ecosystems. This ecosystem difference may arise from a number of general differences in plant–herbivore interactions between terrestrial and aquatic systems (21). Aquatic systems are characterized by larger body-size ratios between herbivores and producers, which might result in lower selectivity of the herbivore for prey species or prey parts (21). Second, it has been suggested that terrestrial producers have higher investment in structural defenses (36), and structurally defended producer species can provide associational resistance to neighboring plants (37). Both aspects may lead to differences in the spatial heterogeneity of consumption and competition between ecosystems. All of these aspects may lead to stronger consumer-mediated coexistence of producer species in terrestrial systems (Fig. 3), but our database does not provide the means to disentangle these hypotheses.
Beyond the ecosystem differences, we found that fertilization and herbivory effects on richness were strongly associated with producer community evenness. Positive herbivore effects on plant richness were confined to productive plant communities and those with low evenness. The response to fertilization became more positive when producer abundances were relatively evenly distributed. The association between consumer effects and productivity agrees well with earlier studies with more limited data sets (27, 28). We extend these previous syntheses by demonstrating how the effects of consumers on producer richness change along a gradient of resource availability rather than simply comparing fertilized versus unfertilized plots. The strong and opposite associations of herbivore and fertilization effects with producer community evenness reconciles the disparate results from isolated experiments through a common mechanism (Fig. 3). Herbivores, on average, increase evenness, but they enhance producer species richness only if evenness is low (16, 17). These results suggest that herbivores tend to disproportionately reduce the abundance of the most abundant taxa and increase richness only when preferred species are most dominant. In producer communities with numerous codominant species, consumer presence tends to cause extinctions and decrease species richness (15, 16). By contrast, fertilization, on average, decreases evenness (Fig. 3). In producer communities with low evenness, fertilization enhances the dominance of already successful competitors (Fig. 3) and accelerates local extinction of rare species (38). In communities where dominance is low and many species are equally rare, fertilization enhances the persistence of rare species, presumably by increasing population sizes (6). Consequently, the evenness of the producer community also determines the interactive outcome of the combined manipulation of herbivory and fertilization, because positive statistical interactions were restricted to producer communities with high evenness.
Our metaanalysis involved an unprecedented number of experiments (274 in total) addressing producer diversity response to herbivory and/or fertilization. Nevertheless, some systems were underrepresented where few studies have been conducted. Although at least 80 studies were used to calculate each average effect size across systems, the system-specific database was narrower, especially for factorial terrestrial studies (n = 5). Given the moderate covariance between explanatory variables (see Methods), we used two separate analyses. The bivariate MA made use of the full data set but neglected the covariation among contextual explanatory variable. The GLM allowed a multivariate analysis reflecting the covariation between factors but required that all relevant variables were reported for a study. Therefore, the GLM comprised a reduced data set and prevented the test of nonlinear responses or higher-order interaction terms. Despite these caveats, the consistency between the MA and GLM analyses strengthen our confidence in the validity of the observed patterns.
Conclusions
Herbivores increase and fertilization decreases producer evenness across ecosystems, but these factors have contrasting effects on producer richness in aquatic and terrestrial ecosystems. The evenness and productivity of the producer community mediates the effects of consumer presence and resource supply on producer richness. In total, these results lead to a general framework for predicting when local manipulations should enhance or decrease plant species richness. Our cross-system quantification of herbivory and fertilization effects and their interactions is a major step toward a general understanding of local control of producer diversity with vital consequences for conservation and management of species diversity in natural systems. The effects of human perturbations of global biogeochemical cycles and food web structure on local-scale diversity will depend on consumer pressure, resource availability, and producer community structure. The response of producer diversity to enhanced global nutrient availability will depend on the stable persistence of consumer populations, whereas human pressure on consumer species will affect producer diversity differently in nutrient-poor compared with nutrient-rich environments.
Methods
This analysis was compiled from the ELSIE database (EcoLogical Synthesis of Interactive Experiments, see metadata on http://knb.ecoinformatics.org/index.jsp). Studies were selected by examining the abstracts of all publications returned from searches on ISI Web of Science by using the search strings [herbivor* or graz* or consum*] and [resourc* or nutrient* or fertili*] and [divers* or species richness or evenness]. We also included data from studies cited in published syntheses (27, 28). A list of studies can be found in SI Table 2.
We used species richness and evenness to characterize two independent aspects of diversity (the number of distinct traits and their abundance distribution within the community) (39, 40). Richness included both species density and species richness measures, whereas evenness comprised different indices, such as Pielou's, Simpson's, or Hill's indices (39). We used the log response ratio as our effect size metric, which is one of the most commonly used effect metrics in ecological metaanalysis (31, 41). Unlike Hedge's d, the log response ratio does not require a measure of sample variability. Moreover, the analysis of treatment responses relative to that of the control is more meaningful than the use of standard deviations to standardize differences between means when comparing between systems. Finally, the relative treatment responses could be compared irrespective of the measure of evenness used.
We used two different approaches to calculate effect sizes for factorial and nonfactorial studies. For factorial experiments, we calculated a main herbivore (HM), a main fertilization (FM), and an interaction effect (INT) as
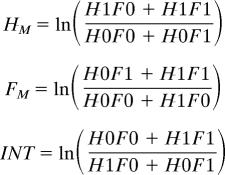 |
where we used the average diversity at grazer presence (H1) and absence (H0) and ambient (F0) and fertilized (F1) treatment combination (“factorial metaanalysis”).
Nonfactorial herbivore (HNF) and fertilization (FNF) effects were calculated as
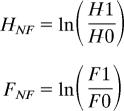 |
By this approach, factorial main effects were directly comparable with effects in nonfactorial experiments, so that without any interaction, a given change in diversity resulted in the same effect size in the factorial and nonfactorial calculation. The factorial interaction effect size denotes whether diversity is higher (positive values) or lower (negative values) than expected from the main effects based on an additive model. Positive interactions can be based on the enhancement of factors favoring diversity or by the mitigation of diversity-reducing effects.
In addition to data on the responses of producer diversity to herbivory and/or fertilization, we recorded information on the environmental context and experimental design for each study. A complete list of the variables and calculations can be found in SI Text. We combined the different aspects of background resource availability (N, P, light, and, for terrestrial studies, water) into one integrative productivity measure (for calculation, see also SI Text and SI Table 3),
As studies reported different numbers of the covariates, statistical tests differed in their degrees of freedom. We used unweighted MA of the log response ratios to calculate average effect sizes for all data and for each of the three study systems. Bootstrapped 95% confidence intervals were used to test whether effect sizes were significantly different from zero. A fixed-model analysis of heterogeneity (41) was used to test for significant differences of average effect sizes among terrestrial, freshwater, and marine ecosystems as well as between factorial and nonfactorial studies. We performed continuous metaanalyses (41) to test how effect sizes on diversity varied with (i) the experimental conditions (size and duration of experiments and latitude of experimental sites), (ii) the ecosystem productivity, (iii) the strength of herbivore or fertilization effects on biomass, and (iv) producer community evenness or richness. We used evenness as explanatory variable for effect sizes on richness and vice versa. For these metaanalyses, we report the slope of the regression and the bootstrapped significance value (999 randomizations).
We observed moderate covariance between the explanatory variables (see SI Text and SI Table 4). To select the optimal model for predicting variation in responses and to account for covariance among the explanatory variables (42), we used the Akaike information criteria (AIC) to select the most parsimonious general linear model (GLM). We report the statistics of the final selected model and estimates of the slopes for the variables included (Table 1).
Supplementary Material
Acknowledgments
Additional data entry was provided by Bettina Höcker, and unpublished data were supplied by Doris Grellmann. The manuscript profited from comments by Claire de Mazancourt, Anja Fitter, Lars Gamfeldt, Stefanie Moorthi, Boris Worm, and two anonymous reviewers. This paper is a publication by the “Trophic Comparison Across Ecosystems” working group at the National Center for Ecological Analysis and Synthesis (NCEAS), a Center funded by National Science Foundation Grant DEB-0072909), the University of California, and University of California (Santa Barbara, CA).
Abbreviations
- GLM
general linear model
- MA
metaanalysis.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0701918104/DC1.
References
- 1.Vitousek PM, Mooney HA, Lubchenco J, Melillo JM. Science. 1997;277:494–499. [Google Scholar]
- 2.Lotze HK, Lenihan HS, Bourque BJ, Bradbury RH, Cooke RG, Kay MC, Kidwell SM, Kirby MX, Peterson CH, Jackson JBC. Science. 2006;312:1806–1809. doi: 10.1126/science.1128035. [DOI] [PubMed] [Google Scholar]
- 3.Vander Zanden MJ, Casselman JM, Rasmussen JB. Nature. 1999;401:464–467. [Google Scholar]
- 4.Holyoak M, Leibold MA, Holt RD. Metacommunities. Chicago: Univ of Chicago Press; 2005. p. 513. [Google Scholar]
- 5.Chesson P. Annu Rev Ecol Syst. 2000;31:343–366. [Google Scholar]
- 6.Tilman D, Pacala S. In: Species Diversity in Ecological Communities. Ricklefs RE, Schluter D, editors. Chicago: Univ of Chicago Press; 1993. pp. 13–25. [Google Scholar]
- 7.Hillebrand H. Oikos. 2003;100:592–600. [Google Scholar]
- 8.Rajaniemi TK. Oikos. 2003;101:449–457. [Google Scholar]
- 9.Olff H, Ritchie ME. Trends Ecol Evol. 1998;13:261–265. doi: 10.1016/s0169-5347(98)01364-0. [DOI] [PubMed] [Google Scholar]
- 10.MacArthur RH. Biol J Linnean Soc. 1969;1:19–30. [Google Scholar]
- 11.Srivastava DS, Lawton JH. Am Nat. 1998;152:510–529. doi: 10.1086/286187. [DOI] [PubMed] [Google Scholar]
- 12.Hutchinson GE. Am Nat. 1959;93:145–159. [Google Scholar]
- 13.Stevens CJ, Dise NB, Mountford JO, Gowing DJ. Science. 2004;303:1876–1879. doi: 10.1126/science.1094678. [DOI] [PubMed] [Google Scholar]
- 14.Suding KN, Collins SL, Gough L, Clark C, Cleland EE, Gross KL, Milchunas DG, Pennings S. Proc Natl Acad Sci USA. 2005;102:4387–4392. doi: 10.1073/pnas.0408648102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hillebrand H, Shurin JB. In: Aquatic Food Webs – an Ecosystem Approach. Belgrano A, Scharler UM, Dunne J, Ulanowicz RE, editors. Oxford: Oxford Univ Press; 2005. pp. 184–197. [Google Scholar]
- 16.Chase JM, Abrams PA, Grover JP, Diehl S, Chesson P, Holt RD, Richards SA, Nisbet RM, Case TJ. Ecol Lett. 2002;5:302–315. [Google Scholar]
- 17.Lubchenco J, Gaines SD. Annu Rev Ecol System. 1981;12:405–437. [Google Scholar]
- 18.Leibold MA. Am Nat. 1996;147:784–812. [Google Scholar]
- 19.Lau JA, Strauss SY. Ecology. 2005;86:2990–2997. [Google Scholar]
- 20.Cebrian J, Lartigue J. Ecol Monogr. 2004;74:237–259. [Google Scholar]
- 21.Shurin JB, Gruner DS, Hillebrand H. Proc R Soc London Ser B. 2006;273:1–9. doi: 10.1098/rspb.2005.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brose U, Jonsson T, Berlow EL, Warren P, Banasek-Richter C, Bersier LF, Blanchard JL, Brey T, Carpenter SR, Blandenier MF, et al. Ecology. 2006;87:2411–2417. doi: 10.1890/0012-9658(2006)87[2411:cbrinf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 23.Elser JJ, Fagan WF, Denno RF, Dobberfuhl DR, Folarin A, Huberty A, Interlandi S, Kilham SS, McCauley E, Schulz KL, et al. Nature. 2000;408:578–580. doi: 10.1038/35046058. [DOI] [PubMed] [Google Scholar]
- 24.Shurin JB, Borer ET, Seabloom EW, Anderson K, Blanchette CA, Broitman B, Cooper SD, Halpern BS. Ecol Lett. 2002;5:785–791. [Google Scholar]
- 25.Borer ET, Seabloom EW, Shurin JB, Anderson KE, Blanchette CA, Broitman B, Cooper SD, Halpern BS. Ecology. 2005;86:528–537. [Google Scholar]
- 26.Halaj J, Wise DH. Am Nat. 2001;157:262–281. doi: 10.1086/319190. [DOI] [PubMed] [Google Scholar]
- 27.Proulx M, Mazumder A. Ecology. 1998;79:2581–2592. [Google Scholar]
- 28.Worm B, Lotze HK, Hillebrand H, Sommer U. Nature. 2002;417:848–851. doi: 10.1038/nature00830. [DOI] [PubMed] [Google Scholar]
- 29.Huston M. Am Nat. 1979;113:81–101. [Google Scholar]
- 30.Kondoh M. Proc R Soc London Ser B. 2001;268:269–271. [Google Scholar]
- 31.Hedges LV, Gurevitch J, Curtis PS. Ecology. 1999;80:1150–1156. [Google Scholar]
- 32.Wassen MJ, Venterink HO, Lapshina ED, Tanneberger F. Nature. 2005;437:547–550. doi: 10.1038/nature03950. [DOI] [PubMed] [Google Scholar]
- 33.Huisman J, Sharples J, Stroom JM, Visser PM, Kardinaal WEA, Verspagen JMH, Sommeijer B. Ecology. 2004;85:2960–2970. [Google Scholar]
- 34.Diehl S, Berger S, Ptacnik R, Wild A. Ecology. 2002;83:399–411. [Google Scholar]
- 35.Sommer U. Limnol Oceanogr. 1994;39:1680–1688. [Google Scholar]
- 36.Strong DR. Ecology. 1992;73:747–754. [Google Scholar]
- 37.Hambäck PA, Agren J, Ericson L. Ecology. 2000;81:1784–1794. [Google Scholar]
- 38.Wilsey BJ, Polley HW. Ecology. 2004;85:2693–2700. [Google Scholar]
- 39.Smith B, Wilson JB. Oikos. 1996;76:70–82. [Google Scholar]
- 40.Wilsey BJ, Chalcraft DR, Bowles CM, Willig MR. Ecology. 2005;86:1178–1184. [Google Scholar]
- 41.Rosenberg MS, Adams DC, Gurevitch J. MetaWin Version 2.0 Statistical Software for Meta-Analysis. Sunderland, MA: Sinauer; 2000. [Google Scholar]
- 42.Johnson JB, Omland KS. Trends Ecol Evol. 2004;19:101–108. doi: 10.1016/j.tree.2003.10.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.