Abstract
Paclitaxel (Taxol) is a well established chemotherapeutic agent for the treatment of solid tumors, but it is limited in its usefulness by the frequent induction of peripheral neuropathy. We found that prolonged exposure of a neuroblastoma cell line and primary rat dorsal root ganglia with therapeutic concentrations of Taxol leads to a reduction in inositol trisphosphate (InsP3)-mediated Ca2+ signaling. We also observed a Taxol-specific reduction in neuronal calcium sensor 1 (NCS-1) protein levels, a known modulator of InsP3 receptor (InsP3R) activity. This reduction was also found in peripheral neuronal tissue from Taxol treated animals. We further observed that short hairpin RNA-mediated NCS-1 knockdown had a similar effect on phosphoinositide-mediated Ca2+ signaling. When NCS-1 protein levels recovered, so did InsP3-mediated Ca2+ signaling. Inhibition of the Ca2+-activated protease μ-calpain prevented alterations in phosphoinositide-mediated Ca2+ signaling and NCS-1 protein levels. We also found that NCS-1 is readily degraded by μ-calpain in vitro and that μ-calpain activity is increased in Taxol but not vehicle-treated cells. From these results, we conclude that prolonged exposure to Taxol activates μ-calpain, which leads to the degradation of NCS-1, which, in turn, attenuates InsP3mediated Ca2+ signaling. These findings provide a previously undescribed approach to understanding and treating Taxol-induced peripheral neuropathy.
Keywords: calcium imaging; dorsal root ganglia; endoplasmic reticulum; polyneuropathy; inositol 1,4,5-trisphosphate receptor
Paclitaxel (Taxol) is a chemotherapeutic drug commonly used in clinical practice for the treatment of solid tumors. One frequent nonhematological side effect of Taxol treatment is a pronounced neurotoxicity, which most commonly manifests as sensory peripheral neuropathy (PNP) (reviewed by ref. 1). Taxol-induced PNP is a dose limiting side effect (2), which despite continuing efforts is still poorly understood.
Many studies point toward a causal role of a disturbed intracellular Ca2+ homeostasis in the pathophysiology of Taxol-induced PNP. In one report the Ca2+ permeable nonselective cation channel, transient receptor potential vanilloid 4 (TRPV4), a receptor which is located in the plasma membrane, was shown to be necessary for Taxol-induced polyneuropathy in rats (3). Other reports showed protective effects of the Ca2+ channel blocker ethosuximide (4) and various drugs that decrease the extra- and intracellular availability of Ca2+ (5). Furthermore, inhibition of the Ca2+-activated calpain proteases has a protective effect against Taxol-induced sensory neuropathy in vivo and Taxol causes activation of both calpains and caspases in PC12 cells (6). Interestingly, other conditions known to be associated with PNP, such as diabetes, are also associated with abnormal Ca2+ homeostasis (7). The comparison of these similar reports strongly suggests that disturbed Ca2+ signaling is one key factor in Taxol-induced PNP and might also be important in other causes of PNP.
We recently identified neuronal Ca2+ sensor-1 (NCS-1) as a novel Taxol-binding protein and showed that Ca2+ oscillations induced by acute exposure to therapeutic concentrations of Taxol are abrogated by NCS-1 knockdown (8). In this study, we aimed to determine whether prolonged exposure to therapeutic concentrations of Taxol alters intracellular Ca2+ signaling in human neuroblastoma cells and primary rat dorsal root ganglia (DRGs). We found that after 6 h of Taxol, but not vehicle, treatment, phosphoinositide-mediated Ca2+ signaling was markedly impaired. This effect is most likely due to degradation of the inositol 1,4,5-trisphosphate receptor (InsP3R) modulator NCS-1 (9), an effect also observed in tissues from animals undergoing Taxol treatment. Intriguingly, Ca2+ signaling recovered after 6 h of Taxol exposure, similar to patients with mild PNP. Furthermore, we showed that Taxol increased calpain activity, calpain degraded NCS-1, and calpain inhibition rescued Ca2+ signaling both in primary rat DRGs and human neuroblastoma cells. These results, in combination with our previous observations of acute effects of Taxol on Ca2+ signaling, introduce a unifying hypothesis for the understanding of Taxol-induced PNP.
Results
Chronic Exposure to Taxol Alters InsP3-Mediated Ca2+ Signaling.
Several studies have addressed the acute effects of Taxol on the Ca2+ homeostasis of various cell lines (10, 11). However, long-term changes in intracellular Ca2+ homeostasis have received much less attention. We recently showed that therapeutic doses of Taxol induce slow Ca2+ oscillations in human neuroblastoma cells (8), a commonly used cell model for peripheral neurons (12). To investigate whether these oscillations still occurred after prolonged exposure to Taxol, we examined the responses of neuroblastoma cells and primary DRG cells after chronic exposure to Taxol.
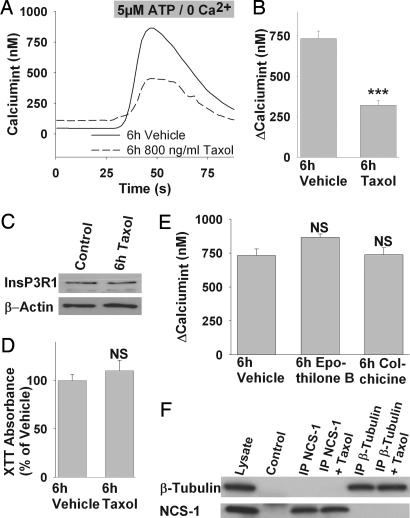
We monitored intracellular Ca2+ changes using the fluorescent Ca2+-sensitive dye Fluo-4/acetoxymethylester (AM) in neuroblastoma cells (SH-SY5Y) incubated for 6 h with 800 ng/ml (937 nM) Taxol. Under these conditions, there was no significant spontaneous activity in Taxol-treated cells compared with vehicle control (not shown). Because oscillations induced by acute exposure to Taxol depend on intact phosphoinositide signaling (8), we tested whether this pathway was functioning properly after prolonged exposure to Taxol. Ca2+ responses were induced by ATP, an extracellular agonist known to initiate InsP3-mediated Ca2+ release through activation of metabotropic P2Y receptors (13). To prevent a confounding signal of the ionotropic P2X receptor family (14), cells were perfused with Ca2+-free (0 Ca2+ and 10 mM EGTA) buffer containing 5 μM ATP. The ability of the cells to respond to ATP was altered after Taxol incubation. Cells treated for 6 h with Taxol before loading with the ratiometric Ca2+ indicator Fura-2 showed a marked reduction in the response amplitude (Fig. 1A). The average increase in intracellular Ca2+ after addition of 5 μM ATP was 733 ± 48 nM (156/7) in vehicle-treated cells and 321 ± 30 nM (136/6) in cells treated with 800 ng/ml Taxol (P < 0.001, Fig. 1B).
Fig. 1.
Chronic exposure to Taxol alters InsP3-mediated Ca2+ signaling in neuroblastoma cells. (A) Representative Ca2+ response to ATP stimulation of cells treated for 6 h with vehicle (solid line) or Taxol (dashed line). (B) The average response amplitude is significantly lower in cells treated with 800 ng/ml Taxol for 6 h compared with vehicle. (C) Taxol treatment (800 ng/ml, 6 h) alters neither InsP3R expression in Western blot analysis nor cell viability (D). (E) Treatment with other microtubule-stabilizing (100 nM Epothilone B) or disrupting drugs (1 μg/ml Colchicine) for 6 h does not impair the response to 5 μM ATP, suggesting a Taxol-specific effect. (F) Coimmunoprecipitation of β-tubulin and NCS-1 from mouse cerebellar lysate. Lanes are: (1) mouse cerebellar lysate, (2) beads treated with preimmune serum but no specific antibody, (3) immunoprecipitate with anti-NCS-1, (4) immunoprecipitate with anti-NCS-1 and 800 ng/ml Taxol, (5) immunoprecipitate with anti-β-tubulin, and (6) immunoprecipitate with anti-β-tubulin and 800 ng/ml Taxol. Upper was probed with anti-β-tubulin, and Lower was probed with anti-NCS-1. ∗∗∗, P < 0.001; NS, not significant.
The basis for the attenuated response was tested. Although Taxol acts to stabilize microtubules, we previously showed that there is no direct interaction between the InsP3R and tubulin (8). A reduction in InsP3R levels could explain the decreased response, but InsP3R expression after 6 h of Taxol exposure was not different compared with vehicle treatment (Fig. 1C). Another possibility was that Taxol exposure depleted endoplasmic reticulum (ER) stores. To address this question, we depleted the ER by adding 5 μM sarcoplasmic reticulum–ER ATPase inhibitor thapsigargin in Ca2+-free medium and calculated the ER-Ca2+ content as area under the release curve. Both Taxol- (114.9 ± 6%) and vehicle- (100 ± 8.7%) treated cells had similar amounts of Ca2+ stored in the ER, making store depletion an unlikely explanation. A further possibility was that the expression of the purinergic receptors P2Y2 or P2Y4 is down-regulated, resulting in a decreased response to ATP. However, we did not observe any significant alteration of either P2Y2 or P2Y4 receptor expression in cells treated with 800 ng/ml Taxol for 6 h [supporting information (SI) Fig. 5 A and B and SI Text]. Additionally, no alterations of the phosphatidylinositol 4-kinase levels were observed after Taxol treatment (SI Fig. 6 and SI Text) and membrane phosphatidylinositol bisphosphate levels were unaltered (not shown), suggesting an intact phosphoinositide metabolism.
Because Taxol has been shown to induce apoptosis, probably through the interaction of Taxol with BCL-2 (15), we tested the possibility that the observed reduction in the response amplitude is due to decreased cell viability. Using a measure of metabolic activity [2,3-bis[2-methoxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxanilide (XTT) assay], it was possible to measure the integrity of cellular metabolism (16). Treatment of neuroblastoma cells with Taxol did not affect the formation of XTT (Fig. 1D), which indicates that cell viability was not impaired after an exposure of 6 h. However, there was a significant reduction of XTT production to 17.9 ± 2.4% (3) in cells treated with 800 ng/ml Taxol for 24 h when compared with vehicle control (P < 0.001, not shown).
Another sensitive indicator of early cell death and apoptosis is the loss of plasma membrane asymmetry (17), which can be measured with annexin-V-FITC labeling. After 6 h of vehicle or Taxol treatment, there was only sparse annexin-V-FITC labeling, whereas 6-h incubation with 1 μM apoptosis inducer staurosporin induced very pronounced annexin-V-FITC staining (not shown). Cell death or impaired cellular viability is therefore unlikely to account for the observed alteration of InsP3-mediated Ca2+ release.
Other Microtubule-Altering Drugs Do Not Affect InsP3-Mediated Ca2+ Signaling.
To further identify whether this effect was due to alterations of the microtubule cytoskeleton or to another Taxol-specific action, we tested the cellular responses after exposure to the microtubule stabilizing agent epothilone B. This reagent has the same microtubule-binding site as Taxol (18) but is ≈10 times more effective (19) and has fewer neurotoxic side effects (reviewed by ref. 20). Because epothilone B is more potent than Taxol, a concentration of 50 ng/ml (100 nM) was used in these experiments. To contrast the microtubule stabilizing actions of Taxol and epothilone B, we also tested the microtubule disrupting drug colchicine at 1 μg/ml (2.5 μM); the use of these reagents allowed us to further examine the role of microtubules on InsP3-mediated Ca2+ signaling.
Cells incubated for 6 h with epothilone B or colchicine did not show any significant difference compared with vehicle when stimulated with 5 μM ATP in Ca2+ free solution. Intracellular Ca2+ increased by 866 ± 25 nM (136/6) in epothilone B-treated cells and 738 ± 51 nM (142/6) in colchicine-treated cells (both not significant by ANOVA; Fig. 1E). Therefore, alterations of the microtubule network do not appear to be responsible for the observed reduced Ca2+ response in Taxol-treated cells.
We recently identified NCS-1, a high-affinity low-capacity Ca2+-binding protein as a modulator of the InsP3R (9) and as a Taxol-binding protein (8). In this context, we were interested whether, in Taxol-treated cells, NCS-1 could be sequestered to the tubulin cytoskeleton, and this association would make NCS-1 unavailable for binding to the InsP3R. The decreased availability of NCS-1 would then lead to reduced InsP3-mediated Ca2+ release. However, no indication was found for an association of tubulin and NCS-1 when tested using coimmunoprecipitation from cerebellar lysate. Only the protein directly associated with the immunoprecipitating antibody could be detected regardless of the presence of Taxol (Fig. 1F). Therefore, it is unlikely that the reduced response to ATP stimulation in Taxol-treated cells is due to sequestration of NCS-1.
Chronic Exposure to Taxol Reduces NCS-1 Levels.
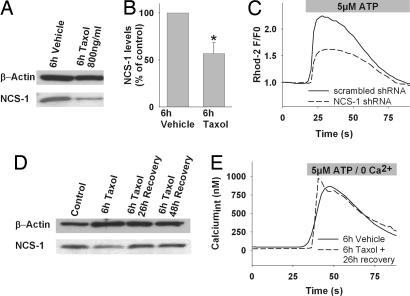
Another possible explanation for the observed diminished Ca2+ response to ATP stimulation is that NCS-1 is degraded in Taxol-treated cells, independent of the microtubule cytoskeleton. Immunoblot analysis of NCS-1 protein levels in cells treated for 6 h with vehicle or 800 ng/ml Taxol revealed a significant Taxol-specific reduction of NCS-1 to 56.9 ± 11.6% compared with vehicle treatment (Fig. 2 A and B). No effect was observed when epothilone B or colchicine was studied (not shown)
Fig. 2.
Chronic exposure to Taxol reduces NCS-1 levels. (A) Representative Western blot, probed for NCS-1, shows decreased immunoreactivity after Taxol treatment, for this protein but not the loading control (β-actin). (B) Normalized NCS-1 immunoreactivity is significantly reduced in Taxol-treated cells (800 ng/ml, 6 h). (C) shRNA-mediated NCS-1 knockdown (dashed line) reduces the Ca2+ response to ATP similar to Taxol treatment. (D) NCS-1 levels recover to baseline 26 h after treatment with Taxol (800 ng/ml, 6 h). (E) Representative Ca2+ response of cells treated for 6 h with vehicle (solid line) or Taxol (800 ng/ml, 6 h; dashed line) followed by a 26-h recovery period. ∗, P < 0.05.
To verify that reduced NCS-1 protein levels can lead to a reduction in the response amplitude, NCS-1 levels were reduced by transient transfection with a vector coexpressing anti-NCS-1 short hairpin RNA (shRNA) and GFP, as described (8). Coexpression with GFP allowed identification of those cells expressing the NCS-1 shRNA, which would contain less NCS-1 protein. Ca2+ transients were monitored by using the red fluorescent dye Rhod-2/AM, plotted as normalized fluorescent ratio F/F0, and the peak of the transient was measured and compared with control cells expressing scrambled shRNA. NCS-1 knockdown cells showed significantly (P < 0.001) reduced response amplitudes, which were on average only 54.5 ± 4.7% compared with scrambled shRNA expressing cells (100 ± 4.8%; Fig. 2C). These results are consistent with the suggestion that reduced NCS-1 protein levels in Taxol-treated cells lead to reduced InsP3-mediated Ca2+ signaling.
One interesting feature of Taxol-induced peripheral polyneuropathy is that many patients with mild sensory neuropathies show a recovery when the treatment is discontinued (21). It was thus of interest to test whether NCS-1 protein levels and Ca2+ signaling recover after cessation of Taxol exposure, or whether the damage was irreversible. Cells were treated either with 800 ng/ml Taxol or vehicle for 6 h and left to recover 26 and 48 h. Within 26 h of recovery, NCS-1 levels in Taxol-treated cells had returned to control levels (Fig. 2D). Taxol-treated cells also showed a complete recovery in ATP-induced Ca2+ signaling after 26 h without Taxol (Fig. 2E), with a mean increase in intracellular Ca2+ of 770 ± 27 nM (184/7) in response to stimulation with 5 μM ATP. These values for the responses in recovered cells were not significantly different from vehicle treatment only (733 ± 48 nM; 156/7).
Chronic Exposure to Taxol Alters InsP3-Mediated Ca2+ Signaling in Primary DRGs.
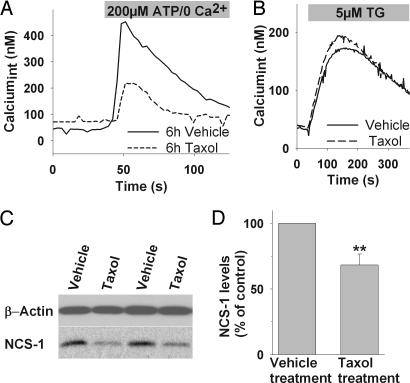
Because our results imply an important role for altered Ca2+ signaling in Taxol-induced polyneuropathy, it was important to determine whether these findings could be reproduced in a primary cell, such as rat DRGs. Previous studies on diabetes-induced abnormalities reported a more efficient detection of alterations in ER-mediated Ca2+ release when cells were preconditioned with a brief depolarization to fill the stores (7). This protocol was used to test the effects of chronic exposure to Taxol. After preconditioning, the average Ca2+ increase in DRGs treated with vehicle for 6 h in response to 200 μM ATP was 216 ± 28 nM (86/4). Taxol-treated DRGs (800 ng/ml) had an attenuated response with an average Ca2+ increase of 117 ± 54 nM (101/4) (P < 0.05; Fig. 3A). This observation supports conclusions acquired in human neuroblastoma cells. Similar to our findings in the cell line, Taxol did not significantly alter ER-Ca2+ content with an average normalized Ca2+ content of 101 ± 9% compared with vehicle treated cells (100 ± 6%; not significant; Fig. 3B). Cell viability was not significantly different in the two groups, with a normalized XTT absorbance of 107 ± 15% relative to the vehicle control (100 ± 18%; not significant).
Fig. 3.
Chronic exposure to Taxol alters InsP3-mediated Ca2+ signaling in primary DRGs. (A) Representative Ca2+ response of DRGs treated for 6 h with vehicle (solid line) or Taxol (800 ng/ml, 6 h; dashed line) to ATP stimulation. (B) Depletion of ER-Ca2+ with thapsigargin (TG) shows comparable amounts of stored Ca2+ in Taxol-treated and untreated cells. (C) Representative Western blot of DRGs isolated from four different animals, treated with Taxol or vehicle, shows that 6-h Taxol treatment leads to reduced NCS-1 levels in vivo. (D) Normalized NCS-1 immunoreactivity is significantly reduced in DRGs isolated from Taxol treated animals (n = 4) compared with animals receiving vehicle treatment (n = 4). ∗∗, P < 0.01.
To study whether NCS-1 levels are altered in vivo, we isolated DRGs from mice treated with Taxol- or sham injections. NCS-1 levels in isolated DRGs from mice treated with Taxol for 6 h were reduced significantly (P < 0.01) to 68 ± 8% (n = 4) compared with vehicle treatment (100%, n = 4) (Fig. 3 C and D). The reproduction of the key observations obtained in the neuroblastoma cell culture in primary DRGs and in tissue of Taxol treated mice supports the validity of the cell model.
Taxol Treatment Activates the NCS-1-Degrading Protease Calpain.
Previous studies on Taxol-induced polyneuropathy suggested that inhibition of the Ca2+-activated protease calpain confers protection against polyneuropathy in vivo (6). In light of our previous observation, that Taxol induces slow Ca2+ oscillations (8), and the present study, that NCS-1 gets degraded over time in Taxol-treated cells, it was important to determine whether calpain activity was altered in the isolated cell models. Measurements of calpain activity using a luminescence-based assay showed that the normalized calpain activity was 121 ± 4% (5), significantly higher (P < 0.01) in Taxol- (800 ng/ml, 6 h) than vehicle-treated control (100 ± 1%).
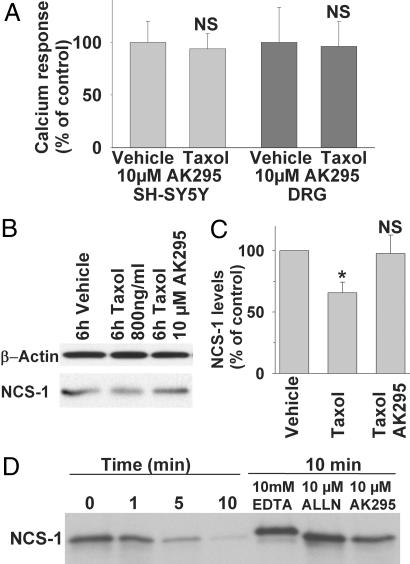
To determine whether calpain activation is related to our finding of Taxol-induced alterations in phosphoinositide-mediated Ca2+ signaling, we incubated both neuroblastoma cells and DRGs with Taxol (800 ng/ml, 6 h) and the cell-permeable calpain inhibitor AK295 (6); controls were incubated with vehicle and AK295. In both cell models, incubation with the calpain inhibitor prevented Taxol-mediated suppression of ATP-induced Ca2+ release. The normalized response amplitude was 96 ± 24% in DRGs and 94 ± 14% in SH-SY5Y cells compared with their vehicle controls (both not significant; Fig. 4A). Interestingly, incubation of cells with Taxol and AK295 prevented the degradation of NCS-1 (98 ± 15% of immunoreactivity compared with vehicle control) that was observed in Taxol-treated cells in the absence of calpain inhibition (Fig. 4 B and C). We also observed that degradation of NCS-1 in Taxol-treated cells is prevented if the cells are preloaded with 10 μM 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate(BAPTA)–AM for 1 h (SI Fig. 7 and SI Text). These findings support the hypothesis that Ca2+-mediated activation of calpain degrades NCS-1.
Fig. 4.
Taxol treatment activates the NCS-1-degrading protease calpain. (A) Addition of the calpain inhibitor AK295 prevents Taxol (800 ng/ml, 6 h)-induced reduction of the response amplitude in DRGs and neuroblastoma cells. (B) Western blot analysis reveals that AK295 also prevents the reduction in NCS-1 immunoreactivity. (C) Normalized NCS-1 immunoreactivity is significantly reduced in Taxol-treated cells but not in cells incubated with 10 μM AK295 (n = 3). (D) NCS-1 is cleaved by μ-calpain. Addition of EDTA or the calpain inhibitors ALLN or AK295 prevents NCS-1 cleavage by μ-calpain. NS, not significant; ∗, P < 0.05.
To confirm a possible NCS-1–μ-calpain interaction, purified μ-calpain was incubated with purified NCS-1 protein at 37°C in the presence of 50 μM free Ca2+, 0 Ca2+ (10 mM EDTA), or with 50 μM free Ca2+ and the calpain inhibitors ALLN or AK295. Calpain rapidly degraded NCS-1 in the presence of activating Ca2+ concentrations, but little to no degradation was observed in the absence of Ca2+ or in the presence of Ca2+ and calpain inhibitors (Fig. 4D). As an aside, we also observed that NCS-1 changes its apparent molecular weight when kept in Ca2+-free conditions, agreeing with the large conformational shift observed upon the binding of Ca2+ (22). Taken together, these results support the conclusion that the observed reduction in ATP-induced Ca2+ response both in human neuroblastoma cells and primary rat DRGs is due to a calpain-mediated degradation of the InsP3R-modulating protein NCS-1.
Discussion
The aim of this study was to investigate the effects of prolonged exposure to submicromolar concentrations of Taxol, as found in patients undergoing chemotherapy for solid tumors (23), on the Ca2+ homeostasis of neuronal cells. After 6 h of Taxol treatment, we found that phosphoinositide-mediated Ca2+ signaling was significantly impaired in these cells but did not observe altered spontaneous activity. The present result is in contrast to those obtained with acute exposure to Taxol, a treatment that induced Ca2+ oscillations (8). This attenuation of signaling was not due to InsP3 or P2YR degradation, store depletion, or decreased cell viability. Furthermore, the reduced signaling appears to be Taxol-specific, because other microtubule-altering drugs, such as colchicine and the Taxol analog epothilone B, did not induce this effect. However, we recently found that acute alterations in intracellular Ca2+ signaling induced by Taxol depend upon the high-affinity low-capacity Ca2+-binding protein NCS-1, and that NCS-1 is a novel Taxol-binding protein (8). Moreover, InsP3R activity is modulated by NCS-1 (9). In this context, it was of interest to determine whether NCS-1 might also play a role in the observed alterations in phosphoinositide signaling after prolonged exposure to Taxol.
In the course of these experiments, we found a significant reduction of NCS-1 protein levels in Taxol-treated cells as well as in tissues obtained from Taxol-treated animals and a decrease in InsP3-mediated Ca2+ signaling in cells. This reduction could be induced directly in cells where NCS-1 levels had been reduced by using shRNA. Intriguingly, NCS-1 levels as well as Ca2+ signaling recovered when exposure to Taxol was stopped. Because previous reports suggested that calpain inhibition confers protection against Taxol-induced PNP (6), we measured calpain activity and found a significant increase in Taxol-treated cells. Calpain inhibition prevented both alterations in phosphoinositide-mediated Ca2+ signaling and NCS-1 degradation. NCS-1 degradation could also be prevented by buffering intracellular Ca2+ with 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate (BAPTA)–AM. We also identified NCS-1 as a μ-calpain substrate, underlining the importance of calpain activation for the altered Ca2+ responses to Taxol.
These are important findings, as they offer an explanation for several previous in vitro and in vivo studies, and they will improve our understanding and possibly the treatment of Taxol-induced PNP. The present results add support to the claim that PNP is not a cremophor EL-vehicle side effect as suggested by various authors (reviewed by ref. 24). Our study agrees with a report in human subjects that shows that pharmacokinetics, not the vehicle, are relevant for Taxol-induced PNP (25). Another important aspect of our study is that we confirm that Taxol exerts biological effects in addition to tubulin stabilization. The present study also shows that InsP3-mediated Ca2+ release was similarly impaired in primary rat DRGs and the human neuroblastoma cell line SH-SY5Y after Taxol exposure.
Our observation, that Ca2+ signaling is impaired before cell viability deteriorates offers an explanation why mild, but not severe, PNP appears reversible in patients (21). In this model, Ca2+ oscillations induced by acute exposure to Taxol (8) would lead to Ca2+-mediated calpain activation. Although the Ca2+-dependent protease μ-calpain can cleave many proteins, an important effect in the context of Taxol comes from the degradation of NCS-1 by calpain. The loss of NCS-1 would then lead in a negative-feedback loop to the cessation of Ca2+ oscillations and to impaired phosphoinositide-mediated Ca2+ signaling. This scenario would be reversible if exposure to Taxol is halted; patients would observe this sequence of events as mild PNP. However, if the patient continued to receive frequent Taxol infusions for longer time periods or at higher doses, two risk factors for Taxol-induced PNP (25), this cascade would be triggered repeatedly with mounting damage to peripheral neurons. Ultimately, if unchecked, the process would lead to necrotic or apoptotic cell death (reviewed by ref. 26).
Intriguingly, knockout of TRPV4, a Ca2+-permeable nonselective cation channel located in the plasma membrane, has been shown to be necessary for Taxol-induced polyneuropathy in rats (3). TRPV4 currents have also been shown to be potentiated by increases in intracellular Ca2+ (27). In light of our results and previous observations of high levels of NCS-1 in DRGs (28), it appears likely that TRP channels amplify Ca2+ signals originating at the ER. These large Ca2+ transients will achieve Ca2+ concentrations that can activate calpain (29).
Although several different processes may be involved, it is of great interest for PNP research that a similar reduction in phosphoinositide-mediated Ca2+ signaling was observed in diabetic rats (7). This suggests a more general involvement of InsP3-mediated signaling and dysregulated Ca2+ homeostasis in DRG (mal)function worth further elucidation. This hypothesis is also supported by a recent study where Ca2+-reducing drugs were shown to ameliorate not only Taxol but also vincristine- and dideoxycytidin-induced PNP (5). Again, the molecular mechanism for induction of the PNP by the latter drugs is not known and may be distinct from that used by Taxol. Nonetheless, the combination of our results and prior reports provide good evidence that disturbed Ca2+ homeostasis is a hallmark event in many PNPs.
We report that prolonged exposure of neuronal cells with low concentrations of Taxol leads to impaired phosphoinositide signaling. These findings introduce an approach to understanding Taxol-induced PNP, provide a mechanism for the positive response to calpain inhibition in animals (by stopping Ca2+ mediated activation of calpain, which leads to NCS-1 degradation), and integrate several existing studies into a unified hypothesis. This information will assist in the understanding of the side effects of Taxol treatment and may help devise new strategies for optimization and management of Taxol therapy.
Materials and Methods
Plasmids.
NCS-1 or scrambled shRNA expressing vectors were used as described before (8).
SH-SY5Y Culture and Transfection.
The human neuroblastoma cell line SH-SY5Y (American Type Culture Collection, Manassas, VA) was cultured and transfected as described (12).
DRG Culture.
DRG isolated from rat neonates (P1–3) were digested in 0.25% collagenase and separated by gentle trituration. Triturated cells were passed through a 70-μm cell strainer to remove cell clumps, and DRGs were plated on polyD-lysine/laminin coated cover slips. DRGs were maintained with Neurobasal-A media supplemented with B-27 (GIBCO–Invitrogen, Carlsbad, CA)/0.5 mM glutamine/fresh nerve growth factor (10 ng/ml) and incubated overnight in a 95% air/5% CO2 humidified atmosphere in an incubator at 37°C, before treatment and imaging. Before imaging, Ca2+ stores were preconditioned by a brief depolarization (<10 s) with standard solution (as described below) containing 84.7 mM NaCl and 50 mM KCl, followed by a 5-min recovery in standard solution with normal (4.7 mM) K+ content. In imaging experiments, DRGs were identified by depolarization with the high K+ solution. Only cells with a Ca2+ increase of at least 200 nM compared with baseline were included in data evaluation.
Treatment of Animals with Taxol.
Treatment of animals with Taxol was approved by the Yale University Animal Care and Use Committee. C57bl6 mice were injected with 60 mg/kg Taxol i.p. and killed 6 h after injection. DRG isolation and Western blot analysis were performed as described in DRG Culture and Immunoprecipitation and Western Blot Analysis.
Live Cell Imaging.
Fura-2.
Cells were loaded with 5 μM Fura-2/AM (Molecular Probes–Invitrogen, Carlsbad, CA) at 37°C for 30 min in a standard solution: 130 mM NaCl/4.7 mM KCl/1 mM MgSO4/1.2 mM KH2PO4/1.3 mM CaCl2/20 mM Hepes/5 mM glucose (pH 7.4)/0.02% pluronic F-127 (Molecular Probes–Invitrogen). After loading, Fura-2 was allowed to deesterify for at least 10 min at room temperature in standard solution. Coverslips were mounted in a perfusion chamber (Warner Instruments, Hamden, CT) and placed on an inverted Zeiss Axiovert 135 stage equipped with Fluar 40×/1.3 and Fluar 20×/0.75 objectives (Zeiss, Thornwood, NY). Fluorescence data were acquired on a personal computer running Imaging Workbench software (INDEC BioSystems, Santa Clara, CA) via a cooled CCD camera (SensiCam, ASI, Eugene, OR). For ATP stimulation experiments, cells were perfused with ATP in Ca2+-free Hepes buffer: 130 mM NaCl/4.7 mM KCl/2.3 mM MgSO4/1.2 mM KH2PO4/10 mM EGTA/20 mM Hepes/5 mM glucose, pH 7.4.
Intracellular free Ca2+ concentration [(Ca2+)int] was derived from background-subtracted F340/F380 fluorescent ratios (R) after in situ calibration (30, 31) according to the following equation: [Ca2+]int (nM) = Kd·Q·(R−Rmin)/(Rmax−R), where Kd is the dissociation constant of fura-2 for Ca2+ at room temperature (225 nM); Q is the fluorescence ratio of the emission intensity excited by 380 nm in the absence of Ca2+ to that during the presence of saturating Ca2+; and Rmin and Rmax are the minimal or maximal fluorescence ratios, respectively. Rmin was measured by perfusion with Ca2+-free Hepes buffer (as described above) containing 10 μM ionomycin. Rmax was obtained by perfusion with standard solution containing 10 mM CaCl2/10 μM ionomycin. ER-Ca2+ was measured by depletion of ER stores with 5 μM sarcoplasmic reticulum–ER ATPase inhibitor thapsigargin over 5 min in Ca2+-free Hepes buffer. Released Ca2+ was calculated by subtracting baseline Ca2+ and plotted over time. The area under the release curve was calculated by using a macro in SigmaPlot 10 (Systat Software, Richmond, CA).
Fluo-4/Rhod-2.
Cells were incubated (30 min at 37°C) in Hepes medium containing 5 μM Fluo-4/AM or 6 μM Rhod-2/AM (Molecular Probes–Invitrogen) together with 0.1% Pluronic F-127 (Molecular Probes–Invitrogen). The Hepes medium contained: 130 mM NaCl/4.7 mM KCl/1 mM MgSO4/1.2 mM KH2PO4/1.3 mM CaCl2/20 mM Hepes/5 mM glucose, pH 7.4. Coverslips were mounted in a chamber (Warner Instruments, Hamden, CT) and transferred to a Zeiss LSM 510 META scanning laser confocal microscope equipped with a C-Apochromat ×40/1.2 water-immersion objective (Zeiss). Images were acquired at 0.5 Hz. All drugs were bath-applied. To identify cells expressing shRNA with GFP, transfected cells were examined by using fluorescence excitation at 488 nm in a multitrack configuration to minimize crosstalk. Cells that responded neither to stimulation (≥20% increase of fluorescence) nor to control stimulation with 3 μM thapsigargin were excluded from evaluation. Ca2+-induced fluorescence intensity ratio F/F0 was plotted as a function of time with F0 as an average of the first five points of the baseline, and the response was quantitated with an IgorPro macro (Wavemetrics, Lake Oswego, OR).
Cell Viability Assays.
XTT assay.
Cell viability was assessed by using the TOX-2 kit (Sigma–Aldrich, St. Louis, MO) according to the manufacturer's protocol as described (32). Results are expressed as percentages with respect to controls (vehicle-treated cells).
Annexin V labeling.
Cells were incubated for 6 h in vehicle, Taxol, or staurosporin. Annexin V labeling was performed and analyzed by using fluorescein isothiocyanate-conjugated annexin V (BD Biosciences, San Jose, CA), as described (32).
Immunoprecipitation and Western Blot Analysis.
Lysate preparation, immunoprecipitation and immunoblotting was performed as described (9). Antibodies used were as follows: anti-NCS-1 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-β actin, and GAPDH (Abcam, Cambridge, MA), anti-β-tubulin (Covance, Berkeley, CA), and anti-InsP3R (33). For immunoprecipitation, cerebellar lysate was incubated with antibody in the presence of 50 μM free Ca2+ and 800 ng/ml Taxol, as indicated in Results. NCS-1 expression blots were quantified by scanning densitometry by using UN-SCAN-IT (Silk Scientific, Orem, UT) normalizing NCS-1 expression to the β-actin-loading control. All Western blot experiments were done with three independent cultures unless stated otherwise.
Calpain Assays.
Activity.
Cells were grown in six-well plates to confluence, treated with vehicle or Taxol for 6 h, rinsed twice with PBS, and lysed with cytobuster protein extraction reagent (Calbiochem, San Diego, CA). The lysate was cleared of debris by centrifugation (5′, 16,000×g), and used for the calpain-Glo protease assay (Promega, Madison, WI) according to the manufacturer's protocol. In this assay, calpain activity is measured with luminescence. The relative luminescence was averaged over 10 sec, background-subtracted, and normalized to the amount of protein in the lysate.
NCS-1 digest.
Purified NCS-1 protein was diluted in assay buffer (10 mM Hepes/10 mM DTT, pH 7.2) with 50 μM free Ca2+ and 110 nM purified μ-calpain (Calbiochem) at 37°C. Samples were taken at regular intervals as indicated in Results, and the reaction was stopped by denaturing the proteins in SDS sample buffer. NCS-1 was visualized with immunoblotting performed as described above.
Statistical Analysis.
Data are expressed as mean ± SEM or as representative traces. (n/N) describes the number of cells studied (n) in N independent cultures. Statistical analysis of the differences between multiple groups was performed by using one-way ANOVA (Dunnett multiple-comparisons test) (Instat; GraphPad, San Diego, CA) for two groups using t test (SigmaPlot, Systat); P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Yung-Chi Cheng for support with the treatment of animals with Pacitaxel and Andreas Jeromin (University of Texas, Austin, TX) and Jolanta Vidugiriene (Promega, Madison, WI) for shRNA reagents. We thank Manuel Estrada, Per Uhlen, Anurag Varshney, and Brenda DeGray for invaluable advice regarding the design of the experiments and thoughtful discussions and comments on the manuscript. This work was supported by a grant from the Robert Leet and Clara Patterson Guthrie Trust (to B.E.E.) and by German National Merit Foundation scholarships (to W.B., K.Z., and F.M.H.).
Abbreviations
- PNP
peripheral neuropathy
- ER
endoplasmic reticulum
- InsP3R
inositol 1,4,5-trisphosphate receptor
- NCS-1
neuronal calcium sensor-1
- DRG
dorsal root ganglion
- shRNA
short hairpin RNA
- AM
acetoxymethylester
- XTT
2,3-bis[2-methoxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxanilide.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0701546104/DC1.
References
- 1.Mielke S, Sparreboom A, Mross K. Eur J Cancer. 2006;42:24–30. doi: 10.1016/j.ejca.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 2.Wasserheit C, Frazein A, Oratz R, Sorich J, Downey A, Hochster H, Chachoua A, Wernz J, Zeleniuch-Jacquotte A, Blum R, et al. J Clin Oncol. 1996;14:1993–1999. doi: 10.1200/JCO.1996.14.7.1993. [DOI] [PubMed] [Google Scholar]
- 3.Alessandri-Haber N, Dina OA, Yeh JJ, Parada CA, Reichling DB, Levine JD. J Neurosci. 2004;24:4444–4452. doi: 10.1523/JNEUROSCI.0242-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flatters SJ, Bennett GJ. Pain. 2004;109:150–161. doi: 10.1016/j.pain.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 5.Siau C, Bennett GJ. Anesth Analg. 2006;102:1485–1490. doi: 10.1213/01.ane.0000204318.35194.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang MS, Davis AA, Culver DG, Wang Q, Powers JC, Glass JD. Brain. 2004;127:671–679. doi: 10.1093/brain/awh078. [DOI] [PubMed] [Google Scholar]
- 7.Kruglikov I, Gryshchenko O, Shutov L, Kostyuk E, Kostyuk P, Voitenko N. Pflügers Arch. 2004;448:395–401. doi: 10.1007/s00424-004-1263-8. [DOI] [PubMed] [Google Scholar]
- 8.Boehmerle W, Splittgerber U, Lazarus MB, McKenzie KM, Johnston DG, Austin DJ, Ehrlich BE. Proc Natl Acad Sci USA. 2006;17:17. doi: 10.1073/pnas.0607240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlecker C, Boehmerle W, Jeromin A, DeGray B, Varshney A, Sharma Y, Szigeti-Buck K, Ehrlich BE. J Clin Invest. 2006;116:1668–1674. doi: 10.1172/JCI22466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kidd JF, Pilkington MF, Schell MJ, Fogarty KE, Skepper JN, Taylor CW, Thorn P. J Biol Chem. 2002;277:6504–6510. doi: 10.1074/jbc.M106802200. [DOI] [PubMed] [Google Scholar]
- 11.Mironov SL, Ivannikov MV, Johansson M. J Biol Chem. 2005;280:715–721. doi: 10.1074/jbc.M409819200. [DOI] [PubMed] [Google Scholar]
- 12.Estrada M, Uhlen P, Ehrlich BE. J Cell Sci. 2006;119:733–743. doi: 10.1242/jcs.02775. [DOI] [PubMed] [Google Scholar]
- 13.Vaziri C, Downes CP. Biochem J. 1992;284:917–922. doi: 10.1042/bj2840917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benham CD. Ann NY Acad Sci. 1990;603:275–285. doi: 10.1111/j.1749-6632.1990.tb37679.x. discussion 285–276. [DOI] [PubMed] [Google Scholar]
- 15.Rodi DJ, Janes RW, Sanganee HJ, Holton RA, Wallace BA, Makowski L. J Mol Biol. 1999;285:197–203. doi: 10.1006/jmbi.1998.2303. [DOI] [PubMed] [Google Scholar]
- 16.Scudiero DA, Shoemaker RH, Paull KD, Monks A, Tierney S, Nofziger TH, Currens MJ, Seniff D, Boyd MR. Cancer Res. 1988;48:4827–4833. [PubMed] [Google Scholar]
- 17.van Engeland M, Nieland LJ, Ramaekers FC, Schutte B, Reutelingsperger CP. Cytometry. 1998;31:1–9. doi: 10.1002/(sici)1097-0320(19980101)31:1<1::aid-cyto1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 18.Bollag DM, McQueney PA, Zhu J, Hensens O, Koupal L, Liesch J, Goetz M, Lazarides E, Woods CM. Cancer Res. 1995;55:2325–2333. [PubMed] [Google Scholar]
- 19.Kowalski RJ, Giannakakou P, Hamel E. J Biol Chem. 1997;272:2534–2541. doi: 10.1074/jbc.272.4.2534. [DOI] [PubMed] [Google Scholar]
- 20.Goodin S, Kane MP, Rubin EH. J Clin Oncol. 2004;22:2015–2025. doi: 10.1200/JCO.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Rowinsky EK, Chaudhry V, Cornblath DR, Donehower RC. J Natl Cancer Inst Monogr. 1993:107–115. [PubMed] [Google Scholar]
- 22.Bourne Y, Dannenberg J, Pollmann V, Marchot P, Pongs O. J Biol Chem. 2001;276:11949–11955. doi: 10.1074/jbc.M009373200. [DOI] [PubMed] [Google Scholar]
- 23.Huizing MT, Keung AC, Rosing H, van der Kuij V, ten Bokkel Huinink WW, Mandjes IM, Dubbelman AC, Pinedo HM, Beijnen JH. J Clin Oncol. 1993;11:2127–2135. doi: 10.1200/JCO.1993.11.11.2127. [DOI] [PubMed] [Google Scholar]
- 24.Gelderblom H, Verweij J, Nooter K, Sparreboom A. Eur J Cancer. 2001;37:1590–1598. doi: 10.1016/s0959-8049(01)00171-x. [DOI] [PubMed] [Google Scholar]
- 25.Mielke S, Sparreboom A, Steinberg SM, Gelderblom H, Unger C, Behringer D, Mross K. Clin Cancer Res. 2005;11:4843–4850. doi: 10.1158/1078-0432.CCR-05-0298. [DOI] [PubMed] [Google Scholar]
- 26.Harwood SM, Yaqoob MM, Allen DA. Ann Clin Biochem. 2005;42:415–431. doi: 10.1258/000456305774538238. [DOI] [PubMed] [Google Scholar]
- 27.Strotmann R, Schultz G, Plant TD. J Biol Chem. 2003;278:26541–26549. doi: 10.1074/jbc.M302590200. [DOI] [PubMed] [Google Scholar]
- 28.Averill S, Robson LG, Jeromin A, Priestley JV. Neuroscience. 2004;123:419–427. doi: 10.1016/j.neuroscience.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 29.Siman R, Baudry M, Lynch G. J Neurochem. 1983;41:950–956. doi: 10.1111/j.1471-4159.1983.tb09039.x. [DOI] [PubMed] [Google Scholar]
- 30.Grynkiewicz G, Poenie M, Tsien RY. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 31.Kao JP. Methods Cell Biol. 1994;40:155–181. doi: 10.1016/s0091-679x(08)61114-0. [DOI] [PubMed] [Google Scholar]
- 32.Estrada M, Varshney A, Ehrlich BE. J Biol Chem. 2006;281:25492–25501. doi: 10.1074/jbc.M603193200. [DOI] [PubMed] [Google Scholar]
- 33.Johenning FW, Zochowski M, Conway SJ, Holmes AB, Koulen P, Ehrlich BE. J Neurosci. 2002;22:5344–5353. doi: 10.1523/JNEUROSCI.22-13-05344.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.