Abstract
Circulating levels of the cytokine interleukin 18 (IL-18) are elevated in obesity. Here, we show that administration of IL-18 suppresses appetite, feed efficiency, and weight regain in food-deprived male and female C57BL/6J mice. Intraperitoneal vs. intracerebroventricular routes of IL-18 administration had similar potency and did not promote formation of a conditioned taste aversion (malaise-like behavior). Mice partially (Il18+/−) or totally (Il18−/−) deficient in IL-18 were hyperphagic by young adulthood, with null mutants then becoming overweight by the fifth month of life. Adult Il18−/− mice gained 2- to 3-fold more weight than WT mice per unit energy consumed of low- or high-fat diet. Indirect calorimetry revealed reduced energy expenditure in female Il18−/− mice and increased respiratory exchange ratios [volume of carbon dioxide production (VCO2)/volume of oxygen consumption (VO2)] in mutants of both sexes. Hyperphagia continued in maturity, with overeating greatest during the mid- to late-dark cycle. Relative white fat-pad mass of Il18−/− mice was ≈2- to 3-fold greater than that of WT, with gonadal, mesenteric, and inguinal depots growing most. The data suggest that endogenous IL-18 signaling modulates food intake, metabolism, and adiposity during adulthood and might be a central or peripheral pharmacological target for controlling energy homeostasis.
Keywords: obesity, food intake, proinflammatory cytokine, body weight, overweight
Approximately 1 billion people worldwide are overweight or obese, conditions that increase mortality, morbidity, and economic costs (1). Identifying molecular controls of energy homeostasis may inform the etiology or treatment of obesity. Interleukin 18 (IL-18), discovered for its proinflammatory, T cell-polarizing, and IFN-γ-inducing properties (2), shares similarities with IL-1β (3) but acts through its own IL-18 receptor complex (4, 5), a member of the IL-1/Toll-like receptor superfamily. Pro-IL-18 is cleaved on demand by caspase-1 (IL-1β converting enzyme), yielding 18-kDa mature IL-18 (pro-IL-1836–192) and a 35-residue N-terminal fragment (pro-IL-181–35). This posttranslational processing allows regulated release of “active” IL-18 from a constitutive intracellular pool of inactive prohormone (6). Like IL-1β, IL-18 is a multifunctional polypeptide, with roles in atherosclerosis and myocardial ischemia–reperfusion injury (3).
In humans, circulating IL-18 levels directly correlate with body mass index, adiposity, insulin resistance, hypertriglyceridemia, and metabolic syndrome (7–9), consistent with findings that obesity and metabolic syndrome are accompanied by a chronic mild inflammatory state (10). Paradoxically, male IL-18-deficient (Il18−/−) mice exhibit late-onset obesity (11). A hypothesis to reconcile these findings is that IL-18 is a homeostatic regulator that opposes excess positive energy balance, wherein elevated IL-18 levels in obesity reflect (inadequate) compensation, analogous to what occurs with the adipocytokine leptin. Accordingly, weight loss decreases (12, 13) and hyperglycemia (14) or a high-fat meal (15) increase circulating IL-18 levels.
In this context, IL-18 may be an endogenous anorectic. Indeed, male Il18−/− mice ate more low-fat chow than C57BL/6J wild-type (WT) mice in a recent study (11). However, Il18−/− mice outweighed controls in that report, so greater intake might have been a consequence of excess weight rather than a cause. Thus, the present study tested the hypothesis that Il18−/− mice overeat before weighing more. Indirect calorimetry was used to determine whether IL-18 deficiency also altered energy expenditure or substrate utilization. Adiposity of Il18−/− mice was studied to determine whether Il18 gene products might modulate fat distribution. Finally, effects of peripheral and central injection of IL-18 and pro-IL-181–35, another IL-18 gene product, on food intake were determined. We report that central and peripheral IL-18 suppress appetite and feed efficiency without malaise and, conversely, that IL-18 deficiency leads to hyperphagia, decreased energy expenditure in females, increased feed efficiency and, ultimately, maturity-onset obesity.
Results
Body Weight.
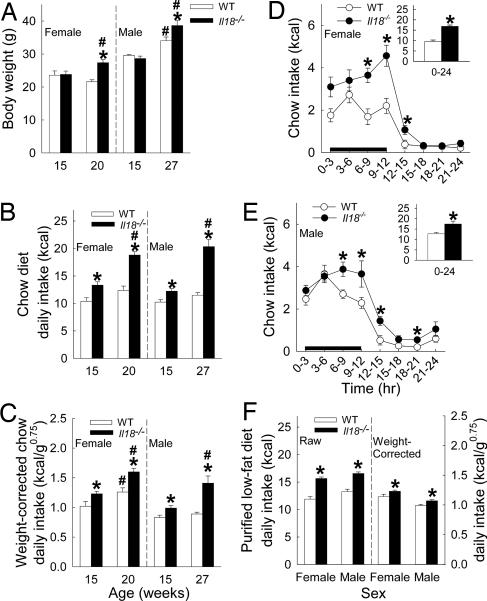
Chow-reared female Il18−/− mice weighed 27% more (5.8 g) than WT mice at 20 but not 15 (1%, 0.2 g) weeks of age, suggesting greater body weight gain during the fifth month of life [genotype × age: F(1,28) = 6.96, P = 0.01; genotype: F(1,28) = 8.30, P < 0.008] (Fig. 1A). The weight of female 20-week-old heterozygote Il18+/− mice was similar to WT mice (M ± SEM: 22.2 ± 0.2 g, n = 6). Male Il18−/− mice were 14% (4.6 g) heavier than WT mice at 27 but not 15 (−3%, −1.0 g) weeks of age, suggesting 2-fold greater weight gain across the intervening 12 weeks [genotype × age: F(1,29) = 9.49, P < 0.005; genotype: F(1,29) = 4.05, P = 0.05] (Fig. 1A).
Fig. 1.
Body weight and low-fat-diet intake in adult male and female Il18−/− and WT mice. Data express mean (+SEM) body weight (A), average daily chow intake on raw- (B) and body weight-corrected (C) bases in young adult and mature mice, chow intake across 24 h in mature females (D) and males (E), and average daily low-fat-purified diet intake (F) on raw (Left) and weight-corrected (Right) bases in mature mice. The black line in D and E indicates the dark cycle. For young adults, female and male WT (n = 7, 7), female and male Il18−/− mice (n = 8, 11); for mature mice, n = 5, 6, 10, and 6, respectively. ∗, P < 0.05 vs. the respective WT group; #, P < 0.05 vs. respective younger group [between-subject Newman–Keuls or Student's t test (Insets)].
Food Intake.
Male and female Il18−/− mice ate more chow than WT mice during 1-week study periods. Hyperphagia was seen at 15 weeks of age, predating body weight differences, and was greater later in adulthood in both male [genotype × age: F(1,29) = 27.76, P < 0.0001; genotype: F(1,29) = 69.37, P < 0.0001], and female Il18−/− mice [genotype × age: F(1,28) = 7.08, P = 0.01; genotype: F(1,28) = 32.41, P < 0.0001] (Fig. 1B). After normalizing for body weight (16), both male [F(1,29) = 42.66, P < 0.0001] and female Il18−/− mice [F(1,28) = 25.48, P < 0.0001] still ate more chow than WT mice (Fig. 1C). Female 20-week-old heterozygote Il18+/− mice (n = 6) also overate chow relative to WT mice [P ≤ 0.01; mean ± SEM: 15.1 ± 0.4 kcal (1 kcal = 4.18 kJ); 1.49 ± 0.04 kcal/g0.75 body weight], but not to the degree of Il18−/− mice (P ≤ 0.05).
Chow hyperphagia in Il18−/− mice varied according to the time of day in both sexes (Fig. 1 D and E) [females, genotype × time: F(7, 98) = 3.13, P = 0.005; genotype: F(1,14) = 53.49, P < 0.0001; males: genotype × time: F(7,77) = 1.88, P = 0.08; genotype: F(1,11) = 11.39, P = 0.006]. Chow intake during the last 6 h of the dark cycle and first 3 h of the light cycle was 117% greater in female (9.3 ± 0.5 vs. 4.3 ± 0.3 kcal, P < 0.0001) and 63% greater in male Il18−/− than in WT mice (9.0 ± 0.7 vs. 5.5 ± 0.2 kcal, P < 0.0004). Chow intake during the other 15 h did not as substantially differ (females: 7.6 ± 0.9 vs. 5.3 ± 0.7 kcal, P > 0.09; males: 8.6 ± 0.7 vs. 7.2 ± 0.6 kcal, P > 0.14). Female heterozygote Il18+/− mice (n = 6) also overate chow relative to WT mice during this 9-h period (P = 0.007; 6.5 ± 0.6 kcal), but not to the degree of Il18−/− mice (P = 0.003).
Il18−/− mice also ate more purified low-fat diet on raw (P < 0.0001) and body-weight-corrected bases (P < 0.03) than WT mice across a 25-day study period (Fig. 1F). Female heterozygote Il18+/− mice (n = 6) likewise ate more purified low-fat diet than WT mice (P < 0.03, 12.6 ± 0.5 kcal per day; P = 0.05, 1.33 ± 0.05 kcal/g0.75) and less than Il18−/− mice on a raw (P = 0.001) but not a weight-corrected basis.
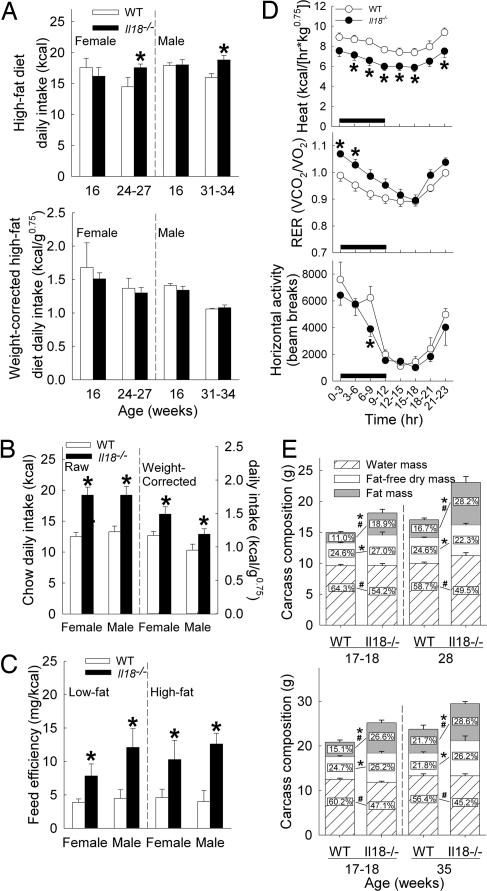
When maintained on high-fat diet, mature female and male Il18−/− mice ingested more kilocalories than WT mice across a 24-day study period (Fig. 2A Upper), but correcting for body weight eliminated this difference (Fig. 2A Lower). Unlike for low-fat diets, female heterozygote Il18+/− mice did not overeat high-fat diet compared with WT mice (P > 0.5, 15.8 ± 1.5 kcal per day, n = 6). Young adult Il18−/− mice also did not eat more high-fat diet than WT mice (Fig. 2A) during a 17-day study period. After being reestablished on chow diet, both female and male mature Il18−/− mice again ate more than WT on both kilocalorie (50% and 44% greater, P < 0.007) and weight-corrected bases (33% and 29% greater, P < 0.04) (Fig. 2B).
Fig. 2.
Food intake and metabolism in adult Il18−/− and WT mice. Data express mean (+SEM) average daily high-fat purified diet intake on raw and body-weight-corrected bases in young adult and mature mice (A); daily intake of mature mice on raw and weight-corrected bases after being returned to chow from high-fat diet (B); feed efficiency (C); heat production, respiratory exchange ratio, and horizontal motor activity of female Il18−/− (n = 7) and WT (n = 6) mice in indirect calorimetry chambers (D); and eviscerated carcass weight and composition (E) in females (Upper) and males (Lower). (Inset) Numbers in E indicate mean relative composition as a function (%) of eviscerated carcass weight, and SEMs for these percents, from left to right, were: females, water: 0.77, 2.73, 0.52, 1.68, fat-free dry mass: 0.58, 1.48, 0.70, 1.64, fat: 0.83, 2.64, 1.14, 2.92; males, water: 1.72, 0.69, 1.78, 0.86, fat-free dry mass: 0.30, 1.44, 1.16, 2.84, and fat: 1.89, 1.87, 2.73, 2.30. The Fig. 1 legend gives sample sizes not otherwise given. ∗, P < 0.05 vs. respective WT group, indicating absolute (g) differences in weight of carcass components (E). #, P < 0.05 vs. respective WT group, indicating differences in relative carcass composition (E) [between-subject Newman–Keuls or Welch's t test (feed efficiency and carcass composition)].
Feed Efficiency and Metabolism.
Across 10-day study periods, female and male adult Il18−/− mice gained 2- to 3-fold more body weight than WT mice per unit energy consumed of purified low-fat (22–29 weeks of age) or high-fat (18 weeks of age) diet, suggesting increased feed efficiency (Fig. 2C). Indirect calorimetry [Fig. 2D; supporting information (SI) Fig. 4] revealed stably reduced oxygen consumption (VO2), carbon dioxide production (VCO2), and heat production, indices of whole-body energy expenditure, in female Il18−/− mice (n = 7) vs. WT (n = 6) mice at these ages. Female Il18−/− mice also had an increased respiratory exchange ratio (RER) early in the dark cycle and showed less horizontal and vertical motor activity than WT mice within the mid- to late-dark cycle. Despite having weights similar to (27.8 ± 2.5 vs. 26.4 ± 2.2 g) and expending less energy than WT mice, female Il18−/− mice ate more (15.3 ± 1.0 vs. 12.1 ± 0.8 kcal, P < 0.05). Male Il18−/− mice (n = 4) did not show altered VO2, VCO2, or heat formation relative to WT mice (n = 4) (SI Fig. 5). However, despite not expending more energy, mutant male mice ate more than WT mice during the test period (18.3 ± 1.6 vs. 13.4 ± 1.3 kcal, weight corrected, 1.16 ± 0.12 vs. 0.86 ± 0.09 kcal/g0.75, P < 0.05) and had an increased RER during the last 6 h of the light cycle (SI Fig. 5), during which they ate 2-fold more than WT mice (3.5 ± 0.5 vs. 1.7 ± 0.4 kcal, P < 0.05).
Adiposity.
Young adult (18 weeks) and mature (30–37 weeks) Il18−/− mice were not only heavier (SI Table 3) but also fattier (Table 1) than age-matched WT mice. Brown fat mass was not disproportionately greater in Il18−/− mice, whereas white fat pads were heavier on both absolute and relative bases. The collective relative white fat-pad mass of Il18−/− mice was ≈2- to 3-fold greater than that of WT mice. Subcutaneous (inguinal) vs. intraabdominal pads (gonadal, mesenteric, and retroperitoneal) were similarly larger. Inguinal and gonadal pads grew most, outweighing those of WT mice by ≈1 g each.
Table 1.
Terminal fat pad weights in Il18−/− and WT mice
| Type of adipose tissue | Female |
Male |
||||||
|---|---|---|---|---|---|---|---|---|
| Young adult |
Mature |
Young adult |
Mature |
|||||
| WT (n = 7) | Il18−/− (n = 8) | WT (n = 5) | Il18−/− (n = 10) | WT (n = 7) | Il18−/−(n = 11) | WT (n = 6) | Il18−/− (n = 6) | |
| Raw fat mass, g | ||||||||
| Brown* | 0.06 ± 0.01 | 0.08 ± 0.01 | 0.08 ± 0.01 | 0.10 ± 0.01 | 0.07 ± 0.01 | 0.09 ± 0.01 | 0.12 ± 0.01 | 0.18 ± 0.02 |
| Inguinal† | 0.29 ± 0.04 | 1.22 ± 0.24 | 0.55 ± 0.04 | 1.71 ± 0.25 | 0.62 ± 0.12 | 1.69 ± 0.09 | 0.94 ± 0.17 | 1.93 ± 0.20 |
| Subcutaneous† | 0.17 ± 0.03 | 0.49 ± 0.08 | 0.33 ± 0.02 | 0.64 ± 0.08 | 0.32 ± 0.06 | 0.96 ± 0.09 | 0.46 ± 0.06 | 0.99 ± 0.10 |
| Gonadal† | 0.21 ± 0.04 | 0.89 ± 0.20 | 0.46 ± 0.05 | 2.02 ± 0.34 | 0.65 ± 0.12 | 1.59 ± 0.09 | 0.91 ± 0.15 | 2.05 ± 0.17 |
| Retroperitoneal† | 0.03 ± 0.01 | 0.15 ± 0.04 | 0.08 ± 0.01 | 0.32 ± 0.06 | 0.17 ± 0.04 | 0.45 ± 0.03 | 0.17 ± 0.03 | 0.51 ± 0.07 |
| Mesenteric† | 0.11 ± 0.01 | 0.24 ± 0.03 | 0.25 ± 0.02 | 0.52 ± 0.08 | 0.15 ± 0.02 | 0.62 ± 0.06 | 0.32 ± 0.05 | 0.67 ± 0.06 |
| Relative fat mass, % | ||||||||
| Brown | 0.4 ± 0.03 | 0.4 ± 0.03 | 0.5 ± 0.03 | 0.4 ± 0.03 | 0.3 ± 0.02 | 0.4 ± 0.02 | 0.5 ± 0.03 | 0.6 ± 0.05 |
| Inguinal† | 1.9 ± 0.2 | 5.9 ± 1.1 | 3.2 ± 0.1 | 7.0 ± 0.8 | 2.9 ± 0.5 | 6.7 ± 0.2 | 3.8 ± 0.5 | 6.5 ± 0.4 |
| Subcutaneous† | 1.1 ± 0.1 | 2.5 ± 0.4 | 1.9 ± 0.1 | 2.7 ± 0.2 | 1.5 ± 0.2 | 3.7 ± 0.3 | 1.9 ± 0.2 | 3.3 ± 0.2 |
| Gonadal† | 1.4 ± 0.2 | 4.2 ± 0.9 | 2.7 ± 0.2 | 8.2 ± 1.2 | 3.0 ± 0.5 | 6.3 ± 0.2 | 3.7 ± 0.5 | 6.9 ± 0.3 |
| Retroperitoneal† | 0.2 ± 0.04 | 0.7 ± 0.2 | 0.4 ± 0.05 | 1.3 ± 0.2 | 0.8 ± 0.2 | 1.8 ± 0.1 | 0.7 ± 0.1 | 1.7 ± 0.2 |
| Mesenteric† | 0.7 ± 0.1 | 1.2 ± 0.1 | 1.5 ± 0.1 | 2.1 ± 0.3 | 0.7 ± 0.1 | 2.4 ± 0.2 | 1.3 ± 0.2 | 2.3 ± 0.2 |
| All white fat pads† | 5.4 ± 0.7 | 14.5 ± 2.5 | 9.7 ± 0.5 | 21.3 ± 2.5 | 8.9 ± 1.4 | 20.8 ± 0.8 | 11.4 ± 1.4 | 20.7 ± 1.1 |
Data express mean± SEM. Carcasses from Il18−/− mice were fattier. Relative mass (% carcass weight) of brown fat did not differ per genotype, P > 0.11. In contrast, each white fat pad was disproportionately larger in male and female Il18−/− mice as compared with WT controls by young adulthood (18 weeks), continuing into maturity (males, 37 weeks; females, 30 weeks of age).
*Values for male and female Il18−/− were greater than respective WT mice (P < 0.05).
†Male, but not female, Il18−/− were greater than respective WT mice (P < 0.05) (Student's and Welch's t tests for groups with equal and unequal variance, respectively).
Chemical analysis of carcass composition (Fig. 2E) also found that fat mass was greater in female (2.1- to 2.4-fold) and male (1.6- to 2.1-fold) Il18−/− than in WT mice, resulting in a disproportionately (%) fatty relative body composition. Conversely, absolute fat-free body mass and total ash content, an index of bone mass, were only marginally greater in female (6–34%) and male (11–53%) Il18−/− mice than in WT mice and not disproportionately (%) so. In fact, relative (%) ash content was lower in young adult male Il18−/− than WT mice (M ± SEM, 2.85 ± 0.10 vs. 3.26 ± 0.04%; P = 0.01).
Exogenous IL-18 Administration.
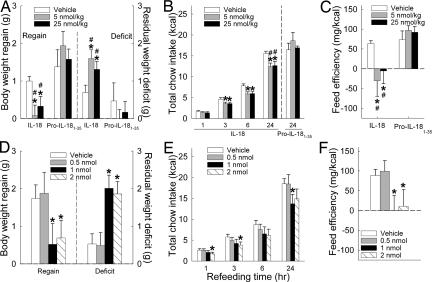
Intraperitoneal injection of IL-18 (5, 25 nmol/kg) reduced body-weight regain and chow intake after food deprivation in WT mice. In males (n = 17), food deprivation led to similar weight loss across future treatments (M ± SEM; vehicle, 1.70 ± 0.15 g; 5 nmol/kg, 1.68 ± 0.12 g; 25 nmol/kg, 1.64 ± 0.13 g). However, IL-18 treatment then prevented significant weight regain during 24 h of refeeding, unlike vehicle-treated conditions (F[2,32] = 4.94, P = 0.01), resulting in a greater postfeeding body-weight deficit (Fig. 3A, F[2,32] = 3.56, P < 0.02). IL-18 treatment reduced incremental food intake (dose: F[2,32] = 7.56, P = 0.002), curbing 3-, 6- and 24-h total intake (Fig. 3B; F ratio, P = 0.06, 0.004, and 0.002, respectively). IL-18 treatment also reduced feed efficiency (Fig. 3C; Friedman test, P < 0.05). IL-18 actions were pharmacologically specific, because equimole injection of pro-IL-181–35 (n = 7) did not yield the same effects (Fig. 3 A–C).
Fig. 3.
Effects of administration of pro-IL-18-derived molecules on energy homeostasis in food-deprived adult male C57BL/6J mice. Mice were pretreated (5 min) with IL-18 (pro-IL-1836–192, i.p., n = 17 or i.c.v., n = 7) or pro-IL-181–35 (i.p., n = 7) just before dark-cycle onset after a 16.5- to 17-h fast. The dose was given in a within-subject counterbalanced design. Data express mean (±SEM) recovery of body weight after i.p. (A) or i.c.v. (D) administration as measured by regain (Left) and residual deficit (Right) in body weight, cumulative refeeding intake of chow after i.p. (B) or i.c.v. treatment (E), and feed efficiency after i.p. (C) or i.c.v. (F) treatment. ∗, P < 0.05 vs. vehicle condition [within-subject Newman–Keuls or Wilcoxon sign-rank test (feed efficiency)], #, P < 0.05 vs. respective dose of pro-IL-181–35 (Welch's t test).
In females (n = 6), i.p. IL-18 treatment also dose-relatedly reduced 3- and 6-h intake after food deprivation (linear dose effects, P ≤ 0.05, P < 0.05 for 25 nmol/kg vs. vehicle; M ± SEM, 3 h, 5.1 ± 0.5, 3.9 ± 0.2, and 3.7 ± 0.3; 6 h, 9.1 ± 0.9, 6.9 ± 0.8, and 6.5 ± 0.3 kcal for vehicle, 5 and 25 nmol/kg, respectively). Equimole pro-IL-181–35 injection again did not suppress intake (not shown).
Intracerebroventricular (i.c.v.) IL-18 injection also blunted recovery of baseline body weight, refeeding, and feed efficiency (Fig. 3 D–F) after food deprivation in male WT mice (n = 8). These actions were seen at doses of 1 and 2, but not 0.5 nmol, per mouse. The i.p. doses of 5 and 25 nmol/kg correspond to mean doses of ≈0.15 and 0.75 nmol per male mouse (M = 30 g), suggesting that the i.p. route was at least as potent as the i.c.v. route in producing these effects.
In females (n = 6), i.c.v. IL-18 treatment also blunted weight regain (0.5, 1, and 2 nmol), feed efficiency (1 nmol), and refeeding after food deprivation (Table 2). Cumulative 3-h food intake was reduced by all IL-18 doses, and 6- and 24-h intake were reduced by 1- and 2-nmol doses. As reference, the effective anorectic i.p. dose was 0.52 nmol per female mouse (M = 21 g), with 0.10 nmol approaching significance (P < 0.10), again suggesting a similar order of potency for i.p. vs. i.c.v. IL-18 administration. Still, i.c.v. IL-18 treatment may have yielded longer-lasting (≥24-h) anorexia than i.p. treatment in female mice.
Table 2.
Effects of i.c.v. IL-18 on weight regain, food intake, and feed efficiency in food-deprived female C5BL/6J mice
| Measure | IL-18 dose |
|||
|---|---|---|---|---|
| Vehicle | 0.5 nmol | 1 nmol | 2 nmol | |
| Body-weight regain, 24 h, g | 1.50 ± 0.11 | 0.14 ± 0.87* | 0.09 ± 0.28* | 0.17 ± 0.85* |
| Total food intake, kcal | ||||
| 1 h | 3.00 ± 0.34 | 2.49 ± 0.17 | 2.31 ± 0.37 | 1.75 ± 0.45* |
| 3 h | 5.91 ± 0.26 | 4.98 ± 0.22* | 3.43 ± 0.43* | 3.79 ± 1.05* |
| 6 h | 9.86 ± 0.43 | 8.16 ± 0.94 | 4.24 ± 0.83* | 5.75 ± 2.10* |
| 24 h | 18.05 ± 0.66 | 16.25 ± 1.35 | 12.17 ± 1.69* | 12.46 ± 3.57* |
| Feed efficiency, mg/kcal | 83.6 ± 7.2 | 3.0 ± 53.5 | −13.2 ± 40.2* | 29.2 ± 38.4 |
Data express mean ± SEM; n = 6. ∗, P < 0.05 vs. vehicle condition (Wilcoxon sign-rank test).
Neither i.p. nor i.c.v. administration of anorectic doses of IL-18 promoted a conditioned taste aversion to a saccharin solution, in contrast to conditioning with an isoanorectic dose of the positive control lithium chloride (SI Text and SI Fig. 6).
IL-18 Levels.
Plasma IL-18 levels were constitutively present (grand M ± SEM, 256.6 ± 21.6 pg/ml; range, 112.5–711.5 pg/ml) and did not vary across 3-h sampling intervals in nondeprived male WT mice (SI Fig. 7). IL-18 immunoreactivity was undetectable in plasma from 7 Il18−/− mice.
Discussion
The present results suggest that the cytokine IL-18 plays a physiologic role in energy homeostasis of adult male and female mice. Mice with null mutations of the Il18 gene were still normal weight at 15 weeks of age but were overweight and fattier by 18–20 weeks of age, suggesting an essential function to oppose positive energy balance beginning from the fourth to fifth months of life, or early maturity. Even after accounting for body-weight differences, Il18−/− mice were hyperphagic on high-carbohydrate diets, and hyperphagia preceded overweight. Heterozygote Il18+/− mice also overate high-carbohydrate diets, indicating that partial deficiency was biologically relevant. At the ages that they began to gain excess weight, adult Il18−/− mice were thriftier with the energy they consumed from low- and high-fat diets. Conversely, acute systemic (i.p.) administration of IL-18, but not equimole treatment with pro-IL-181–35, potently (5–25 nmol/kg) suppressed weight recovery by reducing chow intake and feed efficiency after food deprivation. Central administration (i.c.v.) of IL-18 exerted these actions with similar potency (≈0.5–1 nmol per mouse). The findings support the hypothesis that IL-18 physiologically opposes positive energy balance (11) but suggest it may do so by reducing not only (carbohydrate diet) intake but also feed efficiency.
As compared with WT controls, mice with total (Il18−/−) or partial (Il18+/−) IL-18 deficiency overate two high-carbohydrate/low-fat corn-based diets but did not similarly overeat a high-fat lard-based diet. The lack of high-fat diet hyperphagia was not an artifact of diet order, because mutants again ate more than WT mice when returned to chow diet. Perhaps IL-18−/− mice differentially process carbohydrate- vs. lipid-rich diets or differentially use these macronutrients as fuel. Consistent with this possibility, null mutants exhibited increased utilization of carbohydrates as an energy substrate and previously were observed to have altered glucose clearance and gluconeogenesis (11). On the other hand, from an energy homeostasis perspective (excess kilocalories), IL-18-deficient mice comparably overate all diets available to them, whereas WT mice were selectively hyperphagic on the high-fat diet. Perhaps a “ceiling” on energy intake prevented incrementally greater high-fat diet intake by mutant mice but, with respect to neutral energy balance, they still overate all diets. Studies of IL-18 deficiency that use a high-fat diet that does not promote excess energy intake or that study mice of a genetic background that do not overeat high-fat diets may distinguish between these hypothesized modes of hyperphagia.
Excess body weight of Il18−/− mice was mainly due to white fat accumulation in both s.c. and intraabdominal depots. Greater fattiness was evident at a young age (18 weeks), when body weight had just diverged, exemplified in 4- to 5-fold larger inguinal, gonadal, and retroperitoneal fat pads in young adult Il18−/− females. This finding suggests that IL-18 might help regulate body composition and fat distribution, an intriguing possibility given that human adipose tissue synthesizes and secretes IL-18. Insofar as “obesity-recruited” fat-resident monocyte/macrophage lineage cells are major sources of IL-18 (17), and adipocytes from obese humans secrete 3-fold more IL-18 than those from lean donors (18), perhaps IL-18 acts as a tonic metabolic feedback signal to oppose positive energy balance. Like the lipostatic cytokine leptin, circulating IL-18 levels correlate with body mass index and adiposity, and weight loss decreases IL-18 levels (7).
Il18−/− mice overate by 4 months of age, before they became overweight during the fifth month of life. Hyperphagia was most evident during the last half of the dark cycle but also was seen during portions of the light cycle. Circulating IL-18 levels were constitutively present in WT and did not vary across the day. Thus, circadian variations in IL-18 expression may not explain the propensity of Il18−/− mice to overeat preferentially during the late dark and light cycles. Still, perhaps IL-18 from sources unrelated to appetite control predominate in circulation of lean animals, but local expression of intake-relevant IL-18 does vary across the day. Alternatively, perhaps circadian variations in sensitivity to IL-18 exist, as for the structurally or pathway-related cytokines, IL-1β and IFN-γ (19, 20). The degree to which any relation of IL-18 function to time of day reflects direct circadian influences as opposed to interaction with bodily processes is unclear. Consistent with the latter explanation, energy expenditure was reduced independent of time in mutant females, and RER was increased at different times of day between sexes.
Unlike previous findings (11), the current data suggest that IL-18 modulates not only food intake but also metabolism. Il18−/− mice were more fuel-efficient on both low- and high-fat diets at ≈5 months of age, whereas central or peripheral IL-18 administration reduced feed efficiency. Accordingly, female Il18−/− mice showed reduced whole-body energy expenditure and moved less than WT mice during parts of the dark cycle. However, hypoactivity cannot fully explain reduced energy expenditure of mutant females, because their heat formation was also reduced during the light cycle, when movement was similarly low in both genotypes. Both female and male mutants used less fat and more carbohydrate as an energy substrate, which is evident in higher RER. The high RER (>1.05) of Il18−/− mice suggests a state of greater positive-energy balance and lipogenesis, consistent with their excess intake vs. normal (males) or reduced (females) energy expenditure. IFN-γ, which is induced by IL-18 (also known as IFN-γ-inducing factor), pharmacologically increases resting metabolic rate in humans (21) and mediates not only anorectic but also hypermetabolic responses to inflammatory stimuli (22), making IFN-γ a potential mechanism by which IL-18 alters metabolism.
Anorexia is one sign of the “sickness syndrome,” which occurs during infection and includes fever, lethargy, hypoactivity, and impaired cognition (23). The syndrome, an adaptive response to and signal of illness, is mediated by several cytokines, including IL-1β, IL-6, and TNF. Similar to these molecules, not only is IL-18 anorectic but it also induces sleep and inhibits long-term potentiation (24). However, unlike IL-1β, IL-6, and TNF (25), IL-18 here did not promote fever or a conditioned taste aversion (malaise behavior) at anorectic doses (SI Text). Furthermore, although levels of IL-1β, IL-6, and TNF are low under normal physiologic conditions and released from monocytes/macrophages by inflammatory stimuli, IL-18 is constitutively expressed, derived partly from adipocytes, and with levels that depend upon nutritional state, including fat mass, weight loss, hyperglycemia, and dietary fat intake (12–15). Thus, whereas the role of IL-1 in the control of food intake in healthy individuals is uncertain, IL-18 shares characteristics of adipocytokines that control day-to-day energy homeostasis.
Systemic and central IL-18 treatment opposed weight recovery after food deprivation. Whereas systemic IL-18 injection (i.p., 5 nmol/kg or ≈0.15 nmol) reduced refeeding, feed efficiency, and weight regain in male mice (n = 17) of the current within-subject study, IL-18 (i.v., ≈2 μg per mouse or ≈0.11 nmol) did not alter 24-h intake of nondeprived mice (n = 5) in a previous between-subjects design study. Contrasting results may reflect differences in sample size, study design, route/dose of administration, or feeding status (11). Peripheral IL-18 also suppressed food intake in females (i.p., 25 nmol/kg), with the anorectic potency of i.p. IL-18 comparable to that for the anorectic cytokine leptin (26) but less than for IL-1β, which suppresses feeding at doses (i.p.) of ≈0.3 nmol/kg (27). Consistent with a report that i.c.v. IL-18 (2× per week, 0.56 nmol per mouse) reduced 24-h food intake in male WT mice, central IL-18 also reduced intake here but, unlike the previous study (11), with the same or slightly lower potency than i.p. treatment. The findings raise the possibility of both central and peripheral sites of IL-18 action on energy homeostasis. Potential sources of IL-18 positioned to control energy balance include not only adipose tissue but also anterior pituitary somatotrophs and, to a lesser extent, lactotrophs, corticotrophs, and gonadotrophs; epithelium and lamina propria macrophages of the intestine, where its expression may be provoked by food-related stimuli, and the adrenal cortex (28–32). Relevant central sites of IL-18 synthesis include the hypothalamus, medial habenula, periventricular ependymal cells, cortex, striatum, and glial cells (33–35).
IL-18 joins a growing group of catabolic proinflammatory cytokines that are secreted by adipose tissue, overexpressed in obesity, mediated by signal transducer and activator of transcription 3 (STAT3) and/or Toll-like receptor/myeloid differentiation factor 88 (MyD88) signaling pathways, and that lead to obesity upon loss-of-function mutations. Consistent with the superfamily resemblance of IL-1β with IL-18, Il1R−/− mice also develop maturity-onset obesity (36), and mice deficient in IL-1 receptor antagonist are lean (37). Mice lacking other STAT3- and/or MyD88-mediated cytokines, including IL-6 (38), IL-6/IL-1 (39), leptin (40), and leptin receptor null mutants (41), are obese. However, mice deficient in MyD88, an adaptor protein for Toll-like receptor pathways, show normal body weight and basal food intake (42), whereas mice with inactivation of STAT3 function are obese and hyperphagic (43). The overall results suggest that IL-18 may participate not only in sickness syndromes, but also, via STAT3 pathways, in the day-to-day control of energy homeostasis during maturity. The pharmacological data further suggest that activation of either central or peripheral IL-18 signaling may modulate appetite, feed efficiency, and, ultimately, body weight.
Methods
Subjects.
Subjects were IL-18 knockout (Il18−/−) mice generated on a C57BL/6J background (44) (gifts of Arturo Zychlinsky, New York University, New York), IL-18 heterozygote (Il18+/−) mice, and C57BL/6J WT mice. Three independent cohorts of mutant mice were studied, one from young adulthood (≈15 weeks of age; M ± SEM, 105.1 ± 0.2 days) and one from maturity (≈27 and 20 weeks of age for males and females; 187.8 ± 5.1 and 140.3 ± 4.7 days, respectively) for food intake, body weight, and adiposity studies, and the third cohort for indirect calorimetry studies (≈23–25 and 24–26 weeks for females and males, respectively).
Surgery.
Lateral ventricle guide cannula were stereotaxically secured at anterior/posterior −0.34 and medial/lateral 1.0 from bregma and ventral −1.7 from dura (45).
Drugs and Injections.
Recombinant endotoxin-free mouse IL-18 was obtained commercially (BioSource International, Camarillo, CA; MBL International, Woburn, MA). Pro-IL-181–35 was synthesized at The Scripps Research Institute. Drugs were dissolved in sterile 0.9% saline before i.p. (10 ml/kg) or i.c.v. (2.0 μl/4 min) testing.
Diets.
The chow was Harlan Teklad (Indianapolis, IN) LM-485 Diet 7012 [65% carbohydrate (kcal), 13% fat, metabolizable energy 3.41 kcal/g]. The corn starch/sucrose-based “low-fat” purified diet was Research Diets (New Brunswick, NJ) D12450B (10% fat, 70% carbohydrate, 3.85 kcal/g). The matched “high-fat” lard-based purified diet was D12492 (60% fat, 20% carbohydrate, 5.24 kcal/g).
Body Weight, Food Intake, and Feed Efficiency.
Body weight and food intake were analyzed at twice-weekly intervals (0.01-g precision) 30–60 min before dark-cycle onset. Young adult (15 weeks) chow-reared mice were tested for 1 week on chow diet followed by 17 days on high-fat diet. Mature (27 or 20 weeks) chow-reared mice were tested for 1 week on chow diet, 25 days on purified low-fat diet, and 24 days on purified high-fat diet, reacclimated to chow for 1 week, and then studied for a 1-week period. The circadian profile of food intake was then determined by measuring chow intake of mature mice every 3 h across a 24-h period beginning from dark-cycle onset. Feed efficiency over 10 days was calculated as body weight gained per unit energy intake (mg/kcal) from mice that had been switched from chow to a purified low- (22–28 weeks of age) or high-fat (18 weeks) diet, allowing 1 week acclimation. Food intake was normalized for body weight (16).
For i.p. vs. i.c.v. pharmacological studies, separate groups of adult male (M = 29.67 ± 0.34 and 30.58 ± 0.68 g of weight at treatment, respectively) and female (M = 20.67 ± 0.43 and 21.49 ± 0.36 g) C57BL/6J mice were subjects. Food-deprived (16.5–17 h) mice received i.p. IL-18 (0, 5, and 25 nmol/kg), i.p. pro-IL-181–35 (0, 5, and 25 nmol/kg), or i.c.v. IL-18 (0, 0.5, 1, and 2 nmol) 5 min before they received chow access, 0–30 min before dark cycle onset. Remaining food was measured 1, 3, 6, and 24 h later. Body weights were recorded 24 h before treatment (before the deprivation baseline), at treatment, and after 24-h refeeding. Doses were given in a within-subject counterbalanced design with at least four intervening treatment-free days. Central doses of IL-18 were chosen to resemble total i.p. doses.
Carcass Analysis.
After completing feeding studies, mice were fed chow for 3 days, fasted for 19 h to control recent intake, and decapitated ≈1 h into the dark cycle. Carcasses were frozen (−80°C), and brown adipose tissue and white fat pads were later dissected and returned to the thawed (25°C) carcass, followed by chemical analysis of carcass composition (46).
Metabolic Studies.
Indirect calorimetry (Oxymax, Columbus, OH) was performed in acclimated singly housed purified low-fat diet-fed mice. RER was calculated as the ratio of VCO2 to VO2. Energy expenditure measures {VO2, VCO2, and heat formation [(3.815 + 1.232×RER)×VO2]} were normalized for body weight (16). Chambers with female mice had photobeams to assess locomotor activity.
Conditioned Taste Aversion.
Individually housed adult male C57BL/6J mice were tested in a two-pairing two-bottle choice conditioned taste aversion procedure. A relative decrease in saccharin preference ratio is interpreted as the formation of a conditioned taste aversion.
IL-18 Measurement.
Plasma IL-18 levels from eight groups of five adult C57BL/6J mice decapitated at 3-h intervals across the 24-h day were measured by ELISA (MBL International).
Statistics.
The general linear model and nonparametric tests were used to analyze data that met or violated parametric assumptions, respectively.
Additional Materials and Methods.
For additional materials and methods, see SI Text.
Supplementary Material
Acknowledgments
We thank Tim Nagy and the National Institute of Diabetes and Digestive and Kidney Diseases Program Project-funded University of Alabama–Birmingham Clinical Nutrition Research Unit Small Animal Phenotyping Core for body composition analysis, Amanda Roberts (National Institutes of Health Blueprint Core P30N5057096) for help with indirect calorimetry, and Michael Churchill (The Scripps Research Institute) for pro-IL-181–35. This work was supported by National Institutes of Health Grants DK077616, DK07118, NS43501, and AG28040, as well as the Ellison Medical Foundation.
Abbreviations
- i.c.v.
intracerebroventricular
- RER
respiratory exchange ratio
- STAT3
signal transducer and activator of transcription 3.
Footnotes
Conflict of interest statement: E.P.Z., B.C., M.S.-A., and T.B. have filed an invention disclosure regarding the use of IL-18 and related molecules to control energy homeostasis.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0611523104/DC1.
References
- 1.Yach D, Stuckler D, Brownell KD. Nat Med. 2006;12:62–66. doi: 10.1038/nm0106-62. [DOI] [PubMed] [Google Scholar]
- 2.Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, et al. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 3.Dinarello CA. Am J Clin Nutr. 2006;83:447S–455S. doi: 10.1093/ajcn/83.2.447S. [DOI] [PubMed] [Google Scholar]
- 4.Torigoe K, Ushio S, Okura T, Kobayashi S, Taniai M, Kunikata T, Murakami T, Sanou O, Kojima H, Fujii M, et al. J Biol Chem. 1997;272:25737–25742. doi: 10.1074/jbc.272.41.25737. [DOI] [PubMed] [Google Scholar]
- 5.Born TL, Thomassen E, Bird TA, Sims JE. J Biol Chem. 1998;273:29445–29450. doi: 10.1074/jbc.273.45.29445. [DOI] [PubMed] [Google Scholar]
- 6.Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming MA, Hayashi N, Higashino K, Okamura H, Nakanishi K, et al. Science. 1997;275:206–209. doi: 10.1126/science.275.5297.206. [DOI] [PubMed] [Google Scholar]
- 7.Esposito K, Pontillo A, Ciotola M, Di Palo C, Grella E, Nicoletti G, Giugliano D. J Clin Endocrinol Metab. 2002;87:3864–3866. doi: 10.1210/jcem.87.8.8781. [DOI] [PubMed] [Google Scholar]
- 8.Olusi SO, Al Awadhi A, Abraham M. Horm Res. 2003;60:29–33. doi: 10.1159/000070824. [DOI] [PubMed] [Google Scholar]
- 9.Hung J, McQuillan BM, Chapman CM, Thompson PL, Beilby JP. Arterioscler Thromb Vasc Biol. 2005;25:1268–1273. doi: 10.1161/01.ATV.0000163843.70369.12. [DOI] [PubMed] [Google Scholar]
- 10.Fain JN. Vitam Horm. 2006;74:443–477. doi: 10.1016/S0083-6729(06)74018-3. [DOI] [PubMed] [Google Scholar]
- 11.Netea MG, Joosten LA, Lewis E, Jensen DR, Voshol PJ, Kullberg BJ, Tack CJ, van Krieken H, Kim SH, Stalenhoef AF, et al. Nat Med. 2006;12:650–656. doi: 10.1038/nm1415. [DOI] [PubMed] [Google Scholar]
- 12.Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, Giugliano D. J Am Med Assoc. 2003;289:1799–1804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 13.Schernthaner GH, Kopp HP, Kriwanek S, Krzyzanowska K, Satler M, Koppensteiner R, Schernthaner G. Obes Surg. 2006;16:709–715. doi: 10.1381/096089206777346763. [DOI] [PubMed] [Google Scholar]
- 14.Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D. Circulation. 2002;106:2067–2072. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- 15.Esposito K, Nappo F, Giugliano F, Di Palo C, Ciotola M, Barbieri M, Paolisso G, Giugliano D. Am J Clin Nutr. 2003;78:1135–1140. doi: 10.1093/ajcn/78.6.1135. [DOI] [PubMed] [Google Scholar]
- 16.Kleiber M. The Fire of Life: An Introduction to Animal Energetics. New York: Krieger; 1975. [Google Scholar]
- 17.Fain JN, Tichansky DS, Madan AK. Metabolism. 2006;55:1113–1121. doi: 10.1016/j.metabol.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Skurk T, Kolb H, Muller-Scholze S, Rohrig K, Hauner H, Herder C. Eur J Endocrinol. 2005;152:863–868. doi: 10.1530/eje.1.01897. [DOI] [PubMed] [Google Scholar]
- 19.Lancel M, Mathias S, Faulhaber J, Schiffelholz T. Am J Physiol. 1996;270:R830–R837. doi: 10.1152/ajpregu.1996.270.4.R830. [DOI] [PubMed] [Google Scholar]
- 20.Opp MR, Toth LA. Ann NY Acad Sci. 1997;813:435–436. doi: 10.1111/j.1749-6632.1997.tb51729.x. [DOI] [PubMed] [Google Scholar]
- 21.de Metz J, Sprangers F, Endert E, Ackermans MT, ten Berge IJ, Sauerwein HP, Romijn JA. J Appl Physiol. 1999;86:517–522. doi: 10.1152/jappl.1999.86.2.517. [DOI] [PubMed] [Google Scholar]
- 22.Arsenijevic D, Garcia I, Vesin C, Vesin D, Arsenijevic Y, Seydoux J, Girardier L, Ryffel B, Dulloo A, Richard D. Eur Cytokine Netw. 2000;11:662–668. [PubMed] [Google Scholar]
- 23.Dantzer R. Ann NY Acad Sci. 2001;933:222–234. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- 24.Kubota T, Fang J, Brown RA, Krueger JM. Am J Physiol. 2001;281:R828–R838. doi: 10.1152/ajpregu.2001.281.3.R828. [DOI] [PubMed] [Google Scholar]
- 25.Curran BP, O'Connor JJ. Neurosci Lett. 2003;344:103–106. doi: 10.1016/s0304-3940(03)00440-3. [DOI] [PubMed] [Google Scholar]
- 26.Barrachina MD, Martinez V, Wei JY, Tache Y. Am J Physiol. 1997;272:R1007–R1011. doi: 10.1152/ajpregu.1997.272.3.R1007. [DOI] [PubMed] [Google Scholar]
- 27.Hellerstein MK, Meydani SN, Meydani M, Wu K, Dinarello CA. J Clin Invest. 1989;84:228–235. doi: 10.1172/JCI114145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conti B, Jahng JW, Tinti C, Son JH, Joh TH. J Biol Chem. 1997;272:2035–2037. doi: 10.1074/jbc.272.4.2035. [DOI] [PubMed] [Google Scholar]
- 29.Sugama S, Kim Y, Baker H, Tinti C, Kim H, Joh TH, Conti B. J Immunol. 2000;165:6287–6292. doi: 10.4049/jimmunol.165.11.6287. [DOI] [PubMed] [Google Scholar]
- 30.Penttila IA, Flesch IE, McCue AL, Powell BC, Zhou FH, Read LC, Zola H. Gut. 2003;52:1579–1586. doi: 10.1136/gut.52.11.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagai Y, Watanabe K, Aso H, Ohwada S, Muneta Y, Yamaguchi T. Domest Anim Endocrinol. 2006;30:144–154. doi: 10.1016/j.domaniend.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 32.Wang N, Sugama S, Conti B, Teramoto A, Shibasaki T. J Neuroimmunol. 2006;173:117–125. doi: 10.1016/j.jneuroim.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 33.Culhane AC, Hall MD, Rothwell NJ, Luheshi GN. Mol Psychiatry. 1998;3:362–366. doi: 10.1038/sj.mp.4000389. [DOI] [PubMed] [Google Scholar]
- 34.Conti B, Park LC, Calingasan NY, Kim Y, Kim H, Bae Y, Gibson GE, Joh TH. Brain Res Mol Brain Res. 1999;67:46–52. doi: 10.1016/s0169-328x(99)00034-0. [DOI] [PubMed] [Google Scholar]
- 35.Sugama S, Cho BP, Baker H, Joh TH, Lucero J, Conti B. Brain Res. 2002;958:1–9. doi: 10.1016/s0006-8993(02)03363-2. [DOI] [PubMed] [Google Scholar]
- 36.Garcia MC, Wernstedt I, Berndtsson A, Enge M, Bell M, Hultgren O, Horn M, Ahren B, Enerback S, Ohlsson C, et al. Diabetes. 2006;55:1205–1213. doi: 10.2337/db05-1304. [DOI] [PubMed] [Google Scholar]
- 37.Somm E, Henrichot E, Pernin A, Juge-Aubry CE, Muzzin P, Dayer JM, Nicklin MJ, Meier CA. Diabetes. 2005;54:3503–3509. doi: 10.2337/diabetes.54.12.3503. [DOI] [PubMed] [Google Scholar]
- 38.Wallenius V, Wallenius K, Ahren B, Rudling M, Carlsten H, Dickson SL, Ohlsson C, Jansson JO. Nat Med. 2002;8:75–79. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- 39.Chida D, Osaka T, Hashimoto O, Iwakura Y. Diabetes. 2006;55:971–977. doi: 10.2337/diabetes.55.04.06.db05-1250. [DOI] [PubMed] [Google Scholar]
- 40.Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 41.Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, et al. Cell. 1996;84:491–495. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- 42.Ogimoto K, Harris MK, Jr, Wisse BE. Endocrinology. 2006;147:4445–4453. doi: 10.1210/en.2006-0465. [DOI] [PubMed] [Google Scholar]
- 43.Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, et al. Nature. 2003;421:856–859. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- 44.Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishimoto T, Okamura H, Nakanishi K, Akira S. Immunity. 1998;8:383–390. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- 45.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. New York: Elsevier; 2001. [Google Scholar]
- 46.Zorrilla EP, Iwasaki S, Moss JA, Chang J, Otsuji J, Inoue K, Meijler MM, Janda KD. Proc Natl Acad Sci USA. 2006;103:13226–13231. doi: 10.1073/pnas.0605376103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.