Abstract
Pleiotrophin (PTN, Ptn) is an 18-kDa secretory cytokine expressed in many breast cancers; however, the significance of Ptn expression in breast cancer has not been established. We have now tested three models to determine the role of inappropriate expression of Ptn in breast cancer. Mouse mammary tumor virus (MMTV) promoter-driven Ptn expressed in MMTV-polyoma virus middle T antigen (PyMT)-Ptn mouse breast cancers was first shown to induce rapid growth of morphologically identified foci of “scirrhous” carcinoma and to extensively remodel the microenvironment, including increased tumor angiogenesis and striking increases in mouse protocollagens Iα2, IVα5, and XIα1, and elastin. Ectopic Ptn expression in MCF-7 (human breast cancer)-Ptn cell xenografts also was shown to markedly increase MCF-7-Ptn cell xenograft growth in nude mice; furthermore, it induced extensive remodeling of the microenvironment and tumor angiogenesis. In a coculture model of equal numbers of NIH 3T3 stromal fibroblasts and MCF-7-Ptn cells, PTN secreted from MCF-7-Ptn cells was then shown to induce a more malignant MCF-7-Ptn breast cancer cell phenotype and extensive remodeling of the MCF-7-Ptn/NIH 3T3 cell microenvironment; it up-regulated expression of markers of aggressive breast cancers, including PKCδ and matrix metalloproteinase-9 in both MCF-7-Ptn and NIH 3T3 cells. The morphological phenotypes of MCF-7-Ptn cell xenografts and MCF-7-Ptn cell/NIH 3T3 cell cocultures closely resembled breast cancers in MMTV-PyMT-Ptn mice. Inappropriate expression of Ptn thus promotes breast cancer progression in mice; the data suggest that secretion of PTN through stimulation of the stromal cell microenvironment alone may be sufficient to account for significant features of breast cancer progression.
Keywords: angiogenesis, collagen, polyoma virus middle T, scirrhous carcinoma, stromal fibroblasts
Breast cancers progress through stages of increasing malignancy triggered by genetic and epigenetic mutations that promote their growth, invasiveness, and metastasis. Because mortality from breast cancer is most often due to growth of distant metastases that are not controlled by existing therapies, there is a vital need to better understand the molecular pathogenesis of breast cancer and to identify mutations in breast cancer cells that promote breast cancer progression and lead to metastasis (1).
Pleiotrophin [PTN (the protein), Ptn (the gene)] is a 136-aa heparin-binding cytokine (2, 3) frequently detected in human breast cancers (4). Its expression is constitutive in human breast cancer cells in which it has been tested (5). Furthermore, PTN stimulates functional responses that are known to stimulate breast cancer progression, such as proliferation (2, 3), cytoskeletal rearrangements, and loss of cell–cell adhesion (6, 7), transformation (8), induction of an epithelial to mesenchymal transition (6), and stimulation of tumor angiogenesis (9, 10), in cultured cells or in cells that ectopically express Ptn. These functional responses to PTN are likely to be significant in progression of breast cancers, because interruption of constitutive PTN signaling in human breast cancer cells that inappropriately express Ptn reverses their transformed phenotype in vitro and in vivo (5); these PTN-stimulated functional responses thus support the possibility that mutations in breast cancer cells, which deregulate endogenous Ptn expression, may initiate similar functional responses and lead to breast cancer progression.
In the following studies, three models were tested to determine whether inappropriate expression of Ptn alone is sufficient to induce breast cancer or whether inappropriate expression of Ptn cooperates with different pathogenic mechanisms that stimulate breast cancer progression. Together, the studies demonstrate both in vivo and in vitro that inappropriate expression of Ptn promotes breast cancer progression; the studies also demonstrate that PTN secretion from human breast cancer cells alone is sufficient to promote progression of breast cancer to a more aggressive breast cancer phenotype through activation of stromal cells and extensive remodeling of the microenvironment.
Results
Mouse Mammary Tumor Virus (MMTV)-Ptn Fails to Induce Breast Cancer in MMTV-Ptn Transgenic Mice.
To test whether inappropriate expression of Ptn alone is sufficient to induce breast cancers in mice, MMTV-long terminal repeat-driven-Ptn (MMTV-Ptn) transgenic mice were generated [see supporting information (SI) Materials and Methods and SI Fig. 5]. Female MMTV-Ptn mice had normal mammary gland development and lactation and developed pups in normal numbers and in size equal to that of control mouse pups. Expression of the MMTV-Ptn transgene was detected in high levels in breast epithelial cells. In extensive microscopic examinations of breast tissues, dysplastic changes were not seen. MMTV-Ptn mice were free of detectable breast cancers for 18 months. Thus, in this context, inappropriate expression of Ptn in mouse breast epithelial cells fails to induce breast cancer, and Ptn thus is not an oncogene.
MMTV-Ptn Stimulates Breast Cancer Progression in MMTV-PyMT-Ptn Transgenic Mice.
MMTV-Ptn mice were then bred with MMTV-polyoma virus middle T (PyMT) mice to generate MMTV-PyMT-Ptn (bitransgenic) mice. The MMTV-PyMT transgenic mouse was chosen because PyMT is a potent oncogene; furthermore, the PyMT oncoprotein is known to coopt the Src family, ras, and phosphatidylinositol 3-kinase oncogenic signaling pathways (11). Four distinctly identifiable stages of tumor progression that range from premalignant to malignant invasive ductal carcinomas of the scirrhous subtype (hereafter “scirrhous carcinoma”) (12) occur within a single primary focus. The MMTV-PyMT mouse breast cancers express markers known to be significant in human breast cancers. They also have significant infiltration of inflammatory cells that frequently are found in human breast cancers. The MMTV-PyMT model thus closely recapitulates the many steps of human breast cancer progression.
MMTV-PyMT-Ptn mice developed breast cancers with striking variability in numbers and size, a characteristic of MMTV-PyMT breast cancers described previously (11). Statistically significant differences in time of onset, tumor volume, tumor burden, or average numbers of metastases per lung (recorded as metastasis index) were not found between MMTV-PyMT-Ptn and MMTV-PyMT littermate mice (data not shown). However, the areas occupied by the foci of scirrhous carcinoma in sections of MMTV-PyMT-Ptn mouse breast cancers were increased nearly 2-fold compared with breast cancers in MMTV-PyMT mice (39% vs. 22%) (P < 0.05) (SI Table 1), indicating that the scirrhous carcinoma grows significantly more rapidly in MMTV-PyMT-Ptn breast cancers than in MMTV-PyMT breast cancers, or, perhaps, progression of the breast cancers to the more aggressive scirrhous subtype is markedly accelerated in MMTV-PyMT-Ptn mice. In contrast, the areas occupied by invasive ductal carcinomas that lack the features of the scirrhous carcinoma were not much different in breast cancers in MMTV-PyMT-Ptn and MMTV-PyMT mice (19.2% vs. 16.8%) (SI Table 1).
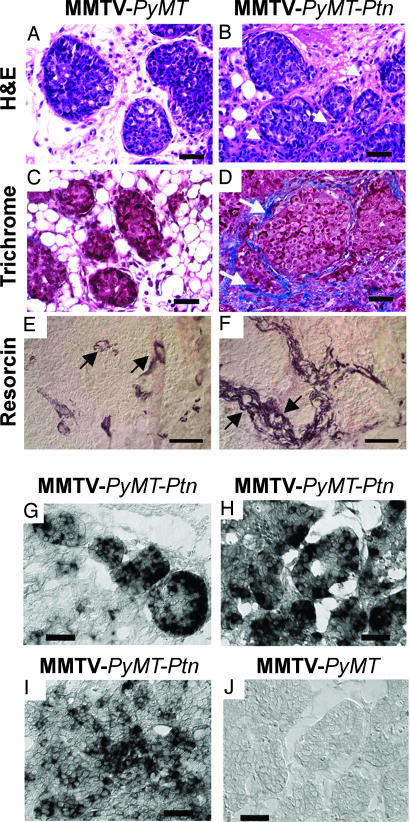
When studied in H&E-stained sections, the nodular foci of scirrhous carcinoma in MMTV-PyMT-Ptn breast cancers were surrounded circumferentially by marked increases in matrix proteins interspersed with increased numbers of morphologically identified “activated stromal fibroblasts” compared with MMTV-PyMT breast cancers (compare Fig. 1 A and B). These matrix proteins surrounding the invasive nodules of scirrhous carcinoma were identified as collagen fibrils in sections of MMTV-PyMT-Ptn mouse breast cancers stained with Masson trichrome (Fig. 1D), whereas only a few collagen fibrils were seen to surround the invasive nodules of scirrhous carcinoma in MMTV-PyMT mice (Fig. 1C). A striking increase in mature elastin fibrils surrounding the invasive nodules of scirrhous carcinoma and surrounding the blood vessels (arrows) also was seen in MMTV-PyMT-Ptn breast cancers (Fig. 1F) compared with MMTV-PyMT breast cancers (Fig. 1E).
Fig. 1.
Histological properties of MMTV-PyMT-Ptn breast cancers compared with MMTV-PyMT breast cancers. Representative images are shown. (A and B) Sections of breast cancers from MMTV-PyMT (A) or MMTV-PyMT-Ptn mice (B) stained with H&E. Note the striking increase in extracellular matrix proteins and stromal fibroblasts (arrow) that surround the foci of breast cancer cells in MMTV-PyMT-Ptn mice. (C and D) Masson trichrome-stained sections of breast cancers from MMTV-PyMT (C) or MMTV-PyMT-Ptn mice (D). Arrows point to the collagen fibrils that surround the foci of invasive nodules of breast cancer cells in MMTV-PyMT-Ptn mice contrasted with the far more limited fibrils and largely fat globules surrounding carcinoma cells in MMTV-PyMT mouse breast cancers. (E and F) Resorcin/fuchsin-stained sections of invasive nodules of breast cancer cells from MMTV-PyMT-Ptn mice (F) and MMTV-PyMT mice (E). Arrows point to the elastin fibrils surrounding the blood vessels. In situ hybridization of Ptn mRNA in paraffin sections of tumors from MMTV-PyMT-Ptn and MMTV-PyMT transgenic mice. (G–J) The antisense human Ptn RNA probe was generated as described in Materials and Methods and used for in situ hybridization of sections of MMTV-PyMT-Ptn and MMTV-PyMT breast cancers. (G) Focus of the scirrhous subtype of invasive ductal carcinomas. (H) Early stage in development of scirrhous subtype of invasive ductal carcinoma. (I) Nonscirrhous invasive ductal carcinomas of MMTV-PyMT-Ptn breast cancers. (J) MMTV-PyMT breast cancers. (Scale bars: 150 μm.)
The basis of the increased collagen staining was then examined; protocollagen Iα2 transcripts were ≈34-fold increased in MMTV-PyMT-Ptn breast cancers when measured by RT-PCR and compared with expression levels in MMTV-PyMT breast cancers. Furthermore, protocollagen IVα5 transcripts were increased ≈53-fold, and protocollagen XIα1 transcripts were increased ≈54-fold; however, the levels of protocollagen Iα1 mRNA were decreased to ≈22% of the levels of procollagen Iα1 in MMTV-PyMT breast cancers. Tropoelastin expression levels were nearly 4-fold increased in breast cancers of MMTV-PyMT-Ptn mice (SI Fig. 6). These findings thus substantiate the striking increases in collagen and elastin as described above in sections of breast cancers of MMTV-PyMT-Ptn mice. A surprising finding, however, was that the dramatic increases in collagen expression is mouse collagen-subtype specific.
Sections of breast cancers were then stained with anti-CD31 antibodies to identify microvascular endothelial cells; it was found that MMTV-PyMT-Ptn breast cancers had an ≈1.5-fold increase in the intratumor microvessel density (IMD) compared with the IMD in breast cancers in MMTV-PyMT mice (SI Table 2). Surprisingly, the average microvessel area in MMTV-PyMT-Ptn breast cancers measured 658 μm2, whereas in breast cancers in MMTV-PyMT mice, it measured 393 μm2 (P < 0.01) (SI Table 2). The blood vessels in MMTV-PyMT-Ptn mice had a normal appearance and were not dysplastic as is characteristic of many epithelial malignancies. Furthermore, as cited above, elastin fibrils that surround the new blood vessels in MMTV-PyMT-Ptn breast cancers were much increased. As for increased collagens and elastin, the increase in IMD and the blood vessels of larger size were consistently localized in close proximity to the foci of scirrhous carcinoma (Fig. 1 E and F, arrows), and, as discussed below, these foci are the sites in MMTV-PyMT-Ptn breast cancers that express the highest levels of the Ptn transgene.
Very high-level expression of the human Ptn transgene was then demonstrated within foci of scirrhous breast carcinomas in sections of MMTV-PyMT breast cancers analyzed by in situ hybridization using human antisense Ptn RNA probes (Fig. 1G). Expression of PTN also was highest within these foci when analyzed by immunohistochemistry (data not shown). High-level expression of Ptn also was seen in later stages of progression of breast cancers with features approaching the scirrhous phenotype (Fig. 1H). Lesser intensity staining was seen in areas of MMTV-PyMT-Ptn breast cancers that were not scirrhous (Fig. 1I); as anticipated, the antisense human Ptn RNA probe failed to hybridize with mRNAs in MMTV-PyMT mouse breast cancers (Fig. 1J), thus establishing the specificity of the probe to the MMTV-Ptn human transgene. It is important that foci of invasive scirrhous carcinoma with the highest levels of expression of Ptn correlated directly with the sites of highest density of collagens and elastin, numbers of morphologically identified activated stromal fibroblasts, and levels of new blood vessels of larger diameter.
PTN Secretion from MCF-7-Ptn Cells Stimulates s.c. Xenograft Growth in Vivo: A Second Model of Breast Cancer Progression.
To test the possibility that secretion of PTN may significantly stimulate breast cancer progression independently of its effect on the breast cancer cell itself, we analyzed a second model in which xenografts of MCF-7 (human breast cancer) cells that express an ectopic Ptn gene (MCF-7-Ptn cells) were compared with xenografts of MCF-7-mock (vector alone) cells in nude mice. MCF-7 cells do not express the receptor protein tyrosine phosphatase β/ζ, the PTN receptor (13), and thus MCF-7-Ptn cells are not activated by PTN through autocrine or paracrine mechanisms. MCF-7-Ptn cell xenografts were first palpated for 7–10 days (plotted as day 1 in Fig. 2A); they then grew rapidly thereafter. MCF-7-Ptn cells coinjected with equal numbers of “surrogate” NIH 3T3 cell stromal fibroblasts xenografts grew even more rapidly. MCF-7 cells alone failed to grow at sites of implantation (Fig. 2A). These data thus suggest that PTN secreted from MCF-7-Ptn cells alone is sufficient to stimulate rapid progression of the MCF-7-Ptn cell xenograft model; furthermore, the data suggest that the stromal fibroblast is a key element in the PTN secretion-dependent progression of MCF-7-Ptn cell xenografts.
Fig. 2.
PTN secretion from MCF-7-Ptn cells stimulates s.c. xenograft growth in vivo. (A) MCF-7-mock cell, MCF-7-Ptn cell, and MCF-7-Ptn/NIH 3T3 cell xenograft growth in flanks of nude mice. Day 1 is the time of detection of the first palpable xenograft. (B) Histological phenotype of sections of MCF-7-mock cell xenografts (H&E). (C) Histological phenotype of sections of MCF-7-Ptn cell xenografts (H&E). (D) Histological phenotype of sections of MCF-7-Ptn Xenografts (Masson trichrome). (Scale bar: 100 μm.)
In sections of MCF-7-Ptn cell xenografts stained with H&E, the MCF-7-Ptn cells were fibroblast-like and more invasive in appearance (Fig. 2C) than MCF-7-Ptn cells in monolayer culture. The nuclei were pleomorphic in shape and the nuclear chromatin appeared to be more densely condensed. The cells formed invasive nodules surrounded by activated stromal fibroblast-like cells intermixed with abundant extracellular matrix proteins identified as collagen in sections stained with Masson trichrome (Fig. 2D). New blood vessels surrounding the invasive nodules of MCF-7-Ptn cells were abundant. In contrast, MCF-7-mock cells were difficult to detect at sites of implantation, and, where found, they were interspersed with fat vacuoles, fibroblasts, and different inflammatory cells (Fig. 2B). The MCF-7-Ptn cell xenografts thus phenotypically resemble the foci of scirrhous carcinoma in MMTV-PyMT-Ptn breast cancers (compare Figs. 1D and 2D). The data thus support the possibility that secretion of PTN from breast cancer cells that inappropriately express Ptn stimulates breast cancer progression.
PTN Secretion from MCF-7-Ptn Cells Stimulates Epithelial Island Formation, Activation of Stromal Fibroblasts, Extensive Remodeling of Microenvironment, and Activation of Markers of Aggressive Breast Cancer in Cocultures of MCF-7-Ptn Cells and NIH 3T3 Cells: A Third Model of Breast Cancer Progression.
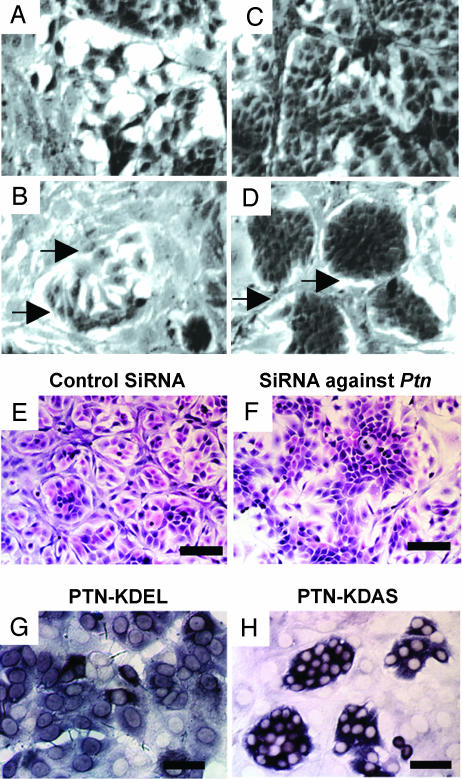
The MCF-7-Ptn cells were then cocultured with equal numbers of NIH 3T3 cells and analyzed by phase-contrast microscopy. The MCF-7-Ptn cells became loosely clustered during logarithmic growth and tightly clustered “epithelial islands” (14) at confluence (Fig. 3 B and D), whereas MCF-7-mock cells in coculture with NIH 3T3 cells failed to form epithelial islands either during logarithmic growth or at confluence (Fig. 3 A and C). In hematoxylin/eosin-stained sections, MCF-7-Ptn cells that expressed an siRNA shown to effectively “knock down” Ptn expression (see Western blot, SI Fig. 7A) failed to form epithelial islands in coculture with NIH 3T3 cells (Fig. 3F). In contrast, MCF-7-Ptn cells that expressed a control (scrambled) siRNA effectively formed epithelial islands (Fig. 3E). Furthermore, by using an anti-human-Ptn probe to detect MCF-7-Ptn cells and distinguish epithelial islands by in situ hybridization, it was shown that MCF-7 cells that expressed Ptn with a C-terminal KDEL endoplasmic reticulum retention sequence (15) (MCF-7-Ptn-KDEL cells), which effectively blocked secretion of PTN from the MCF-7-Ptn-KDEL cells (see Western blot, SI Fig. 7B), failed to form epithelial islands in coculture with NIH 3T3 cells (Fig. 3G). However, the MCF-7-Ptn cells that express Ptn with a C-terminal KDAS (control) sequence readily formed epithelial islands (Fig. 3H); thus, in this model, epithelial island formation in cocultures of MCF-7-Ptn cells with NIH 3T3 cells is MCF-7 cell-Ptn expression-dependent, MCF-7 cell PTN secretion-dependent, and surrogate NIH 3T3 stromal cell-dependent (coculture).
Fig. 3.
MCF-7 cells stably transfected with Ptn (MCF-7-Ptn) cells form epithelial islands in coculture with NIH 3T3 cells that depend on Ptn expression and PTN secretion. Representative images are shown. (A and B) Epithelial island formation. Cocultures of equal numbers of MCF-7-mock cells (A) or MCF-7-Ptn cells (B) with NIH 3T3 cells in logarithmic phase observed by using phase-contrast microscopy. (C and D) Cocultures of equal numbers of MCF-7-mock (C) or MCF-7-Ptn cells (D) with NIH 3T3 cells at confluence. Arrows point to acellular areas surrounding epithelial islands. Phase-contrast microscopy. (Scale bar: 100 μm.) (E and F) Loss of epithelial island formation by knockdown of Ptn expression. siRNA reduces epithelial island formation in cocultures of MCF-7-Ptn with NIH 3T3 cells (F), but MCF-7-Ptn cells with a control (scrambled) siRNA formed clearly defined epithelial islands (E). H&E-stained slides. (Scale bar: 200 μm.) (G and H) Anti-human Ptn in situ hybridization of MCF-7-Ptn-KDEL and MCF-7-Ptn-KDAS cells in coculture with NIH 3T3 cells. (H and G) Epithelial islands form when MCF-7-Ptn-KDAS (H) but not MCF-7-Ptn-KDEL (G) cells are cocultured with NIH 3T3 cells. (Scale bar: 100 μm.)
The MCF-7-Ptn cells in cocultures of MCF-7-Ptn cells and NIH 3T3 cells within epithelial islands were found to be more fibroblast-like (Fig. 3 B and D) than MCF-7-Ptn cells in monolayer culture or MCF-7-mock cells in coculture with NIH 3T3 cells (Fig. 3 A and C); the MCF-7-Ptn cells overlapped one another and thus were not cell–cell contact limited (Fig. 3D). An ≈1.5- to 2.0-fold increase in MCF-7-Ptn cells in epithelial islands was found in comparison with MCF-7-mock cells in coculture with NIH 3T3 cells. The epithelial islands were surrounded by fibrillar proteinaceous bands (recognized as acellular areas) that are in immediate contiguity to the epithelial islands. They were intermixed with more elongated, more spindle-shaped NIH 3T3 cells than NIH 3T3 cells in monolayer culture or NIH 3T3 cells in coculture with mock MCF-7 cells; surprisingly again, the morphological features of the epithelial islands in MCF-7-Ptn/NIH 3T3 cell cocultures were very similar to the nodular foci of scirrhous breast cancers in MMTV-PyMT-Ptn mice and in MCF-7-Ptn cell xenografts (compare Figs. 1D, 2D, and 3D).
MCF-7-Ptn cell/NIH 3T3 cell cocultures were then stained with antitropoelastin antibodies; a marked increase in immunoreactivity was found in NIH 3T3 cells cocultured with MCF-7-Ptn cells (Fig. 4D), which was most intense in NIH 3T3 cells that surround the MCF-7-Ptn epithelial islands. Tropoelastin expression also was readily detected in the MCF-7-Ptn cells within epithelial islands (Fig. 4D) but not in MCF-7-mock cells in coculture with NIH 3T3 cells (Fig. 4C) nor in MCF-7-Ptn cells in monolayer culture (data not shown). A slight increase in anti-tropoelastin immunoreactivity was found in PTN-stimulated NIH 3T3 cells (Fig. 4B), compared with nonstimulated NIH 3T3 cells (Fig. 4A). Protocollagen gene expression also was seen in high levels in NIH 3T3 cells in coculture with MCF-7-Ptn cells; however, the appearance of fibrillar collagen in cocultures was delayed, perhaps, it is speculated, because collagens were degraded by metalloproteinases such as matrix metalloproteinase (MMP)-9 (see below).
Fig. 4.
Extracellular remodeling by PTN in cocultures of MCF-7-Ptn and NIH 3T3 cells. (A–D) Detection of tropoelastin using immunofluorescence in NIH 3T3 cells treated with 50 ng/ml PTN for 1 h (B), untreated NIH 3T3 cells (A), cocultures of MCF-7-mock with NIH 3T3 cells (C), and cocultures of MCF-7-Ptn with NIH 3T3 cells (D). (Scale bar: 50 μm.) (E and F) Detection of phospho-PKCδ (Thr505) using immunofluorescent microscopy in cocultures of MCF-7-Ptn transfected by siRNAs against Ptn (F) or control siRNAs (E) with NIH 3T3 cells. (Scale bar: 100 μm). (G) Gelatin zymography of supernatants of MCF-7 (lane 1), MCF-7-mock (lane 2), and MCF-7-Ptn (lane 3) cells cocultured with NIH 3T3 cells. (H–K) The 105-kDa band corresponds to murine pro-MMP-9, and the 92-kDa band corresponds to human pro-MMP-9. Detection of pro-MMP-9 using immunocytochemistry in monocultures of MCF-7-mock cells (H) and MCF-7-Ptn cells (I) or cocultures of NIH 3T3 cells with MCF-7-mock cells (J) or MCF-7-Ptn cells (K).
PKCδ is a marker of aggressive breast cancers; it is known to be activated in both breast cancer cells and activated stromal fibroblasts (16); PTN furthermore is known to activate PKC activity in PTN-stimulated cells (7). Activated PKCδ in turn activates pro-MMP-9 (17), and activated MMP-9 degrades collagens, releasing collagen peptides that themselves are growth stimulatory (18). Activated PKCδ was readily identified with immunofluorescent microscopy in both NIH 3T3 stromal fibroblasts and the MCF-7-Ptn cells transfected with the control “scrambled” siRNA in coculture with NIH 3T3 cells (Fig. 4E). In contrast, markedly reduced levels of activated PKCδ were found in cocultures of MCF-7-Ptn cells transfected with the Ptn-specific siRNA and NIH 3T3 cells (Fig. 4F). The data thus support the fact that secretion of PTN from MCF-7-Ptn cells in coculture with NIH 3T3 cells leads to a marked activation of PKCδ, a known marker of aggressive breast cancers, in both MCF-7-Ptn cells and NIH 3T3 cells.
Conditioned media from MCF-7, MCF-7-mock, and MCF-7-Ptn cells in coculture with NIH 3T3 cells were then examined by gelatin zymography (19). Bands of ≈92 and ≈105 kDa that correspond to human pro-MMP-9 and murine pro-MMP-9 were detected in gels of conditioned media from MCF-7-Ptn/NIH 3T3 cell cocultures (Fig. 4G, lane 3). Human pro-MMP-9 also was readily detected by immunohistochemistry using anti-pro-MMP9 antibodies in MCF-7-mock and MCF-7-Ptn cells cocultured with NIH 3T3 cells (Fig. 4 J and K) but not when these cells were examined in monoculture (Fig. 4 H and I). The highest levels of pro-MMP-9 were detected in MCF-7-Ptn cells cocultured with NIH 3T3 cells (Fig. 4K); the levels were significantly higher compared with MCF-7-mock cells in coculture with NIH 3T3 cells (Fig. 4J). Importantly, human and murine pro-MMP-9 were found in very limited amounts in conditioned media of cocultures of MCF-7 (Fig. 4G, lane 1) or MCF-7-mock cells with NIH 3T3 cells (Fig. 4G, lane 2) in comparison with levels of murine and human pro-MMP-9 in media from MCF-7-Ptn cells with NIH 3T3 cells (Fig. 4G, lane 3). Thus, the high level of expression of MMP-9 in both MCF-7-Ptn and NIH 3T3 cells in cocultures of MCF-7-Ptn and NIH 3T3 cells leads to export of MMP-9 in media; export of MMP-9 is minimal if it occurs at all from MCF-7-mock or MCF-7 cells in coculture with NIH 3T3 cells.
Discussion
These studies demonstrate that inappropriate expression of Ptn alone in MMTV-Ptn transgenic mice is not sufficient to induce breast cancer; thus, in this context, PTN is not an oncogenic protein. However, inappropriate expression of Ptn driven by MMTV in MMTV-PyMT-Ptn mice induced more rapid growth of foci of breast cancers with the histological phenotype of scirrhous carcinoma; it induced truly remarkable increases in new collagen and elastin deposition, extensive extracellular matrix remodeling, increased tumor angiogenesis, and increased size of healthy-appearing new blood vessels. The foci of scirrhous carcinoma in MMTV-PyMT-Ptn mice express very high levels of the MMTV-Ptn transgene and, thus, high-level expression of MMTV-Ptn correlates directly with foci of scirrhous carcinoma and, furthermore, with the remarkable increases in collagen, elastin, and tumor angiogenesis and increased size of new blood vessels within the MMTV-PyMT-Ptn breast cancers. Secretion of PTN from MCF-7-Ptn cells alone in two other models used in this study also was shown to be sufficient alone to stimulate progression of breast cancer cells and induce a remarkable remodeling of the microenvironment with striking increases in collagens, elastin, and, in the case of MCF-7-Ptn xenografts in nude mice, tumor angiogenesis. Surprisingly, the histological patterns of the MCF-7-Ptn epithelial islands in MCF-7-Ptn and NIH 3T3 cell cocultures and xenografts of MCF-7-Ptn cells in nude mice closely resemble the histological pattern of breast cancers in MMTV-PyMT-Ptn mice, namely scirrhous carcinomas. Thus, constitutive PTN-signaling in MMTV-PyMT-Ptn mice promotes progression of mouse breast cancer and induces a breast cancer phenotype in MMTV-PyMT-Ptn mice akin to scirrhous carcinoma in humans. Because the scirrhous subtype of invasive ductal carcinoma is among the most aggressive human breast cancers (20), the study raises the possibility that inappropriate expression of Ptn may be an important factor in the pathogenesis of scirrhous invasive ductal carcinoma.
Our data furthermore suggest the potential that PTN expression may account for many of the features of scirrhous carcinoma seen in the breast cancers in MMTV-PyMT-Ptn mice. PTN stimulates new collagen of different subtypes and new elastin synthesis (L. Ezquerra, G. Herradon, and T.F.D., unpublished data). Collagen fragments are known to stimulate growth of carcinoma cells and to stimulate antiapoptotic pathways, favoring growth of the carcinoma cells (21). Through activation of integrins and “outside-in” signaling, both elastin and collagens are known to activate the ERK pathway in epithelial cells and function to prevent caspase 8 activation and apoptosis (18). Thus, the data generated suggest the possibility that the increased synthesis of extracellular matrix proteins is an important contributor to the increased growth of these foci of scirrhous carcinoma in MMTV-PyMT-Ptn breast cancers. PTN also is known to stimulate new blood vessel formation and tumor angiogenesis (10, 22), and, in these studies, secretion of PTN leads to up-regulation of two well recognized markers of aggressive breast cancers, PKCδ and MMP-9, in both breast cancer cells and stromal fibroblasts.
Activated stromal fibroblasts are often identified by characteristic morphological features and different biomarkers, such as α-smooth muscle actin; however, more recently, activated stromal fibroblasts have been characterized by different functional responses that result from their activation, such as expression of the extracellular matrix proteins collagens, elastin, and MMPs and release of growth factors and cytokines (23). These studies thus identify PTN as one factor that activates stromal fibroblasts to induce many features of aggressive breast cancers, and, thus, it is suggested that inappropriate expression of Ptn may be very important in breast cancer progression. The importance of the epithelial cancer cell-stromal fibroblast interactions is well established (24), but only limited progress has been made in identifying the factors that activate stromal cells to initiate cross-talk and tumor progression. These studies thus demonstrate that activated stromal cells are central in the models used in this study; they are responsible for the synthesis, deposition, and remodeling of the extracellular matrix protein and release of factors that stimulate the growth and malignant phenotype of the carcinoma cells themselves (25). The studies thus indicate that PTN secreted from the breast cancer cells is the mechanism of stromal cell activation; the studies also identify the fact that PTN alone is sufficient to stimulate in activated fibroblasts many of the critical signaling pathways needed to aggressively promote breast cancer progression.
Materials and Methods
Cell Culture, Transfections, and Tissue and Cell Staining.
Human breast cancer MCF-7 and murine fibroblast NIH 3T3 cells were obtained from American Type Culture Collection (Manassas, VA) and maintained in minimum essential medium or Dulbecco's modified Eagle's medium supplemented with 10% FBS at 37°C and 5% CO2.
Cells were transfected with the different constructs described in SI Materials and Methods by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's recommendations.
MCF-7 and NIH 3T3 cell cocultures were fixed with 4% paraformaldehyde and either stained with H&E or blocked with PBS containing 1% inactivated FBS and 0.1% Triton X-100 and incubated with antitropoelastin antibodies, anti-phospho-PKCδ (Thr505) antibodies (Cell Signaling, Danvers, MA) or anti-pro-MMP-9 antibodies that recognize human and mouse pro-MMP-6 (Calbiochem, San Diego, CA) and, subsequently, with FITC-conjugated or HRP-conjugated secondary antibodies.
The tissues excised from euthanized animals were fixed in 10% zinc formalin and embedded in paraffin. The specimens were sectioned and stained with H&E, Masson trichrome for collagen, and resorcin/fuchsin for elastin.
Transgenic Constructs, Transgenic Mice.
See SI Materials and Methods and SI Fig. 5. All mice were housed in the animal facility of The Scripps Research Institute and maintained according to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
IMD and in Situ Hybridization.
In situ hybridization and IMD were performed as described (10, 26). For details, see SI Materials and Methods.
Western Blot Analysis and Gelatin Zymography.
Western blot analysis and gelatin zymography were performed as described (19, 27). See SI Materials and Methods.
Statistics.
Results are presented as means ± SEM. Student's t test was used to determine the statistical significance of tumor onset time, the time of tumor detection, tumor size, and scirrhous area of scirrhous foci in breast cancers.
Supplementary Material
Acknowledgments
We thank Francis Chisari (The Scripps Research Institute) for assistance with the microscopic examination of sections of the MMTV breast cancers. This work was supported by National Institutes of Health Grant R01-CA084400. Y.C. was supported by training grants from The Skaggs Institute. P.P.-P. was supported by National Institutes of Health Grant 2 T32 DK007022-26. This is manuscript 18433-MEM from The Scripps Research Institute.
Abbreviations
- PTN
pleiotrophin
- MMTV
mouse mammary tumor virus
- IMD
intratumor microvessel density
- MMP
matrix metalloproteinase.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0704366104/DC1.
References
- 1.Hanahan D, Weinberg RA. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Milner PG, Li YS, Hoffman RM, Kodner CM, Siegel NR, Deuel TF. Biochem Biophys Res Commun. 1989;165:1096–1103. doi: 10.1016/0006-291x(89)92715-0. [DOI] [PubMed] [Google Scholar]
- 3.Li YS, Milner PG, Chauhan AK, Watson MA, Hoffman RM, Kodner CM, Milbrandt J, Deuel TF. Science. 1990;250:1690–1694. doi: 10.1126/science.2270483. [DOI] [PubMed] [Google Scholar]
- 4.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, et al. Proc Natl Acad Sci USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang N, Zhong R, Wang ZY, Deuel TF. J Biol Chem. 1997;272:16733–16736. doi: 10.1074/jbc.272.27.16733. [DOI] [PubMed] [Google Scholar]
- 6.Perez-Pinera P, Alcantara S, Dimitrov T, Vega JA, Deuel TF. Proc Natl Acad Sci USA. 2006;103:17795–17800. doi: 10.1073/pnas.0607299103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pariser H, Herradon G, Ezquerra L, Perez-Pinera P, Deuel TF. Proc Natl Acad Sci USA. 2005;102:12407–12412. doi: 10.1073/pnas.0505901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chauhan AK, Li YS, Deuel TF. Proc Natl Acad Sci USA. 1993;90:679–682. doi: 10.1073/pnas.90.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choudhuri R, Zhang HT, Donnini S, Ziche M, Bicknell R. Cancer Res. 1997;57:1814–1819. [PubMed] [Google Scholar]
- 10.Zhang N, Zhong R, Perez-Pinera P, Herradon G, Ezquerra L, Wang ZY, Deuel TF. Biochem Biophys Res Commun. 2006;343:653–658. doi: 10.1016/j.bbrc.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Lin EY, Jones JG, Li P, Zhu L, Whitney KD, Muller WJ, Pollard JW. Am J Pathol. 2003;163:2113–2126. doi: 10.1016/S0002-9440(10)63568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsuchiya S, Li F. Med Mol Morphol. 2005;38:216–224. doi: 10.1007/s00795-005-0300-9. [DOI] [PubMed] [Google Scholar]
- 13.Meng K, Rodriguez-Pena A, Dimitrov T, Chen W, Yamin M, Noda M, Deuel TF. Proc Natl Acad Sci USA. 2000;97:2603–2608. doi: 10.1073/pnas.020487997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maeda T, Alexander CM, Friedl A. Cancer Res. 2004;64:612–621. doi: 10.1158/0008-5472.can-03-2439. [DOI] [PubMed] [Google Scholar]
- 15.Munro S, Pelham HR. Cell. 1987;48:899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- 16.Kiley SC, Clark KJ, Duddy SK, Welch DR, Jaken S. Oncogene. 1999;18:6748–6757. doi: 10.1038/sj.onc.1203101. [DOI] [PubMed] [Google Scholar]
- 17.Woo JH, Lim JH, Kim YH, Suh SI, Min do S, Chang JS, Lee YH, Park JW, Kwon TK. Oncogene. 2004;23:1845–1853. doi: 10.1038/sj.onc.1207307. [DOI] [PubMed] [Google Scholar]
- 18.Sanders MA, Basson MD. J Biol Chem. 2000;275:38040–38047. doi: 10.1074/jbc.M003871200. [DOI] [PubMed] [Google Scholar]
- 19.Zuka M, Okada Y, Nemori R, Fukuda A, Takekoshi N, Nakanishi I, Katsuda S. Appl Immunohistochem Mol Morphol. 2003;11:78–84. doi: 10.1097/00129039-200303000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Debnath J, Brugge JS. Nat Rev Cancer. 2005;5:675–688. doi: 10.1038/nrc1695. [DOI] [PubMed] [Google Scholar]
- 21.Ruhl M, Sahin E, Johannsen M, Somasundaram R, Manski D, Riecken EO, Schuppan D. J Biol Chem. 1999;274:34361–34368. doi: 10.1074/jbc.274.48.34361. [DOI] [PubMed] [Google Scholar]
- 22.Christman KL, Fang Q, Kim AJ, Sievers RE, Fok HH, Candia AF, Colley KJ, Herradon G, Ezquerra L, Deuel TF, et al. Biochem Biophys Res Commun. 2005;332:1146–1152. doi: 10.1016/j.bbrc.2005.04.174. [DOI] [PubMed] [Google Scholar]
- 23.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 24.Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, Huang H, Porter D, Hu M, Chin L, Richardson A, et al. Cancer Cell. 2004;6:17–32. doi: 10.1016/j.ccr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Kalluri R. Nat Rev Cancer. 2003;3:422–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 26.Silos-Santiago I, Yeh HJ, Gurrieri MA, Guillerman RP, Li YS, Wolf J, Snider W, Deuel TF. J Neurobiol. 1996;31:283–296. doi: 10.1002/(SICI)1097-4695(199611)31:3<283::AID-NEU2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 27.Pariser H, Perez-Pinera P, Ezquerra L, Herradon G, Deuel TF. Biochem Biophys Res Commun. 2005;335:232–239. doi: 10.1016/j.bbrc.2005.07.060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.