Abstract
Background
Molecular hydrogen is an environmentally-clean fuel and the reversible (bi-directional) hydrogenase of the cyanobacterium Synechocystis sp. PCC 6803 as well as the native Escherichia coli hydrogenase 3 hold great promise for hydrogen generation. These enzymes perform the simple reaction 2H+ + 2e- ↔ H2 (g).
Results
Hydrogen yields were enhanced up to 41-fold by cloning the bidirectional hydrogenase (encoded by hoxEFUYH) from the cyanobacterium into E. coli. Using an optimized medium, E. coli cells expressing hoxEFUYH also produced twice as much hydrogen as the well-studied Enterobacter aerogenes HU-101, and hydrogen gas bubbles are clearly visible from the cultures. Overexpression of HoxU alone (small diaphorase subunit) accounts for 43% of the additional hydrogen produced by HoxEFUYH. In addition, hydrogen production in E. coli mutants with defects in the native formate hydrogenlyase system show that the cyanobacterial hydrogenase depends on both the native E. coli hydrogenase 3 as well as on its maturation proteins. Hydrogen absorption by cells expressing hoxEFUYH was up to 10 times lower than cells which lack the cloned cyanobacterial hydrogenase; hence, the enhanced hydrogen production in the presence of hoxEFUYH is due to inhibition of hydrogen uptake activity in E. coli. Hydrogen uptake by cells expressing hoxEFUYH was suppressed in three wild-type strains and in two hycE mutants but not in a double mutant defective in hydrogenase 1 and hydrogenase 2; hence, the active cyanobacterial locus suppresses hydrogen uptake by hydrogenase 1 and hydrogenase 2 but not by hydrogenase 3. Differential gene expression indicated that overexpression of HoxEFUYH does not alter expression of the native E. coli hydrogenase system; instead, biofilm-related genes are differentially regulated by expression of the cyanobacterial enzymes which resulted in 2-fold elevated biofilm formation. This appears to be the first enhanced hydrogen production by cloning a cyanobacterial enzyme into a heterologous host.
Conclusion
Enhanced hydrogen production in E. coli cells expressing the cyanobacterial HoxEFUYH is by inhibiting hydrogen uptake of both hydrogenase 1 and hydrogenase 2.
Background
Cyanobacteria are diverse, ancient (present 3.5 billion years ago), photosynthetic, and photoautotrophic, and it is believed that these bacteria evolved to become chloroplasts in plant cells [1]. Cyanobacteria have at least three enzymes involved in hydrogen synthesis/metabolism: (i) nitrogenase which produces hydrogen as nitrogen is converted to ammonia, (ii) uptake hydrogenase which consumes hydrogen produced by nitrogenase, and (iii) a bi-directional hydrogenase which can both consume and produce hydrogen [1]. The bi-directional hydrogenase was employed here since hydrogen production via the nitrogenase requires substantially more energy from the cell (16 ATP per mole of hydrogen) so it would be a less-energy-efficient system (N2 + 8H+ + 8e- +16ATP → 2NH3 + H2 + 16ADP + 16Pi) [1].
The reversible (bi-directional) hydrogenase enzyme of Synechocystis sp. PCC 6803 produces hydrogen via the reaction 2H+ + 2e ↔ H2 (g) [1]; the source of the two electrons is NADH. The genes (hoxEFUYH) encoding this enzyme were identified and indicate that HoxFU are iron-sulfur proteins that bind NADH (diaphorase) and that the large hydrogenase subunit HoxH contains six conserved sites for binding the Ni-Fe cofactor [2]. The small hydrogenase subunit HoxY may bind a [4Fe-4S] cluster [1]. The function of HoxE is not clear but it may be a bridging subunit in the membrane [3]. We chose this bacterium since it is well-characterized with the complete genome (3,573,470 bp) sequenced in 1996 [4]; hence, the hydrogenase is readily cloned. Transcription of HoxEFUYH is regulated by the LexA transcription activator, which specifically binds to the promoter region of the hox operon [5,6]. Note that the hydrogenase enzyme is sensitive to oxygen [7], so the assays are performed anaerobically.
Hydrogenase enzymes in E. coli are involved in two distinct modes of hydrogen metabolism: hydrogen production via hydrogenase 3 and hydrogen uptake by hydrogenases 1 and 2 [8]. Hydrogenase 1 (encoded by hyaABCDEF), hydrogenase 2 (encoded by hybOABCDEFG), and hydrogenase 3 (encoded by hycABCDEFGHI) have nickel, iron, and three non-protein diatomic ligands (cyanide and carbon monoxide) in the active site which rely on the auxiliary proteins HypABCDEF (metalochaperones for NiFe insertion) and SlyD (nickel insertion) for maturation as well as may possibly rely on the chaperones GroEL/GroES [9]. Hydrogenase 1 and hydrogenase 2 are αβ heterodimers of a small subunit and a Ni-Fe containing catalytic large subunit and are present in the inner membrane facing the periplasmic space [10-12]. In E. coli, hydrogen is produced by hydrogenase 3 in the formate hydrogenlyase system (FHL) [13]. hycE encodes the large subunit of hydrogenase 3, and hycA encodes the repressor gene of the FHL system including the hyc operon [14]. HycI protease catalyses a C-terminal proteolytic cleavage of the HycE large subunit, and HypA, HypB, HypC, HypD, HypE, and HypF are required for metallocenter assembly [15]. Ordinarily, cyanobacteria employ photosynthesis fueled by light energy to produce hydrogen. However, if an active hydrogenase from a cyanobacterium may be expressed in E. coli, it is possible to use the energy from simple sugars (e.g., from agricultural products and wastes) to produce hydrogen. Other advantages of using E. coli are that the use of energy from sugar rather than light avoids relying on the availability of light and avoids the production of oxygen as occurs during photosynthesis. Oxygen as an impurity in hydrogen arising from photosynthetic activity is undesirable for fuel cells based on enzyme electrodes [16] and is undesirable as a fire hazard [17]. Hence, large production of hydrogen is more advantageous via fermentation rather than photochemical production [17].
Hydrogen is a 100% renewable fuel that burns cleanly, is efficient, and generates no toxic by-products [7]. Hydrogen is also the preferred choice for fuel cells. Not only is H2 a clean fuel, producing only water as its by-product, it actually has a higher energy content than oil (142 MJ/kg for H2 vs. 44.2 MJ/kg for oil), and is thus more efficient. Most of the H2 now produced globally is by the process of steam reforming and the water-gas shift reaction, or as a by-product of petroleum refining and chemicals production [18]. Use of biological methods of H2 production promises significant energy reduction costs, as these processes do not require extensive heating (or extensive electricity as in electrolysis plants). Here we report the cloning of an active cyanobacterial enzyme complex into E. coli to enhance hydrogen production primarily by limiting hydrogen uptake by the native E. coli hydrogenases.
Results
Enhanced E. coli hydrogen production by HoxEFUYH
To create a recombinant system which produces hydrogen via fermentation, we cloned the hydrogenase locus (hoxEFUYH) of Synechocystis sp. PCC 6803 into the well-studied bacterium E. coli. DNA sequencing and restriction enzyme digests showed the correct locus was cloned; our plasmid was designated pBS(Kan)Synhox (Figure 1A).
Figure 1.
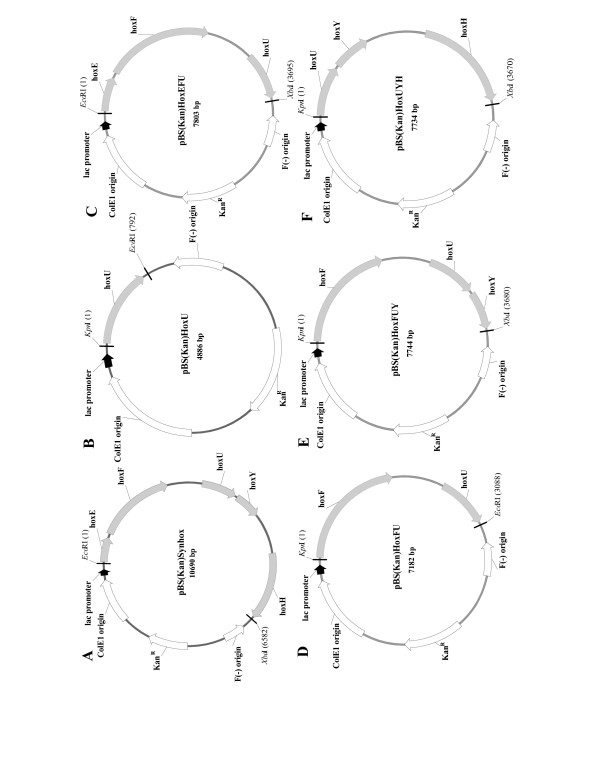
Vectors pBS(Kan)Synhox (A), pBS(Kan)HoxU (B), pBS(Kan)HoxEFU (C), pBS(Kan)HoxFU (D), pBS(Kan)HoxFUY (D), and pBS(Kan)HoxUYH (E). KanR is the kanamycin resistance gene. The five genes coding for the hydrogenaseare hoxEFUYH.
Native E. coli TG1 produces hydrogen via the FHL system during mixed-acid fermentations [19], and TG1 expressing the cyanobacterial hydrogenase produced 3-fold more hydrogen than cells which lacked the hoxEFUYH locus (22 ± 1 vs. 7 ± 1 μmol/mg protein) after 6 h in complex medium. More hydrogen was measured at least 23 times for cells expressing hoxEFUYH relative to the negative control that lacks the cyanobacterial locus so the effect is reproducible. The negative controls of both autoclaved TG1/pBS(Kan)Synhox and autoclaved E. aerogenes HU-101 did not produce hydrogen. Note that co-elution with pure hydrogen confirmed that hydrogen was produced by the E. coli cells and also our retention time for hydrogen was consistent with literature values (22.8 vs. 22 sec) [20]. In addition, the recombinant E. coli expressing the cyanobacterial hydrogenase produced about 2-fold more hydrogen after 6 h in complex medium than the positive control E. aerogenes HU-101 (11 ± 0.3 μmol/mg protein), a well-studied producer of hydrogen [21,22]. Furthermore, hydrogen gas bubbles were clearly more visible in the recombinant strain compared to the host which lacked the cyanobacteria locus and also visible in the positive control but were not visible with autoclaved samples (Figure 2). Therefore, the recombinant E. coli strain produces significantly higher quantities of hydrogen gas.
Figure 2.

Hydrogen production with E. coli TG1/pBS(Kan)Synhox and E. aerogenes HU-101 (hydrogen bubbles shown). Representative samples shown from hydrogen assay experiments in complex medium with glucose (repeated 3 times).
Optimization of medium and time course of hydrogen production
The cyanobacterial genes were fused to a lac promoter in pBS(Kan); therefore, the expression of hoxEFUYH will be suppressed by catabolite repression if glucose is included in complex medium. To search for an optimal medium for producing hydrogen, glucose was replaced with fructose, galactose, maltose, lactose, glycerin, citrate, and succinate in complex medium. Hydrogen production in complex medium with fructose, galactose or maltose was 20% more than that with glucose in TG1/pBS(Kan)Synhox whereas the other carbon sources did not improve hydrogen production.
To investigate hydrogen production with TG1/pBS(Kan)Synhox in more detail, a hydrogen time course experiment was performed. Hydrogen produced by E. coli TG1 cells with or without HoxEFUYH was maximum within 1.5 h (Figure 3). Furthermore, from 1.5 h to 6 h, hydrogen produced in the absence of HoxEFUYH decreased 2.1 ± 0.4-fold more rapidly than that from cells with HoxEFUYH (Figure 3); hence, the hydrogen formed in the presence of HoxEFUYH was more stable. This suggested that hydrogen uptake is inhibited by expression of active HoxEFUYH. Note that after 18 h, the hydrogen yield from cells expressing hoxEFUYH was over 41 times more than that of the wild-type strain (Figure 3).
Figure 3.
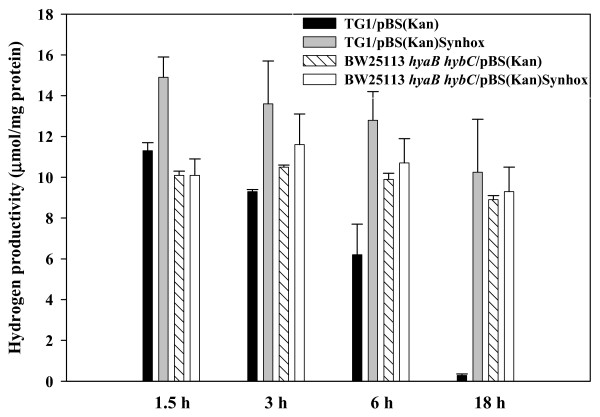
Time course of hydrogen productivity (via gas chromatography) incomplex medium for TG1/pBS(Kan), TG1/pBS(Kan)Synhox, BW25113 hyaB hybC/pBS(Kan), and BW25113 hyaB hybC/pBS(Kan)Synhox.
Enhanced hydrogen production depends on native hydrogenase 3
To ascertain if elements of the native E. coli host FHL system impact the cyanobacteria hydrogenase system, hydrogenase activities of the cyanobacteria and the E. coli FHL were assayed in a series of mutants that lack either E. coli hydrogenase 3 of the FHL (HD705) or lack the maturation machinery required for assembling hydrogenases in E. coli (SE1497 and PMD23). We also examined a mutant that lacked the transcriptional activator FhlA, and thus would not express genes encoding the FHL complex (SE1174) as well as assayed a mutant that cannot form selenoproteins (WL400) and thus is unable to produce active formate dehydrogenase H (formate dehydrogenase H is sole electron donor for hydrogenase 3). Since the parent strain MC4100 with pBS(Kan)Synhox produced hydrogen but the FHL mutants (HD705, SE1174, SE1497 PMD23, and WL400) harboring pBS(Kan)Synhox did not produce hydrogen gas, active expression of the cyanobacteria hydrogenase system relies on both an active E. coli hydrogenase 3 as well as the maturation proteins of the host and cannot simply be due to HoxEFUYH acting as an electron donor to HYD3.
Role of the cyanobacterial proteins HoxEFUYH
To observe the expression of the recombinant enzymes, SDS-PAGE was performed. As shown in Figure 4, HoxU (26 kDa) from the cyanobacterium was clearly expressed in TG1/pBS(Kan)Synhox that produced hydrogen gas in both complex medium and LB medium while the other proteins (HoxE, 18 kDa; HoxF, 62 kDa; HoxY, 23 kDa; HoxH, 51 kDa) were not observed. As expected, HoxU was not observed in TG1/pBS(Kan) (negative control). Also, the expression of HoxU was greater in complex medium relative to LB medium and complex medium produced more hydrogen. Hence, HoxU of Synechocystis sp. PCC 6803 may play a major role in the elevated hydrogen production in E. coli.
Figure 4.
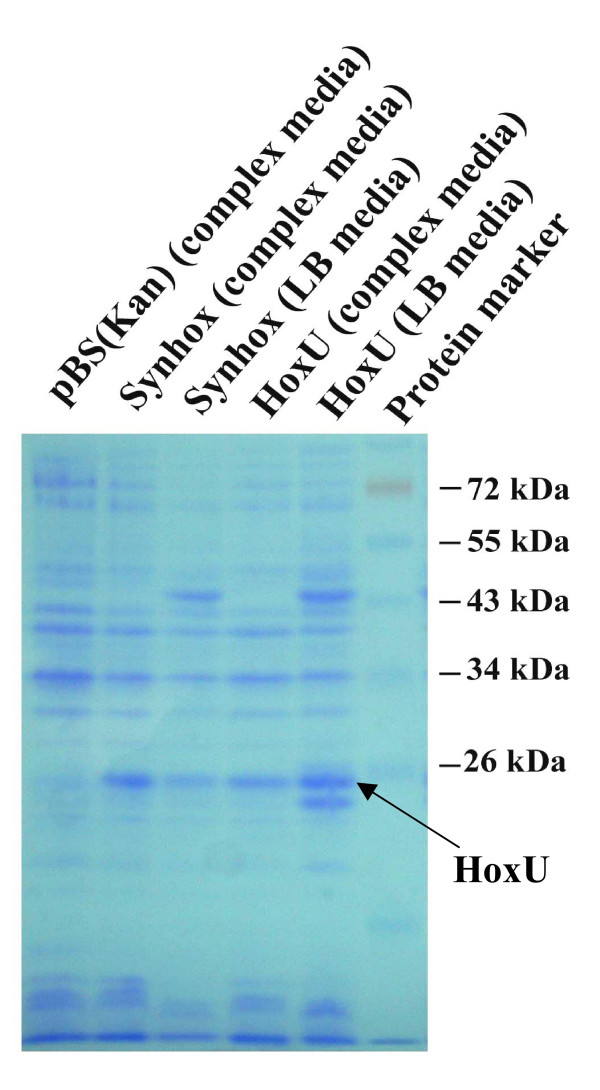
SDS-PAGE of HoxEFUYH and HoxU expression from E. coli TG1/pBS(Kan)Synhox and TG1/pBS(Kan)HoxU grown anaerobically in complex media and LB media. Arrow indicates the expression of HoxU. pBS(Kan) is TG1/pBS(Kan) (negative control), and Synhox is TG1/pBS(Kan)Synhox.
To discern if only HoxU is required for producing more hydrogen, hoxU was cloned under the lac promoter (designated as pBS(Kan)HoxU, Figure 1B), and hydrogen production in TG1 harboring pBS(Kan)HoxU was examined. TG1/pBS(Kan)HoxU produced more hydrogen than TG1/pBS(Kan) but only 44.6 ± 7.3% of that of TG1/pBS(Kan)Synhox. These results indicate that the additional hydrogen produced by expressing HoxEFUYH is not solely the result of active HoxU; hence, other active HoxEFUYH proteins are required for producing hydrogen.
To determine the importance of the other cyanobacterial enzymes for hydrogen production, pBS(Kan)HoxEFU (Figure 1C), pBS(Kan)HoxFU (Figure 1D), pBS(Kan)HoxFUY (Figure 1E), and pBS(Kan)HoxUYH (Figure 1F) were also constructed and hydrogen production in TG1 with these plasmids was tested. Hydrogen production in TG1/pBS(Kan)HoxEFU and TG1/pBS(Kan)HoxFU showed 48 ± 7% and 57 ± 2% of the hydrogen produced by TG1/pBS(Kan)Synhox; hence, these strains had about half the hydrogen produced by cloning either HoxEFUYH or the almost same value as that by expressing only HoxU and they confirm the importance of HoxU. In contrast, TG1/pBS(Kan)HoxFUY and TG1/pBS(Kan)HoxUYH produced 81 ± 22% and 113 ± 31% of the hydrogen produced by TG1/pBS(Kan)Synhox, respectively; therefore, hydrogen production in TG1/pBS(Kan)HoxUYH was somewhat better than that in TG1/pBS(Kan)Synhox indicating the importance of proteins HoxU, HoxY, and HoxH.
Mechanism of enhanced hydrogen production is via inhibition of hydrogen uptake
Since the hydrogen time course experiment (Figure 3) showed the hydrogen produced by cells expressing hoxEFUYH was more stable than hydrogen from cells which lacked this locus and given that the greater hydrogen production seen upon cloning the cyanobacterial locus was dependent on both the native hydrogenase maturation proteins and active hydrogenase 3, we theorized that HoxEFUYH may be influencing hydrogen uptake. Recall that hydrogenase 1 and 2 are involved in hydrogen uptake only [10,11]. Hence, hydrogen uptake activity was measured three ways to investigate this hypothesis. As shown in Table 1, E. coli TG1 expressing HoxEFUYH had 3.3 times less hydrogen uptake compared to the negative control TG1/pBS(Kan) and E. coli MC4100 had similar results. These hydrogen uptake results were corroborated by using a plate assay for reversible hydrogenase activity which showed 10-fold less hydrogen uptake upon expressing HoxEFUYH and by a GC-based hydrogen up-take assay which indicated that H2 uptake activity in TG1/pBS(Kan)Synhox is 2.1 ± 0.2-fold less than TG1/pBS(Kan) over 0 to 6 h. Hence, the active cyanobacterial enzymes (HoxEFUYH) inhibit hydrogen uptake consistently in E. coli.
Table 1.
Hydrogen uptake activity with various E. coli BW25113 mutants in complex medium as determined by the oxidized methylviologen-based H2 uptake assay after 5 min
| Strain | Description | Hydrogen uptake | |
| nmol/min/mg proteina | relative | ||
| E. coli BW25113/pBS(Kan) | wild type | 94 ± 12 | 3.3 |
| E. coli BW25113/pBS(Kan)Synhox | wild type + HoxEFUYH | 29 ± 7 | 1 |
| E. coli BW25113 hyaA/pBS(Kan)Synhox | ΔhyaA (defective hydrogenase 1) + HoxEFUYH | 59 ± 21 | 2.0 |
| E. coli BW25113 hyaB/pBS(Kan)Synhox | ΔhyaB (defective hydrogenase 1) + HoxEFUYH | 59 ± 6 | 2.0 |
| E. coli BW25113 hybB/pBS(Kan)Synhox | ΔhybB (defective hydrogenase 2) + HoxEFUYH | 73 ± 13 | 2.5 |
| E. coli BW25113 hybC/pBS(Kan)Synhox | ΔhybC (defective hydrogenase 2) + HoxEFUYH | 80 ± 11 | 2.8 |
| E. coli BW25113 hycE/pBS(Kan) | ΔhycE (defective hydrogenase 3) | 25 ± 0.8 | 0.9 |
| E. coli BW25113 hycE/pBS(Kan)Synhox | ΔhycE (defective hydrogenase 3) + HoxEFUYH | 7 ± 2 | 0.3 |
| E. coli BW25113 hycG/pBS(Kan)Synhox | ΔhycG (defective hydrogenase 3) + HoxEFUYH | 10 ± 2 | 0.4 |
| E. coli BW25113 hyaB hybC/pBS(Kan) | ΔhyaB and ΔhybC (defective hydrogenase 1 and 2) | 21 ± 1 | 0.7 |
| E. coli BW25113 hyaB hybC/pBS(Kan)Synhox | ΔhyaB and ΔhybC (defective hydrogenase 1 and 2) + HoxEFUYH | 21.6 ± 0.3 | 0.8 |
| E. coli BW25113 hyaB hybC hycE/pBS(Kan) | ΔhyaB, ΔhybC, and ΔhycE (defective hydrogenase 1, 2, and 3) | 1.8 ± 0.4 | 0.06 |
| E. coli MC4100/pBS(Kan) | wild type | 35 ± 15 | 2.3 |
| E. coli MC4100/pBS(Kan)Synhox | wild type + HoxEFUYH | 15 ± 6 | 1 |
| E. coli TG1/pBS(Kan) | wild type | 16 ± 7 | 3.3 |
| E. coli TG1/pBS(Kan)Synhox | wild type + HoxEFUYH | 5 ± 3 | 1 |
| E. coli HD705/pBS(Kan) | defective hydrogenase 3 | 8 ± 1 | 5.4 |
| E. coli HD705/pBS(Kan)Synhox | defective hydrogenase 3 + HoxEFUYH | 2 ± 2 | 1 |
Standard deviations shown from one representative experiment with 2 replicates. a IPTG was added for hydrogen uptake (1 mM) assays activity for 6 h in complex medium.
To determine by which of the three native E. coli hydrogenases that hydrogen uptake was affected by HoxEFUYH, a series of isogenic mutants of E. coli BW25113 was used (Table 1). Using two mutants for hydrogenase 1 (hyaA and hyaB), hydrogen uptake was measured and found to increase consistently 2-fold upon removing hydrogenase 1 in the presence of HoxEFUYH; hence, HoxEFUYH decreases hydrogen uptake via hydrogenase 1. Similarly, using two mutants for hydrogenase 2 (hybB and hybC), hydrogen uptake increased consistently 2.5- to 2.8-fold (Table 1) upon removing hydrogenase 2 in the presence of HoxEFUYH; hence, HoxEFUYH decreases hydrogen uptake via hydrogenase 2. In contrast, upon adding HoxEFUYH, isogenic mutations that eliminate hydrogenase 3 activity (hycE and hycG) (Table 1) decreased hydrogen uptake (rather than increasing it). In addition, hydrogen uptake in the hyaB hybC double mutant that eliminated hydrogenase 1 and 2 activity was identical to that of the wild-type strains expressing hoxEFUYH and the expression of hoxEFUYH had no effect in the double mutant (Table 1). Corroborating these results, hydrogen production by BW25113 hyaB hybC/pBS(Kan)Synhox was the same as BW25113 hyaB hybC/pBS(Kan) (Figure 3). On the other hand, a triple mutant (hyaB hybC hycE; defective in hydrogenase 1, 2, and 3) with pBS(Kan) or pBS(Kan)Synhox did not produce hydrogen (data not shown), indicating that hydrogenase 3 is essential for producing hydrogen. Therefore, HoxEFUYH works through hydrogenase 1 and hydrogenase 2 rather than through hydrogenase 3, and the decrease in hydrogen uptake seen with the hydrogenase 3 mutants is due to HoxEFUYH inhibition of the remaining active hydrogenase 1 and 2 enzymes.
DNA microarrays
To investigate whether cloning of the cyanobacterial hoxEFUYH merely increased hydrogen production by up-regulating the native E. coli hydrogenase system, we examined differential gene expression upon expression of hoxEFUYH from pBS(Kan)Synhox. The microarrays showed that gene expression for the hya, hyb, hyc, and hyp operons was not altered between TG1/pBS(Kan)Synhox and TG1/pBS(Kan); hence, functional HoxEFUYH is necessary for enhanced hydrogen production in E. coli (Additional file 1). Surprisingly, the differential gene expression indicated that primarily biofilm-related genes are regulated by expressing HoxEFUYH as shown in Table 2. To investigate the effect of expression of HoxEFUYH on biofilm formation, a 96-well polystyrene plate assay was performed. E. coli TG1 expressing HoxEFUYH produced 1.7 ± 0.2 times more biofilm than TG1/pBS(Kan) under anaerobic conditions in LB with 0.2% glucose after 48 h at 37°C.
Table 2.
Differentially-expressed, biofilm-related genes upon expression of HoxEFUYH in E. coli TG1 in complex medium at 37°C after 6 h. References indicate the relevant biofilm publication
| Gene name | b # | Fold change | Gene function | Reference |
| ydfX | b1568 | 34.3 | hypothetical protein | [31] |
| tnaL | b3707 | 24.3 | tryptophanase leader peptide | [31, 34] |
| tnaA | b3708 | 13.9 | tryptophanase | [31] |
| sdhC | b0721 | 10.6 | succinate dehydrogenase membrane protein | [31, 32] |
| sdhD | b0722 | 9.2 | succinate dehydrogenase membrane protein | [32, 34] |
| treB | b4240 | 9.2 | subunit of EIITre | [34] |
| treC | b4239 | 6.1 | trehalose-6-phosphate hydrolase | [31-33] |
| pspA | b1304 | 9.2 | regulatory protein for the phage shock protein operon | [32] |
| pspB | b1305 | 8.0 | stimulates PspC-mediated transcriptional activation of the psp operon | [32] |
| yhaN | b3109 | 8.6 | hypothetical ORF | [31] |
| tdcC | b3116 | 8.6 | threonine STP transporter | [32] |
| tdcD | b3115 | 8.0 | propionate kinase/acetate kinase C | [32] |
| gltA | b0720 | 8.0 | subunit of citrate synthase | [32] |
| yciD | b1256 | 8.0 | subunit of Colicin S4 Transport System | [31] |
| gatA | b2094 | 6.1 | subunit of EIIGat | [31, 34] |
| gatB | b2093 | 7.5 | subunit of EIIGat | [31] |
| ibpB | b3686 | 6.5 | small heat shock protein | [33, 34] |
| nmpC | b0553 | 6.1 | outer membrane porin protein | [31, 34] |
| Mdh | b3236 | 5.7 | subunit of malate dehydrogenase | [32] |
| sucC | b0728 | 5.3 | succinyl-CoA synthetase, βsubunit | [32] |
| fumB | b4122 | - 14.9 | subunit of fumarase B | [31-33] |
| yigE | b3814 | - 11.3 | conserved protein | [31, 32] |
| ydeO | b1499 | - 10.6 | transcriptional activator | [31, 32] |
| yjjJ | b4385 | - 9.2 | conserved protein | [31, 32] |
| yihP | b3877 | - 8.0 | GPH transporter | [32, 33] |
| yjeN | b4157 | - 8.0 | conserved protein | [32] |
| yfjT | b2637 | - 7.5 | CP4-57 prophage predicted protein | [32] |
| yjhS | b4309 | - 6.5 | conserved protein | [32] |
| yghG | b2971 | - 5.3 | unknown function | [32, 33] |
| yicE | b3654 | - 5.3 | NCS2 transporter | [31, 32] |
| tort | b0994 | - 3.0 | unknown inducer | [32-34] |
Discussion
To date, no cyanobacterial hydrogenase protein has been actively expressed in E. coli. Functional expression here of the cyanobacterial hydrogenase components in E. coli allows for both mutagenesis for structure/function determinations as well as for enhanced hydrogen production via saturation mutagenesis and DNA shuffling [23]. Also, E. coli cells offer two advantages over normally photosynthetic microbes regarding protein evolution. First, the transformation efficiency of E. coli (greater than 109 transformants per microgram of plasmid) is at least three orders of magnitude greater than those of photosynthetic bacteria [24]. A rapid E. coli-based genetic selection method for hydrogenase activity would enable the sampling of thousands of different hydrogenase mutants in a single day. Second, hydrogen production by cyanobacteria requires light, and oxygen is produced through photosynthesis; oxygen production is undesirable for fuel cells; therefore, it is more desirable to clone the hydrogenase into E. coli rather than cyanobacteria since E. coli is a facultative anaerobe.
In cyanobacterium Synechocystis sp. PCC 6803, HoxFU are iron-sulfur proteins that bind NADH (diaphorase) [25]. Also, HoxU is thought to serve as the bridging unit in the link between respiration and the hydrogenase [26]. In addition, cyanobacteria Anacystis nidulans SAUG 1402-1 and Anacystis sp. PCC 7942 showed reduced hydrogen-evolution catalyzed by the bidirectional hydrogenase upon mutation of hoxU [27]; these indicate that HoxU is important for hydrogenase activity. Our SDS-PAGE results demonstrated that the HoxU protein (26 kDa) was clearly expressed in TG1/pBS(Kan)Synhox and that the expression of HoxU increased according to increasing hydrogen production. Since only HoxU was seen with SDS-PAGE, we surmised that it may have its own promoter. Corroborating this, between the stop codon of hoxF and the start codon of hoxU there is a gap of 705 bp that contains six putative promoters upstream of hoxU based on promoter prediction software [28]. The expression of HoxU is probably regulated independently by at least one of these promoters; this suggested that controlling expression of HoxU may provide significant insight for elevating hydrogen production, which proved correct since cloning only hoxU accounted for about half of the hydrogen produced by cloning hoxEFUYH. This shows the other cyanobacterial proteins (HoxEFYH), although not clearly observed with SDS-PAGE, are beneficial for producing hydrogen. Since expressing HoxUYH in E. coli TG1 yields nearly the same effect as expressing all of HoxEFUYH via TG1/pBS(Kan)Synhox, HoxUYH are clearly important for enhanced hydrogen production.
Hydrogen production was enhanced as much as 41-fold via production of the active cyanobacterial HoxEFUYH, and the hydrogen produced using TG1/pBS(Kan)Synhox was more stable in comparison to that of TG1/pBS(Kan); this indicated that TG1/pBS(Kan)Synhox has reduced hydrogen uptake activity. Since any mutation related to hydrogenase 3 eliminated the benefit of expressing the cyanobacterial hydrogenase, it is clear that hydrogenase 3 is necessary for the HoxEFUYH effect since only hydrogenase 3 produces hydrogen in E. coli whereas HoxEFUYH maintains this hydrogen that is produced by limiting hydrogen uptake by hydrogenase 1 and 2. In accordance with this interpretation, hydrogen uptake was found to be 5 to 10 times lower upon expression of HoxEFUYH. Also, the microarray analysis shows that native hydrogenase gene expression is not affected by enhanced hydrogen production with TG1/pBS(Kan)Synhox, so there are no transcriptional effects related to cloning hoxEFUYH. Taken together, these results show expression of HoxEFUYH increases hydrogen production by reducing hydrogen uptake of the native E. coli hydrogenases, but hydrogenase 3 is required to produce the hydrogen in the first place. Note that hydrogenase 1 and 2 have hydrogen uptake activity [10,11], and hydrogenase 3 has hydrogen production activity (hydrogenase 3 is the primary source of hydrogen gas production in E. coli [29] as hydrogenase 4 is inactive [30])
In contrast to the native hydrogenase gene expression, the DNA microarrays indicated the expression of many biofilm-related genes were altered upon expression of HoxEFUYH (Table 2), and this altered expression led to an increase in biofilm formation. Interestingly, our study of temporal gene expression in E. coli K-12 biofilms [31] shows that the genes related to hydrogenase 1 (hyaABCDE) and to hydrogenase 2 (hybBC) are transiently repressed, that the genes related to hydrogenase 3 (hycBF) and hydrogenase 4 (hyfBC) are up-regulated in the process of biofilm formation, and that some hya or hyf mutants produce 3- to 7-fold more biofilm. So the fact that expression of HoxEFUYH affects biofilm genes (Table 2) and that hydrogenase genes are routinely found in biofilm studies [31-34] suggest that biofilm formation is related to enhanced hydrogen production. Hence, it appears that biofilm formation may repress hydrogen uptake activity or induce hydrogen production, either of which would result in enhanced hydrogen production. In the future, we will need to ascertain whether biofilm formation is directly related to enhanced hydrogen production and how HoxEFUYH represses hydrogen uptake activity in E. coli.
To the best of our knowledge, on a protein basis, Citrobacter sp. Y19 (65 μmol/mg/h) [35], Rhodopseudomonas palustris JA1 (56 μmol/mg/h) [36], Rhodopseudomonas palustris P4 (41 μmol/mg/h) [37], and Klebsiella oxytoca HP1 (30 μmol/mg/h) [38] all have higher maximum hydrogen activity compared to our TG1/pBS(Kan)Synhox recombinant (6 μmol/mg/h); however, these organisms are more fastidious than E. coli. Therefore, E. coli holds promise for producing hydrogen as a more robust model host for heterologous expression of hydrogenases. Along these lines, Yoshida et al. [39] recently increased native hydrogenase expression in E. coli by inactivating the HycA FHL repressor and by overexpressing the FhlA transcriptional activator for the hyc and hyp operons with the result that 2.8-fold more hydrogen was generated (further increases, up to 250 μmol/mg/h, were obtained using a novel high-density reactor and formate addition). This approach suggests similar genetic changes (along with the aforementioned DNA shuffling) may be used to increase further hydrogen production using both the native and cyanobacterial hydrogenases.
Conclusion
E. coli TG1 cells with pBS(Kan)Synhox yielded 41 times more hydrogen after 18 h than those with empty vector pBS(Kan) due to active HoxEFUYH from Synechocystis sp. PCC 6803 (primarily through active HoxUYH). The mechanism for this enhanced hydrogen production is that hydrogen is formed first by hydrogenase 3 (so the HoxEFUYH effect relies on active hydrogenase 3 and its maturation proteins), then HoxEFUYH inhibits hydrogen uptake by E. coli native hydrogenase 1 and hydrogenase 2. In effect, a novel way to reduce reversible hydrogen formation has been discovered using a cyanobacterial locus.
Methods
Bacterial strains, growth, and total protein
Strains are shown in Table 3. E. coli cells containing pBS(Kan) and derivatives were initially streaked from -80°C glycerol stocks on Luria-Bertani (LB) agar plates [40] containing 100 μg/mL kanamycin and incubated at 37°C. After growth on LB agar plates, these strains were cultured from a fresh single colony in LB medium [40] or complex medium [22] supplemented with 100 μg/mL kanamycin at 37°C with shaking at 250 rpm (New Brunswick Scientific Co., Edison, NJ). Wild-type E. coli K-12 BW25113 was obtained from the Yale University CGSC Stock Center, and its isogenic deletion mutants (Keio collection) were obtained from the Genome Analysis Project in Japan [41]. Enterobacter aerogenes HU-101 [22] was obtained from National Institute of Technology and Evaluation Biological Resource Center, Japan (accession number 100048) and was used as a positive control for hydrogen production; this strain was grown in complex medium. To optimize the medium for producing hydrogen, fructose (20 g, J.T. Baker Chemical, Phillipsburg, NJ), galactose (20 g, Aldrich Chemical, Milwaukee, WI), maltose (10 g, Sigma Chemical, St. Louis, MO), lactose (10 g, Sigma Chemical), glycerol (40 g, Fisher Scientific, Fair Lawn, NJ), citrate (30 g, Fisher Scientific) or succinate (40 g, Fisher Scientific) were substituted for glucose (20 g, Fisher Scientific) in complex medium. Plasmids pBS(Kan) [42] and pBS(Kan)Synhox (below) were electroporated into the formate hydrogenlyase mutants (Table 3). Cell growth was measured using turbidity at 600 nm, and total protein concentrations for E. coli and E. aerogenes HU-101 were 0.22 mg/OD/mL or 0.34 mg/OD/mL, respectively (Protein assay kit, Sigma Diagnostics, St. Louis, MO).
Table 3.
Strains and plasmids used. KmR, CmR and ApR are kanamycin, chloramphenicol and ampicillin resistance, respectively.
| Strains and plasmids | Genotype | Source |
| Strains | ||
| E. coli TG1 | supE thi-1 Δ(lac-proAB) Δ(mcrB-hsdSM)5 (rK - mK -) [F' traD36 proAB lacIqZΔM15] | [51] |
| E. coli MC4100 | F- araD139 ΔlacU169 rpsL thi fla | [52] |
| E. coli HD705 | MC4100 ΔhycE; defective in large subunit of the hydrogenase 3 subunit | [14] |
| E. coli SE1174 | thi-1 leu-6 suc-10 bioA2? galT27 rpsL129 chlC3 λ- fhlA102::Tn10; defective transcription of the hyc operon and fdhF | [53] |
| E. coli SE1497 | cysC43 srl-300::Tn10 thr-1 leu-6 thi-1 proA2 galK2 ara-14 xyl-5 mtl-1 lacY1 his-4 argE3 rpsL31 tsx-33 Δ(srl-fhlA); large deletion in the 59 min region removes the hyc, hyp or fhlA genes | [54] |
| E. coli PMD23 | MC4100 ΔhypF ; defective in full maturation of hydrogenase | K. T. Shanmugam |
| E. coli WL400 | selD (CmR) | [55] |
| E. coli BW25113 | lacIqrrnBT14ΔlacZWJ16hsdR514 ΔaraBADAH33ΔrhaBADLD78 | Yale CGSG Stock Center |
| E. coli BW25113 ΔhyaA Δkan | E. coli JW0954 [41] Δkan; defective in small subunit of hydrogenase 1 | this study |
| E. coli BW25113 ΔhyaB Δkan | E. coli JW0955 [41]Δkan; defective in large subunit of hydrogenase 1 | this study |
| E. coli BW25113 ΔhybB Δkan | E. coli JW5494 [41] Δkan; defective in probable cytochrome Ni/Fe component of hydrogenase 2 | this study |
| E. coli BW25113 ΔhybC Δkan | E. coli JW2962 [41]Δkan; defective in probable large subunit of hydrogenase 2 | this study |
| E. coli BW25113 ΔhycE Δkan | E. coli JW2691 [41] Δkan; defective in large subunit of hydrogenase 3 | this study |
| E. coli BW25113 ΔhycG Δkan | E. coli JW2689 [41]Δkan; defective in subunit of hydrogenase 3 and formate hydrogenlyase complex | this study |
| E. coli MW1000 | BW25113 ΔhyaB ΔhybC Δkan; defective in large subunit of hydrogenase 1 and 2 | this study |
| E. coli MW1001 | BW25113 ΔhyaB ΔhybC ΔhycE Δkan; defective in large subunit of hydrogenases 1, 2, and 3 | this study |
| E. aerogenes HU-101 | Hydrogen-producing bacteria (positive control strain; wild type) | NBRC |
| Plasmids | ||
| pBS(Kan)Synhox | pBS(Kan) plac::hoxEFUYH; KmR, expresses hydrogenase genes derived from Synechocystis sp. PCC 6803 | this study |
| pBS(Kan)HoxU | pBS(Kan) plac::hoxU; KmR, expresses HoxU derived from Synechocystis sp. PCC 6803 | this study |
| pBS(Kan)HoxEFU | pBS(Kan) plac::hoxEFU; KmR, expresses HoxEFU derived from Synechocystis sp. PCC 6803 | this study |
| pBS(Kan)HoxFU | pBS(Kan) plac::hoxFU; KmR, expresses HoxFU derived from Synechocystis sp. PCC 6803 | this study |
| pBS(Kan)HoxFUY | pBS(Kan) plac::hoxFUY; KmR, expresses HoxFUY derived from Synechocystis sp. PCC 6803 | this study |
| pBS(Kan)HoxUYH | pBS(Kan) plac::hoxUYH; KmR, expresses HoxUYH derived from Synechocystis sp. PCC 6803 | this study |
| pCP20 | ApR and CmR plasmid with temperature-sensitive replication and thermal induction of FLP synthesis | [43] |
Eliminating kanamycin resistance and P1 transduction
Plasmid pCP20 [43] was used as described previously [44] to eliminate the kanamycin resistance gene (kanR) from the isogenic BW25113 mutants (Keio strains) defective in hydrogenase 1, hydrogenase 2, and hydrogenase 3 so that pBS(Kan)Synhox could be added and so that a double and triple mutant could be constructed (Table 3). P1 transduction [45] and pCP20 were used to create E. coli MW1000 (hyaB hybC Δkan) from BW25113 hybC Δkan by transferring hyaB kanR via P1 transduction and using pCP20 to eliminate the kanamycin resistance marker. Similarly, MW1001 (hyaB hybC hycE Δkan) was created from BW25113 hyaB hybC Δkan by transferring hycE kanR and then eliminating the kanamycin resistance marker.
Constructing pBS(Kan)Synhox
Synechocystis sp. PCC 6803 genomic DNA was obtained using an UltraClean Microbial DNA Isolation Kit (Mo Bio Laboratories, Solana Beach, CA). The 6500 bp chromosomal DNA fragment encoding hoxEFUYH was amplified using Taq and Pfu polymerases mixture (1:1) using primers SynHoxEcoR1 Front [5'-CCAATCATGAATTCGCTGTATTGCTCCTTTTTGAGG-3'] and SynHoxXbaI Rear [5'-GACATTGAGTTCTTCTAGATATGCCTCGGTG-3'] with 30 cycles and 55°C annealing. The PCR product was cloned into the multiple cloning site in pBS(Kan) [42] after double digestion with XbaI and EcoRI to create pBS(Kan)Synhox (Figure 1A). Plasmid DNA was isolated using a Midi or Mini Kit (Qiagen, Inc., Chatsworth, CA), and polymerase chain reaction (PCR) products were purified with a Wizard® PCR Preps DNA Purification System (Promega Corporation, Madison, WI). The correct plasmid was verified by digesting the plasmid with the restriction enzymes EcoRI/XbaI, AvrII, MfeI/SnaBI, and PflMI. In addition, the beginning of hoxE and the end of hoxH of the cloned loci were sequenced [23] to show the presence of the hoxEFUYH locus using primers 5'-GACCATGATTACGCCAAGCGCGC-3' and 5'-GGGCGAATTGGAGCTCC-3', respectively.
Constructing pBS(Kan)HoxU, pBS(Kan)HoxEFU, pBS(Kan)HoxFU, pBS(Kan)HoxFUY and pBS(Kan)UYH
The 803 bp, 3804 bp, 3113 bp, 3705 bp, and 3811 bp DNA fragments for hoxU, hoxEFU, hoxFU, hoxFUY, and hoxUYH were amplified from pBS(Kan)Synhox using Pfu polymerase with primers for HoxU (HoxUKpnI Front 5'-ACAATTTAGGTACCTCATTAACAAAGGAGTTTTTGGCCAATGTC-3' and HoxUEcoRI Rear 5'-ATGATTAAGAATTCAGAAATGATGTTAAAAGTTC-3'), for HoxEFU (HoxEFU Front 5'-ACAGCTATGACCATGATTACGCC-3' and HoxEFUXbaI Rear 5'-TAGCCATGTCTAGAAGTTTAGAAATGATGTTAAAAG-3'), for HoxFU (HoxFUKpnI Front 5'-TCTAGTTGGGTACCTGATTAATTGTTAAGGAGGTTAAACCCCATGGAC-3' and HoxFUEcoRI Rear 5'-ATGATTAAGAATTCAGAAATGATGTTAAAAGTTC-3'), for HoxFUY (HoxFUYKpnI Front 5'-TCTAGTTGGGTACCTGATTAATTGTTAAGGAGGTTAAACCCCATGGAC-3' and HoxFUYXbaI Rear [5'-ATCTCCTGTCTAGATATTTTGCAAACTGTTTAG-3'), and for HoxUYH (HoxUYHKpnI Front 5'-ACAATTTAGGTACCTCATTAACAAAGGAGTTTTTGGCCAATGTC-3'] and HoxUYH Rear [5'-AAGGCGAT TAAGTTGGGTAACGC-3') with 30 cycles and 54°C annealing. The PCR products were cloned into the multiple cloning site in pBS(Kan) [42] after double digestion with KpnI and EcoRI to create pBS(Kan)HoxU (Figure 1B), with EcoRI and XbaI to create pBS(Kan)HoxEFU (Figure 1C), with KpnI and EcoRI to create pBS(Kan)HoxFU (Figure 1D), with KpnI and XbaI to create pBS(Kan)HoxFUY (Figure 1E), and with KpnI and XbaI to create pBS(Kan)HoxUYH (Figure 1F). The correct plasmids were verified by digesting the plasmid with the restriction enzymes KpnI/BamHI, AvaI, BanI, DraI, PvuI and PvuII for pBS(Kan)HoxU, KpnI/XbaI, BspHI, NcoI, XhoI, PvuII and ApaI for pBS(Kan)HoxEFU, KpnI/SacI, BspHI, PstI, PvuII, NcoI and ApaI for pBS(Kan)HoxFU, KpnI/SacI, BspHI, PvuII, NcoI and ApaI for pBS(Kan)HoxFUY, and KpnI/SacI, BspHI, AvaI, BclI and PvuII for pBS(Kan)HoxUYH.
Hydrogen assay and SDS-PAGE
Overnight, aerobic cultures (25 mL) were used to inoculate 75 mL of the complex medium (with 5 mM IPTG for the E. coli strains) in 250 mL shake flasks, and these cultures were sparged for 5 min with nitrogen, sealed, and incubated anaerobically at 37°C for 6 h to induce expression of the cyanobacterial hydrogenase system. After 6 h the cultures were poured anaerobically into a 250 mL centrifuge tubes in a glove box, and centrifuged (7350 × g) for 10 min at 4°C. The supernatant was decanted in the glove box, and 25 mL of complex medium (including 5 mM IPTG for E. coli cells) was added. Sealed crimp-top vials (27 mL) were sparged for 5 min with nitrogen, and 20 mL of the cell suspension was added to the bottles which were incubated at 37°C with shaking for 1.5 to 24 h. The amount of hydrogen generated in the head space of the recombinant system was measured using a 50 μL of aliquot by gas chromatography (GC) using a 6890 N gas chromatograph (Agilent Technologies, Glastonbury, CT) equipped with a 80–100 mesh Porapak Q column (Suppelco, Bellefonte, PA) and a thermal conductivity detector. The injector and detector were maintained at 100°C and 200°C respectively. The nitrogen carrier gas flow rate was maintained at 20 mL/min. The column temperature was 70°C. Under these conditions, the retention time for hydrogen was 0.38 min and the sensitivity is about 0.1 μmol. Retention times were determined by comparisons to neat standards as well as by co-elution with standards. Hydrogen productivity was calculated as μmol H2/mg total protein. Expression of recombinant proteins from samples actively expressing hydrogen was analyzed with standard Laemmli discontinuous SDS-PAGE (12%) [40].
Hydrogen uptake assays
Hydrogen uptake activities were measured as the increase in absorbance as oxidized methylviologen (MV) (ε604 = 13.9 mM-1 cm-1[21]) is reduced [MV+2 + 1/2H2→ MV+1 + H+] as reported previously [46] except whole cells were used rather than lysed cells. Cells were prepared as for the hydrogen assay except 1 mM IPTG was added to induce expression of the cyanobacterial hydrogenase system for 6 h, then the cell pellets from 1.5 mL were resuspended with 1 mL Tris buffer (50 mM, pH 8.0) in the anaerobic glove box. Oxidized MV (MV+2, colorless) solution (1 mL, 0.8 mM in 50 mM Tris buffer, pH 8) was sparged first with nitrogen gas for 10 min to remove oxygen to prevent residual oxygen from oxidizing any reduced MV (MV+1, purple) that is formed by the hydrogenases, was poured into cuvettes that were sealed with rubber stoppers, and was sparged with pure hydrogen gas for 10 min. Whole cell suspensions (0.5 mL) were mixed into the cuvettes and the change in absorbance during 5 min was monitored using a spectrophotometer (Varian, Walnut Creek, CA). Two independent cultures were used.
To corroborate the MV uptake assay, a filter paper assay for reversible hydrogenase activity was used based on a method described previously [47]. Filter paper (Whatman 541; Whatman plc, 27 Great West Road, UK) was immersed in MV solution (18 mM in 6 mM Tris buffer, pH 7.5), was air-dried with a hair dryer, and was firmly pressed on agar plates containing equal-size of colonies. The filter paper was then incubated at room temperature in a moist atmosphere of pure hydrogen in a Gas-Pak anaerobic chamber. The cells and adjacent white filter turned from white to blue-purple in approximately 10 min.
For the GC-based hydrogen uptake assay, cells were prepared as for the hydrogen assay except 1 mM IPTG was added to induce expression of the HoxEFUYH enzymes and after 6 hours, the cell pellets from 100 mL were resuspended with 25 mL phosphate buffer (100 mM, pH 7.5) including 1 mM IPTG in the anaerobic glove box. The cell suspension (20 mL) was added to sealed crimp-top vials (27 mL). The bottles were sparged with hydrogen for 10 min, were incubated at 37°C with shaking for 3 to 6 h, and the amount of hydrogen in the head space was measured as described above.
Microarray analysis
To isolate RNA from hydrogen producing cells, TG1/pBS(Kan)Synhox and TG1/pBS(Kan) were cultured in complex medium with fructose as for the hydrogenase assay. RNA was isolated as described previously [48] with the RNeasy kit (Qiagen, Inc.). To inhibit RNase and ensure high-quality RNA, β-mercaptoethanol, which acts as a reducing agent to irreversibly denature RNase, and guanidinium isothiocyanate contained in the RLT buffer (RNA Lysis Tissue, RNeasy mini kit: Qiagen, Inc.), which is a strong but temporary RNase-denaturing agent, were utilized. The E. coli GeneChip antisense genome array was used (part no. 900381, Affymetrix, Inc., Central Expressway, Santa Clara, CA), and contains probe sets for all 4,290 open reading frames, rRNA, tRNA, and 1,350 intergenic regions. Analysis of the microarray data was as previously described [32], and the data have been deposited in the NCBI Gene Expression Omnibus [49] and are accessible through accession numbers GSM129630 and GSM129631.
Ninety-six-well biofilm assay
Biofilm formation was quantified in 96-well polystyrene plates as reported previously [50]. Biofilm formation of E. coli TG1 with pBS(Kan)Synhox and pBS(Kan) was measured in LB supplemented with 0.2% glucose under anaerobic conditions using a Gas-Pak system. Thirty replicate wells were averaged to obtain each data point. Three independent cultures were used.
Authors' contributions
TM performed all the experiments except for constructing pBS(Kan)Synhox which was made by GV. WTS suggested some experiments with hydrogenase mutants, and TKW contributed to experimental design, authored some of the manuscript, and managed the project. All authors read and approved the final manuscript.
Supplementary Material
Microarray data for induced and repressed genes upon expressing the cyanobacterial locus. The data indicate differential gene expression in Escherichia coli TG1 cells upon expressing the cyanobacterial hydrogenase locus hoxEFUYH derived from Synechocystis sp. PCC6803.
Acknowledgments
Acknowledgements
This research was supported by DARPA (HR0011-06-1-0001).
Contributor Information
Toshinari Maeda, Email: toshinari.maeda@chemail.tamu.edu.
Gönül Vardar, Email: gonul@hawaii.edu.
William T Self, Email: wself@mail.ucf.edu.
Thomas K Wood, Email: thomas.wood@chemail.tamu.edu.
References
- Tamagnini P, Axelsson R, Lindberg P, Oxelfelt F, Wünschiers R, Lindblad P. Hydrogenases and hydrogen metabolism of cyanobacteria. Microbiol Mol Biol Rev. 2002;66:1–20. doi: 10.1128/MMBR.66.1.1-20.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf T, De Lacey AL, Friedrich B. Functional analysis by site-directed mutagenesis of the NAD+-reducing hydrogenase from Ralstonia eutropha. J Bacteriol. 2002;184:6280–6288. doi: 10.1128/JB.184.22.6280-6288.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz O, Boison G, Salzmann H, Bothe H, Schütz K, Wang SH, Happe T. HoxE-a subunit specific for the pentameric bidirectional hydrogenase complex (HoxEFUYH) of cyanobacteria. Biochim Biophys Acta. 2002;1554:66–74. doi: 10.1016/S0005-2728(02)00214-1. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, Nakamura Y, Miyajima N, Hirosawa M, Sugiura M, Sasamoto S, Kimura T, Hosouchi T, Matsuno A, Muraki A, Nakazaki N, Naruo K, Okumura S, Shimpo S, Takeuchi C, Wada T, Watanabe A, Yamada M, Yasuda M, Tabata S. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 1996;3:109–136. doi: 10.1093/dnares/3.3.109. [DOI] [PubMed] [Google Scholar]
- Gutekunst K, Phunpruch S, Schwarz C, Schuchardt S, Schulz-Friedrich R, Appel J. LexA regulates the bidirectional hydrogenase in the cyanobacterium Synechocystis sp. PCC 6803 as a transcription activator. Mol Microbiol. 2005;58:810–823. doi: 10.1111/j.1365-2958.2005.04867.x. [DOI] [PubMed] [Google Scholar]
- Oliveira P, Lindblad P. LexA, a transcription regulator binding in the promoter region of the bidirectional hydrogenase in the cyanobacterium Synechocystis sp. PCC 6803. FEMS Microbiol Lett. 2005;251:59–66. doi: 10.1016/j.femsle.2005.07.024. [DOI] [PubMed] [Google Scholar]
- Hansel A, Lindblad P. Toward optimization of cyanobacteria as biotechnologically relevant producers of molecular hydrogen, a clean and renewable energy source. Appl Microbiol Biotechnol. 1998;50:153–160. doi: 10.1007/s002530051270. [DOI] [Google Scholar]
- Adams MW, Mortenson LE, Chen JS. Hydrogenase. Biochim Biophys Acta. 1980;594:105–176. doi: 10.1016/0304-4173(80)90007-5. [DOI] [PubMed] [Google Scholar]
- Zhang JW, Butland G, Greenblatt JF, Emili A, Zamble DB. A role for SlyD in the Escherichia coli hydrogenase biosynthetic pathway. J Biol Chem. 2005;280:4360–4366. doi: 10.1074/jbc.M411799200. [DOI] [PubMed] [Google Scholar]
- Ballantine SP, Boxer DH. Isolation and characterisation of a soluble active fragment of hydrogenase isoenzyme 2 from the membranes of anaerobically grown Escherichia coli. Eur J Biochem. 1986;156:277–284. doi: 10.1111/j.1432-1033.1986.tb09578.x. [DOI] [PubMed] [Google Scholar]
- King PW, Przybyla AE. Response of hya expression to external pH in Escherichia coli. J Bacteriol. 1999;181:5250–5256. doi: 10.1128/jb.181.17.5250-5256.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawers RG, Boxer DH. Purification and properties of membrane-bound hydrogenase isoenzyme 1 from anaerobically grown Escherichia coli K12. Eur J Biochem. 1986;156:265–275. doi: 10.1111/j.1432-1033.1986.tb09577.x. [DOI] [PubMed] [Google Scholar]
- Sawers RG, Ballantine SP, Boxer DH. Differential expression of hydrogenase isoenzymes in Escherichia coli K-12: evidence for a third isoenzyme. J Bacteriol. 1985;164:1324–1331. doi: 10.1128/jb.164.3.1324-1331.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter M, Böhm R, Böck A. Mutational analysis of the operon (hyc) determining hydrogenase 3 formation in Escherichia coli. Mol Microbiol. 1992;6:1523–1532. doi: 10.1111/j.1365-2958.1992.tb00873.x. [DOI] [PubMed] [Google Scholar]
- Drapal N, Böck A. Interaction of the hydrogenase accessory protein HypC with HycE, the large subunit of Escherichia coli hydrogenase 3 during enzyme maturation. Biochemistry. 1998;37:2941–2948. doi: 10.1021/bi9720078. [DOI] [PubMed] [Google Scholar]
- Morozov SV, Voronin OG, Karyakina E, Zorin NA, Cosnier S, Karyakin AA. Tolerance to oxygen of hydrogen enzyme electrodes. Electrochem Commun. 2006;8:851–854. doi: 10.1016/j.elecom.2006.03.007. [DOI] [Google Scholar]
- Das D, Veziroglu TN. Hydrogen production by biological processes: a survey of literature. Int J Hydrogen Ener. 2001;26:13–28. doi: 10.1016/S0360-3199(00)00058-6. [DOI] [Google Scholar]
- Dicks AL. Hydrogen generation from natural gas for the fuel cell systems of tomorrow. J Power Sour. 1996;61:113–124. doi: 10.1016/S0378-7753(96)02347-6. [DOI] [Google Scholar]
- Penfold DW, Forster CF, Macaskie LE. Increased hydrogen production by Escherichia coli strain HD701 in comparison with the wild-type parent strain MC4100. Enzyme Microb Technol. 2003;33:185–189. doi: 10.1016/S0141-0229(03)00115-7. [DOI] [Google Scholar]
- Markov SA, Thomas AD, Bazin MJ, Hall DO. Photoproduction of hydrogen by cyanobacteria under partial vacuum in batch culture or in a photobioreactor. Int J Hydrogen Energy. 1997;22:521–524. doi: 10.1016/S0360-3199(96)00134-6. [DOI] [Google Scholar]
- Nakashimada Y, Rachman MA, Kakizono T, Nisho N. Hydrogen production of Enterobacter aerogenes altered by extracellular and intracellular redox states. Int J Hydrogen Energy. 2002;27:1399–1405. doi: 10.1016/S0360-3199(02)00128-3. [DOI] [Google Scholar]
- Rachman MA, Furutani Y, Nakashimada Y, Kakizono T, Nishio N. Enhanced hydrogen production in altered mixed acid fermentation of glucose by Enterobacter aerogenes. J Ferment Bioeng. 1997;83:358–363. doi: 10.1016/S0922-338X(97)80142-0. [DOI] [Google Scholar]
- Fishman A, Tao Y, Rui L, Wood TK. Controlling the regiospecific oxidation of aromatics via active site engineering of toluene para-monooxygenase of Ralstonia pickettii PKO1. J Biol Chem. 2005;280:506–514. doi: 10.1074/jbc.M410320200. [DOI] [PubMed] [Google Scholar]
- Smith SA, Tabita FR. Positive and negative selection of mutant forms of prokaryotic (cyanobacterial) ribulose-1,5-bisphosphate carboxylase/oxygenase. J Mol Biol. 2003;331:557–569. doi: 10.1016/S0022-2836(03)00786-1. [DOI] [PubMed] [Google Scholar]
- Appel J, Schulz R. Sequence analysis of an operon of a NAD(P)-reducing nickel hydrogenase from the cyanobacterium Synechocystis sp. PCC 6803 gives additional evidence for direct coupling of the enzyme to NAD(P)H-dehydrogenase (complex I) Biochim Biophys Acta. 1996;1298:141–147. doi: 10.1016/s0167-4838(96)00176-8. [DOI] [PubMed] [Google Scholar]
- Schmitz O, Bothe H. The diaphorase subunit HoxU of the bidirectional hydrogenase as electron transferring protein in cyanobacterial respiration? Naturwissenschaften. 1996;83:525–527. doi: 10.1007/BF01141957. [DOI] [PubMed] [Google Scholar]
- Boison G, Schmitz O, Schmitz B, Bothe H. Unusual gene arrangement of the bidirectional hydrogenase and functional analysis of its diaphorase subunit HoxU in respiration of the unicellular cyanobacterium Anacystis nidulans. Curr Microbiol. 1998;36:253–258. doi: 10.1007/s002849900305. [DOI] [PubMed] [Google Scholar]
- Neural Network Promoter Prediction http://www.fruitfly.org/seq_tools/promoter.html
- Mnatsakanyan N, Bagramyan K, Trchounian A. Hydrogenase 3 but not hydrogenase 4 is major in hydrogen gas production by Escherichia coli formate hydrogenlyase at acidic pH and in the presence of external formate. Cell Biochem Biophys. 2004;41:357–366. doi: 10.1385/CBB:41:3:357. [DOI] [PubMed] [Google Scholar]
- Self WT, Hasona A, Shanmugam KT. Expression and regulation of a silent operon, hyf, coding for hydrogenase 4 isoenzyme in Escherichia coli. J Bacteriol. 2004;186:580–587. doi: 10.1128/JB.186.2.580-587.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domka J, Lee J, Bansal T, Wood TK. Temporal gene-expression in Escherichia coli K-12 biofilms. Environ Microbiol. 2007;9:332–346. doi: 10.1111/j.1462-2920.2006.01143.x. [DOI] [PubMed] [Google Scholar]
- Domka J, Lee J, Wood TK. YliH (BssR) and YceP (BssS) regulate Escherichia coli K-12 biofilm formation by influencing cell signaling. Appl Environ Microbiol. 2006;72:2449–2459. doi: 10.1128/AEM.72.4.2449-2459.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg M, Kaye IK, Peti W, Wood TK. YdgG (TqsA) controls biofilm formation in Escherichia coli K-12 through autoinducer 2 transport. J Bacteriol. 2006;188:587–598. doi: 10.1128/JB.188.2.587-598.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schembri MA, Kjærgaard K, Klemm P. Global Gene Expression in Escherichia coli Biofilms. Molecular Microbiology. 2003;48:253–267. doi: 10.1046/j.1365-2958.2003.03432.x. [DOI] [PubMed] [Google Scholar]
- Oh YK, Seol EH, Kim JR, Park S. Fermentative biohydrogen by a new chemoheterotrophic bacterium Citrobacter sp. Y19. Int J Hydrogen Energy. 2003;28:1353–1359. doi: 10.1016/S0360-3199(03)00024-7. [DOI] [Google Scholar]
- Archana A, Sasikala C, Ramana Ch V. Augmentation of H2 photoproduction in Rhodopseudomonas palustris by N-heterocyclic aromatic compounds. Biotechnol Lett. 2003;25:79–82. doi: 10.1023/A:1021717424268. [DOI] [PubMed] [Google Scholar]
- Jung GY, Jung HO, Kim JR, Ahn Y, Park S. Isolation and characterization of Rhodopseudomonas palustris P4 which utilizes CO with the production of H2. Biotechnol Lett. 1999;21:525–529. doi: 10.1023/A:1005560630351. [DOI] [Google Scholar]
- Minnan L, Jinli H, Xiaobin W, Huijuan X, Jinzao C, Chuannan L, Fengzhang Z, Liangshu X. Isolation and characterization of a high H2-producing strain Klebsiella oxytoca HP1 from a hot spring. Res Microbiol. 2005;156:76–81. doi: 10.1016/j.resmic.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Yoshida A, Nishimura T, Kawaguchi H, Inui M, Yukawa H. Enhanced hydrogen production from formic acid by formate hydrogen lyase-overexpressing Escherichia coli strains. Appl Environ Microbiol. 2005;71:6762–6768. doi: 10.1128/AEM.71.11.6762-6768.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning, A Laboratory Manual. 2. Cold Spring Harbor, NY , Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol. 2006;2:2006.0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canada KA, Iwashita S, Shim H, Wood TK. Directed evolution of toluene ortho-monooxygenase for enhanced 1-naphthol synthesis and chlorinated ethene degradation. J Bacteriol. 2002;184:344–349. doi: 10.1128/JB.184.2.344-349.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherepanov PP, Wackernagel W. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene. 1995;158:9–14. doi: 10.1016/0378-1119(95)00193-A. [DOI] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhavy TJ, Berman ML, Enquist LW. Experiments with gene fusions. Cold Spring Habor, N.Y. , Cold Spring Harbor Laboratories; 1984. [Google Scholar]
- Fiebig K, Friedrich B. Purification of the F420-reducing hydrogenase from Methanosarcina barkeri (strain Fusaro) Eur J Biochem. 1989;184:79–88. doi: 10.1111/j.1432-1033.1989.tb14992.x. [DOI] [PubMed] [Google Scholar]
- Glick BR, Wang PY, Schneider H, Martin WG. Identification and partial characterization of an Escherichia coli mutant with altered hydrogenase activity. Can J Biochem. 1980;58:361–367. doi: 10.1139/v80-058. [DOI] [PubMed] [Google Scholar]
- Ren D, Bedzyk LA, Thomas SM, Ye RW, Wood TK. Gene expression in Escherichia coli biofilms. Appl Microbiol Biotechnol. 2004;64:515–524. doi: 10.1007/s00253-003-1517-y. [DOI] [PubMed] [Google Scholar]
- Barrett T, Suzek TO, Troup DB, Wilhite SE, Ngau WC, Ledoux P, Rudnev D, Lash AE, Fujibuchi W, Edgar R. NCBI GEO: mining millions of expression profiles--database and tools. Nucleic Acids Res. 2005;33:D562–6. doi: 10.1093/nar/gki022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Zuo R, Gonzalez A, Bedzyk LA, Wood TK. Differential gene expression to investigate Escherichia coli biofilm inhibition by plant extract ursolic acid. Appl Environ Microbiol. 2005;71:4022–4034. doi: 10.1128/AEM.71.7.4022-4034.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson TJ. in Ph. D. thesis. Cambridge, England , Cambridge University; 1984. [Google Scholar]
- Casadaban MJ. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976;104:541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Sankar P, Lee JH, Shanmugam KT. Gene-product relationships of fhlA and fdv genes of Escherichia coli. J Bacteriol. 1988;170:5440–5445. doi: 10.1128/jb.170.12.5440-5445.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self WT, Shanmugam KT. Isolation and characterization of mutated FhlA proteins which activate transcription of the hyc operon (formate hydrogenlyase) of Escherichia coli in the absence of molybdate. FEMS Microbiol Lett. 2000;184:47–52. doi: 10.1111/j.1574-6968.2000.tb08988.x. [DOI] [PubMed] [Google Scholar]
- Lacourciere GM, Levine RL, Stadtman TC. Direct detection of potential selenium delivery proteins by using an Escherichia coli strain unable to incorporate selenium from selenite into proteins. Proc Natl Acad Sci U S A. 2002;99:9150–9153. doi: 10.1073/pnas.142291199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Microarray data for induced and repressed genes upon expressing the cyanobacterial locus. The data indicate differential gene expression in Escherichia coli TG1 cells upon expressing the cyanobacterial hydrogenase locus hoxEFUYH derived from Synechocystis sp. PCC6803.


