Abstract
Hepcidin is a peptide hormone secreted by the liver that plays a central role in the regulation of iron homeostasis. Increased hepcidin levels result in anemia while decreased expression is the causative feature in most primary iron overload diseases. Mutations in hemochromatosis type 2 (HFE2), which encodes the protein hemojuvelin (HJV), result in the absence of hepcidin and an early-onset form of iron overload disease. HJV is a bone morphogenetic protein (BMP) coreceptor and HJV mutants have impaired BMP signaling. In this issue of the JCI, Babitt and colleagues show that BMPs are autocrine hormones that induce hepcidin expression (see the related article beginning on page 1933). Administration of a recombinant, soluble form of HJV decreased hepcidin expression and increased serum iron levels by mobilizing iron from splenic stores. These results demonstrate that recombinant HJV may be a useful therapeutic agent for treatment of the anemia of chronic disease, a disorder resulting from high levels of hepcidin expression.
Regulation of iron homeostasis
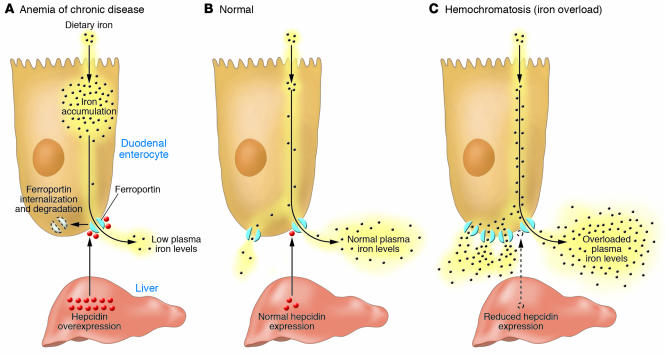
In all species, the concentration of iron in biological fluids is tightly regulated to provide iron as needed and to avoid toxicity, because iron excess can lead to the generation of reactive oxygen species. Iron homeostasis in mammals is regulated at the level of intestinal absorption, as there is no excretory pathway for iron. Hepcidin, a circulating peptide hormone, is the master regulator of systemic iron homeostasis, coordinating the use and storage of iron with iron acquisition (1). This hormone is primarily produced by hepatocytes and is a negative regulator of iron entry into plasma (Figure 1). Hepcidin acts by binding to ferroportin, an iron transporter present on cells of the intestinal duodenum, macrophages, and cells of the placenta. Binding of hepcidin induces ferroportin internalization and degradation (2). The loss of ferroportin from the cell surface prevents iron entry into plasma (Figure 1A). Decreased iron entry into plasma results in low transferrin saturation, and less iron is delivered to the developing erythroblast. Conversely, decreased expression of hepcidin leads to increased cell surface ferroportin and increased iron absorption (Figure 1C).
Figure 1. Hepcidin-mediated regulation of iron homeostasis.
(A) Increased hepcidin expression by the liver results from inflammatory stimuli. High levels of hepcidin in the bloodstream result in the internalization and degradation of the iron exporter ferroportin. Loss of cell surface ferroportin results in macrophage iron loading, low plasma iron levels, and decreased erythropoiesis due to decreased transferrin-bound iron. The decreased erythropoiesis gives rise to the anemia of chronic disease. (B) Normal hepcidin levels, in response to iron demand, regulate the level of iron import into plasma, normal transferrin saturation, and normal levels of erythropoiesis. (C) Hemochromatosis, or iron overload, results from insufficient hepcidin levels, causing increased iron import into plasma, high transferrin saturation, and excess iron deposition in the liver.
Plasma hepcidin levels are regulated by different stimuli, including cytokines, plasma iron, anemia, and hypoxia. Dysregulation of hepcidin expression results in iron disorders. Overexpression of hepcidin leads to the anemia of chronic disease, while low hepcidin production results in hereditary hemochromatosis with consequent iron accumulation in vital organs (Figure 1). Most hereditary iron disorders result from inadequate hepcidin production relative to the degree of tissue iron accumulation. Impaired hepcidin expression has been shown to result from mutations in any of 4 different genes: transferrin receptor 2 (TFR2), hemochromatosis (HFE), hemochromatosis type 2 (HFE2), and hepcidin antimicrobial peptide (HAMP). Mutations in HAMP, the gene that encodes hepcidin, result in iron overload disease, as the absence of hepcidin permits constitutively high iron absorption. The role for other genes (TFR2, HFE, and HFE2) in the regulation of hepcidin production has been unclear. A study presented in this issue of the JCI by Babitt et al. (3) provides a mechanistic understanding of the role of hemojuvelin (HJV; encoded by HFE2) in the regulation of hepcidin production.
Regulation of hepcidin expression
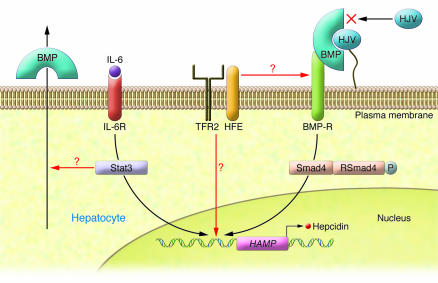
Regulation of hepcidin expression appears to occur at the level of transcription (Figure 2). Inflammatory cytokines, predominately IL-6, induce transcription of HAMP in hepatocytes. This induction involves the activation of Stat3 (3) and binding of Stat3 to a regulatory element in the HAMP promoter (4–6).
Figure 2. Transcriptional regulation of hepcidin by the BMP/Smad pathway.
Hepcidin transcription depends upon signaling through BMP receptors (BMP-Rs) and downstream Smads. BMPs can act as autocrine or paracrine hormones. Binding of BMP to cell surface HJV positions BMP to activate BMP receptors. Activation of BMP receptors leads to the generation of phosphorylated RSmads, which dimerize with Smad4. The RSmad/Smad4 heterodimer translocates into the nucleus and activates transcription of the HAMP gene, which encodes hepcidin. Soluble HJV binding to BMP prevents the formation of a cell surface BMP-HJV complex and blocks activation of BMP receptors. Inflammatory cytokines such as IL-6 bind to IL-6 receptors (IL-6Rs), activating Stat3, which also binds to the HAMP promoter. Stat3 activation requires the presence of Smad4, as deletion of the Smad4 gene prevents IL-6 induction of hepcidin. Smad4 is downstream of TFR2 and HFE, which suggests that the signal provided by these proteins also activates the HAMP promoter or that these membrane proteins affect BMP receptor signal transmission. In their study in this issue of the JCI, Babitt and colleagues demonstrate in vivo that soluble HJV binds BMPs produced by the liver, leading to alteration in iron homeostasis (3).
A second mode of hepcidin regulation depends upon signaling through the bone morphogenetic protein/Smad (BMP/Smad) pathway. BMPs are cytokines in the TGF-β family. These ligands play key roles in regulating cell proliferation, differentiation, apoptosis, and migration in tissues. In particular, BMPs play essential roles in cardiac, neural, and cartilaginous differentiation. All BMPs share a common signaling pathway, which involves binding of BMP to type I and type II cell serine/threonine kinase receptors, forming a BMP–type I/type II receptor complex. This complex results in the phosphorylation of an intracellular protein termed RSmad. The specificity of BMP ligand responses is due to the multiplicity of more than 20 BMPs, 7 type I receptors, 5 type II receptors, and 5 RSmads (7). The RSmads, however, combine with a single, common Smad family member, Smad4 (also referred to as Co-Smad), and that complex translocates into the nucleus and activates transcription of target genes (Figure 2). Wang et al. demonstrated that deletion of Smad4 results in embryonic lethality but that liver-specific inactivation of Smad4 results in loss of hepcidin synthesis and an iron overload phenotype similar to the phenotype seen in hepcidin-knockout mice (8). Babitt et al. also showed that mice with a deletion in the Smad4 gene were unable to synthesize hepcidin in response to inflammatory stimuli or to iron load (3). This result was the first to our knowledge to show that the BMP/Smad4 pathway is critical to hepcidin expression.
BMP signaling by HJV regulates hepcidin expression
Babitt and colleagues previously demonstrated that HJV acts as a BMP coreceptor in vitro, which facilitates the activation of the BMP–type I/type II receptor complex (9). Mutations in HFE2, the gene that encodes HJV, lead to early-onset iron overload disease (10). This form of juvenile hemochromatosis is typified by the absence of hepcidin and leads to heart disease, liver iron overload, and diabetes and is indistinguishable from the effects of HAMP mutations in patients. HJV is a glycosylphosphatidyl inositol–linked cell surface protein expressed predominantly in skeletal muscle and hepatocytes. The most informative structural feature of HJV is that it has homology to a small family of proteins involved in neural guidance. Members of this family (e.g., RBMCA, RGMCB, and DRAGON) bind BMPs and enhance BMP-mediated signaling (10). These previous results suggested that HJV’s ability to function as a BMP coreceptor was required for HJV to alter hepcidin expression and affect systemic iron homeostasis.
In vivo effect of BMP and HJV
In the present study, Babitt and colleagues extend their observations on the relationship between HJV and BMPs in hepcidin expression (3). First, they demonstrate that BMP receptor expression is selective for specific members of the TGF-β superfamily and is not activated by TGF-β, activins, or all BMPs. Second, they show in vitro that hepatocytes secrete BMPs and that siRNA silencing of specific BMPs reduces hepcidin expression. Thus, BMPs are autocrine regulators. Third, they show in vivo that injection of BMPs into wild-type mice increases hepcidin expression and concomitantly decreases serum iron levels. Fourth, they demonstrate that a recombinant, soluble form of HJV binds BMPs and acts as a BMP antagonist, decreasing hepcidin transcription. The observation that soluble BMPs decrease hepcidin expression in hepatocytes in vitro was shown previously (11). To our knowledge, Babitt et al. (3) are the first to show that soluble HJV inhibits hepcidin expression in vivo. Injection of soluble HJV for a period of weeks resulted in increased serum iron and increased liver iron. Soluble HJV reduced hepcidin transcripts in liver, decreasing the amount of phosphorylated RSmad. This observation provides proof that the effect of soluble HJV is to reduce BMP signaling events. The authors also show that soluble HJV reduces hepcidin expression in response to IL-6 (3). The observation that soluble HJV administration leads to increased liver and plasma iron but decreased hepcidin expression indicates that BMP signaling can modify hepcidin expression in response to other stimuli. These results are aligned with the observations of Wang et al. that neither iron nor IL-6 can induce hepcidin expression in mice with a liver-specific Smad4 gene deletion (8).
There are several reasons why these results are important. First, they suggest the possibility of pharmacological intervention for disorders resulting from excessive hepcidin production, as seen in the anemia of chronic disease. Second, they provide a mechanistic explanation for the role of HJV in iron overload disease. Third, the observation that soluble HJV affects hepcidin production provides an explanation for what was initially, to us, a curious observation that the highest expression of HJV is found in skeletal muscle. The recent finding that HJV is released from skeletal muscle in iron deficiency suggests that this tissue may play a critical role in iron homeostasis (12).
BMPs are critical for expression of hepcidin, which leads to the question: Is expression of BMPs regulated in hepatocytes? The present study (3) and that previously reported by Wang et al. (8) provide compelling data that the BMP signaling pathway is critical for IL-6 induction, prompting us to ask: How do Stat3 and Smad signaling interact? These pathways might interact at the level of the HAMP/hepcidin promoter, but an interesting possibility is that activated Stat3 might regulate hepatocyte BMP expression, as activated Stat3 in other tissues induces the expression of BMPs (13). Finally, how do HFE and TFR2 interact with BMPs and the Smad signaling system? Addition of BMPs to hepatocytes cultured from mice with targeted deletions in the HFE or TFR2 genes showed induction of hepcidin to levels that were similar to those in wild-type hepatocytes (14). This observation, together with the finding that Smad4 signaling is required for hepcidin expression regardless of stimuli, suggests that HFE and TFR2 might work upstream of BMP. A recent study has shown that HFE and TFR2 interact and that this interaction may provide a downstream signal that affects hepcidin expression (15). Does the HFE/TFR2 complex interact with the cell surface BMP/HJV/BMP receptor complex, or do intracellular signals provided by the HFE/TFR2 complex act on the HAMP/hepcidin promoter? The finding that the BMP/Smad system is fundamental to hepcidin expression provides a valuable starting point to deciphering these issues.
Acknowledgments
The research work in the laboratory of J. Kaplan is supported by NIH grants DK070947 and DK30534. The authors express their appreciation to Richard Ajioka for assistance with Figure 2.
Footnotes
Nonstandard abbreviations used: BMP, bone morphogenetic protein; HJV, hemojuvelin.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 117:1755–1758 (2007). doi:10.1172/JCI32701.
See the related article beginning on page 1933.
References
- 1.Nemeth E., Ganz T. Regulation of iron metabolism by hepcidin. Annu. Rev. Nutr. 2006;26:323–342. doi: 10.1146/annurev.nutr.26.061505.111303. [DOI] [PubMed] [Google Scholar]
- 2.Nemeth E., et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 3.Babitt J.L., et al. Modulation of bone morphogenetic protein signaling in vivo regulates systemic iron balance. J. Clin. Invest. 2007;117:1933–1939. doi: 10.1172/JCI31342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wrighting D.M., Andrews N.C. Interleukin-6 induces hepcidin expression through STAT3. Blood. 2006;108:3204–3209. doi: 10.1182/blood-2006-06-027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pietrangelo A., et al. STAT3 is required for IL-6-gp130-dependent activation of hepcidin in vivo. Gastroenterology. 2007;132:294–300. doi: 10.1053/j.gastro.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 6.Verga Falzacappa M.V., et al. STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood. 2007;109:353–358. doi: 10.1182/blood-2006-07-033969. [DOI] [PubMed] [Google Scholar]
- 7.Massague J., Seoane J., Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 8.Wang R.H., et al. A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression. Cell Metab. 2005;2:399–409. doi: 10.1016/j.cmet.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Babitt J.L., et al. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat. Genet. 2006;38:531–539. doi: 10.1038/ng1777. [DOI] [PubMed] [Google Scholar]
- 10.Papanikolaou G., et al. Mutations in HFE2 cause iron overload in chromosome 1q-linked juvenile hemochromatosis. Nat. Genet. 2004;36:77–82. doi: 10.1038/ng1274. [DOI] [PubMed] [Google Scholar]
- 11.Lin L., Goldberg Y.P., Ganz T. Competitive regulation of hepcidin mRNA by soluble and cell-associated hemojuvelin. Blood. 2005;106:2884–2889. doi: 10.1182/blood-2005-05-1845. [DOI] [PubMed] [Google Scholar]
- 12.Zhang A.S., et al. Evidence that inhibition of hemojuvelin shedding in response to iron is mediated through neogenin. J. Biol. Chem. 2007;282:12547–12556. doi: 10.1074/jbc.M608788200. [DOI] [PubMed] [Google Scholar]
- 13. Fukuda, S., et al. 2007. Potentiation of astrogliogenesis by STAT3-mediated activation of BMP-Smad signaling in neural stem cells. Mol. Cell. Biol. doi:10.1128/MCB.02435-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Truksa J., Peng H., Lee P., Beutler E. Bone morphogenetic proteins 2, 4, and 9 stimulate murine hepcidin 1 expression independently of Hfe, transferrin receptor 2 (Tfr2), and IL-6. Proc. Natl. Acad. Sci. U. S. A. 2006;103:10289–10293. doi: 10.1073/pnas.0603124103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goswami T., Andrews N.C. Hereditary hemochromatosis protein, HFE, interaction with transferrin receptor 2 suggests a molecular mechanism for mammalian iron sensing. J. Biol. Chem. 2006;281:28494–28498. doi: 10.1074/jbc.C600197200. [DOI] [PubMed] [Google Scholar]