Abstract
The leukotoxins [9(10)- and 12(13)-EpOME] are produced by activated inflammatory leukocytes such as neutrophils. High EpOME levels are observed in disorders such as acute respiratory distress syndrome and in patients with extensive burns. Although the physiological significance of the EpOMEs remains poorly understood, in some systems, the EpOMEs act as a protoxin, with their corresponding epoxide hydrolase metabolites, 9,10- and 12,13-DiHOME, specifically exerting toxicity. Both the EpOMEs and the DiHOMEs were also recently shown to have neutrophil chemotactic activity. We evaluated whether the neutrophil respiratory burst, a surge of oxidant production thought to play an important role in limiting certain bacterial and fungal infections, is modulated by members of the EpOME metabolic pathway. We present evidence that the DiHOMEs suppress the neutrophil respiratory burst by a mechanism distinct from that of respiratory burst inhibitors such as cyclosporin H or lipoxin A4, which inhibit multiple aspects of neutrophil activation.
Keywords: DHET, DiHOME, leukotoxin, neutrophil, p47phox, superoxide
1. Introduction
The leukotoxins (±)9(10)-epoxy-12Z- and (±)12(13)-epoxy-9Z-octadecenoic acid [9(10)- and 12(13)]-EpOME (figure 1A) are produced by inflammatory leukocytes such as neutrophils and macrophages (Ozawa et al 1988b; Zhang et al 1995; Hayakawa et al 1986). Plasma levels of the EpOMEs are elevated in patients suffering from extensive burns and elevated EpOME levels are associated with acute respiratory distress syndrome (ARDS), a systemic failure of organ systems frequently observed in trauma victims (Hayakawa et al 1990; Ozawa et al 1988a; Kosaka et al 1994). Elevated EpOME levels are observed in lungtissue and lavage after experimental exposure of rats to inhaled oxidants (Sevanian et al 1979). In animal models, administration of EpOME causes pulmonary oedema, vasodilation and cardiac failure, suggesting the possibility that EpOME-associated toxicity contributes to ARDS (Ishizaki et al 1995; Siegfried et al 1990; Sugiyama et al 1987; Hu et al 1988).
Figure 1.
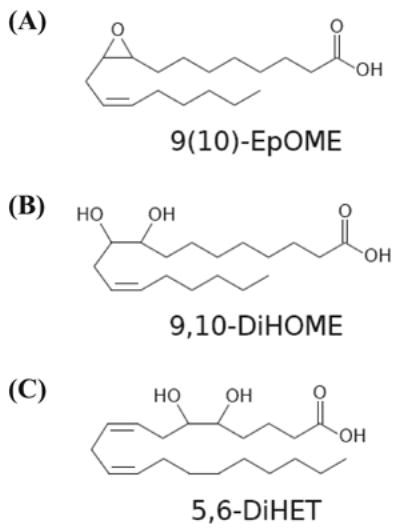
(A) Representative cytochrome P450 and sEH metabolites of linoleic acid and arachidonic acid. (B) One of the monoepoxide derivatives of linoleic acid, 9(10)-EpOME and the corresponding sEH metabolite, 9,10-DiHOME. (C) Structure of a dihydroxy derivative of arachidonic acid, 5,6-DiHET.
Although epoxides such as the EpOMEs are sometimes considered reactive toxins, most 1,2-aliphatic epoxides are not potent alkylating agents. Several studies suggest that the active entities which mediate EpOME-associated toxicity are not the EpOMEs, but their soluble epoxide hydrolase (sEH) metabolites, (±)9,10-dihydroxy-12Z- and (±)12,13-dihydroxy-9Z-octadecenoic acid (9,10- and 12,13-DiHOME, respectively) (Moghaddam et al 1997; Zheng et al 2001). DiHOMEs are detected in human urine under normal conditions (Zurek et al 2002; Newman et al 2002). In an animal model, elevated plasma DiHOME concentrations are observed upon exposure to tobacco smoke. This effect can be largely blocked by administration of an sEH inhibitor (Smith et al 2005). The DiHOMEs exert a number of effects on mammalian cells including stimulating MCF-7 cell proliferation and acting as an endocrine disrupter (Markaverich et al 2005), modulating the sodium cation current in cardiac cells (Harrell and Stimers 2002), and increasing cellular oxidative stress (Viswanathan et al 2003). In mammalian cells, the DiHOMEs specifically inhibit mitochondrial function (Sisemore et al 2001; Moran et al 1997), suggesting one possible mechanism underlying EpOME-associated toxicity.
Neutrophils are a significant component of the “non-specific” immune response. These immune cells are activated by stimuli such as binding of Gram-negative bacteria or by soluble stimuli such as chemotactic peptides, triggering processes including the respiratory burst, adherence to endothelial cells and release of secretory granules. Neutrophil influx and activation are hallmarks of ARDS (review in Yao et al 1998). Furthermore, EpOME synthesis is enhanced in activated neutrophils (review in Ishizaki et al 1999), suggesting that neutrophils may contribute to the elevated EpOME levels observed in ARDS patients. Whether EpOMEs in turn influence neutrophil activity has remained unclear until the EpOMEs and DiHOMEs were shown to be neutrophil chemotactic agents (Totani et al 2000). We evaluated whether members of the EpOME metabolic pathway influence another neutrophil function, the respiratory burst. This oxidative burst, produced by a molecular complex termed the NADPH oxidase, contributes to the immune-mediated elimination of a number of microorganisms (reviewed in Hampton et al 1998; Segal 2005). We provide evidence that members of the EpOME metabolic pathway, the DiHOMEs, specifically inhibit the respiratory burst.
2. Materials and methods
2.1 Reagents
Biolyte 3/10 ampholyte solution was purchased from BioRad (Hercules, CA, USA). Dithiothreitol and urea were purchased from Fisher Scientific. WST-1 was purchased from Dojindo Molecular Technologies, Inc. (Gaithersburg, MD, USA). Linoleic acid, methyl arachidonate and methyl linoleate were purchased from Nuchek Prep. (Elysian, MN, USA). All other reagents were purchased either from Sigma-Aldrich (St Louis, MO, USA) or ICN Biomedicals (Solon, OH, USA). Dibutyryl cyclic adenosine monophosphate (AMP) was dissolved as a stock solution in 9:1 ethanol:water which was stored at −70°C for no longer than 2–3 months (loss of activity was observed on prolonged storage). A mixture of methyl 9(10)-EpOME and 12(13)-EpOME was synthesized as described (Greene et al 2000b); a mixture of methyl 5(6), 8(9), 11(12), and 14(15)-epoxyeicosatrienoates (EpETEs) was synthesized in an analogous fashion. Methyl, propyl, and butyl esters of stearic acid were synthesized as described (Greene et al 2000a). Methyl threo-9,10-dihydroxyocta-12Z-enoate and threo-12,13-dihydroxyocta-9Z-enoate (9,10- and 12,13-DiHOME) were synthesized by acidic hydrolysis of methyl 9(10)-EpOME and 12(13)-EpOME as described (Greene et al 2000b); methyl 5,6-, 8,9-, 11,12-, and 14,15-DiHET were synthesized in an analogous fashion. Methyl 5,6-, 8,9-, 11,12-, and 14,15-DiHET were purified by reverse-phase HPLC (Brownlee Spheri-5 250×4.6 RP-18 column, 1 ml/min, acetonitrile:water:acetic acid from 40: 60:0.1 to 55:45:0.1 in 7.1 min; to 65:35:0.1 in 12.5 min; to 74:26:0.1 in 22 min). 9(10)- and 12(13)-EpOME and 9,10- and 12,13-DiHOME were synthesized by base hydrolysis of the corresponding methyl esters as described for 8(9)-EET (Falck et al 1990). Structures and purity of EpOMEs, DiHOMEs, DiHETs and the corresponding methyl esters were supported by NMR analysis and by chromatography. For chromatographic analysis by gas chromatography, after the compounds were incubated overnight with the silylating agent BSA/0.1% TCMS, they were analysed by gas chromatography/mass spectroscopy (GC/MS) (HP 5973/6890 GC/MS, using 30×0.25×0.25 DB-5MS, DB-17MS, or DB-XLB columns, 1 ml/min constant flow, oven programme: 50–224°C at 30°C/min, 224–245 at 2°C/min, 245–260 at 4°C/min, 260–320 at 10°C/min) where they gave a single peak based on total ion current and the expected fragmentation pattern. On chromatographic analysis by TLC, compounds were ultraviolet (UV)-transparent at 254 nm/280 nm and showed single spots with epoxide-selective reagents (4-nitrobenzylpyridine), diol-selective reagents (lead tetraacetate) and charring agents (sulphuric acid and phosphomolybdic acid).
2.2 Cell culture
HL-60 cells were obtained from Dr Dallas Hyde (University of California, Davis, USA) or ATCC (Manassas, VA, USA) and maintained in RPMI 1640 supplemented with 10% foetal calf serum. Non-differentiated cells were maintained at 1.5×105 to 8×105 cells/ml and were passaged every 2–3 days. In some experiments, HL-60 cells were treated with 500 μM dibutyryl cyclic AMP for 3.5–4.5 days.
2.3 Neutrophil respiratory burst assays
WST-1-reducing activity was evaluated essentially as described (Tan and Berridge 2000). In short, 1×106 cells were resuspended in 1 ml 300 μM WST-1 in HBSS. Cells were incubated at 37° C for 15 min and centrifuged at 1310 g for 5 min; the supernatant was then transferred to a cuvette and absorbance at 440 nm quantified.
To assess nitroblue tetrazolium-reducing activity, 50 μl of a 16.7 mg/ml solution of nitroblue tetrazolium in water was added to 500 μl of cells suspended at 4 to 5×105 cells/ml in growth medium. Cells were incubated at 37°C for 20 min, rinsed in PBS, and examined by light microscopy for the presence of the reduced (dark purple) nitroblue diformazan. At least 100 cells were counted for each sample.
Lucigenin-dependent chemiluminescence was measured essentially as described (Li et al 1998). In brief, 1×106 neutrophil-like HL-60 cells were resuspended in PBS, 0.1% glucose, 0.5 mM MgCl2, 0.7 mM CaCl2 and the solution adjusted to 10 μM myxothiazol, 10 μM rotenone, and 5 μM lucigenin. Additional stimuli were added as designated in the figures or text. The cell suspension was transferred to a 35 mm tissue culture dish in a luminometer (Turner Designs TD-20/20) and luminescence integrated over 10 or 15 min intervals. The cytochrome C reduction assay was not employed since some fatty acids are reported to interfere with cytochrome C reduction (Hardy et al 1994).
2.4 β-Glucuronidase release assay
β-Glucuronidase activity was assessed essentially as previously described (Tiberghien et al 1999). In brief, 5×105 cells were resuspended in 138 mM NaCl, 6 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 5.5 mM D-glucose, 1 mg/ml bovine serum albumin, 20 mM HEPES (pH 7.4) and adjusted to 5 μM cytochalasin B. After stimulation (as indicated in the figure 7B or text), the cells were incubated at 37°C for 10 min, 195 μl supernatant was then removed and added to 50 μl 25 mM p-nitrophenol β-glucuronide. After incubation for 5–6 h, 50 μl 0.3 M NaOH was added and absorbance at 410 nm evaluated.
Figure 7.
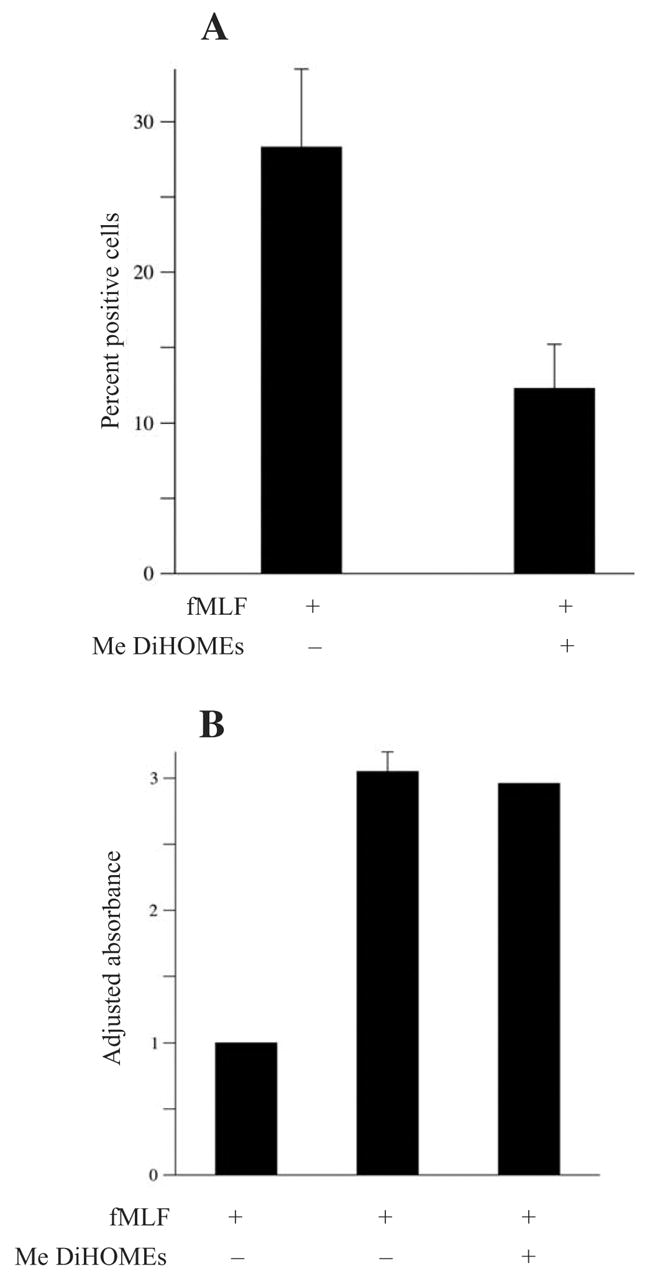
(A) Methyl 9,10-/12,13-DiHOME inhibits fMLF-induced nitroblue tetrazolium reduction. Granulocytic HL-60 cells were incubated with 1 μM fMLF and cotreated with either 200 μM methyl 9,10-/12,13-DiHOME or vehicle as indicated. Nitroblue tetrazolium reduction was evaluated as described in Materials and methods. Error bars represent population standard error of two independent experiments. The P value is 0.05. (B) Methyl 9,10-/12,13-DiHOME does not inhibit fMLF-induced β-glucuronidase release. Granulocytic HL-60 cells were stimulated with 1 μM fMLF and cotreated with 200 μM methyl 9,10-/12,13-DiHOME. β-Glucuronidase release was evaluated as described in Materials and methods. Results represent three independent experiments. Error bars represent population standard error. Since the magnitude of β-glucuronidase varied substantially between experiments, results were normalized based on the minimal and maximal response in each data set. P values (with respect to the vehicle control) were P < 0.01 for both fMLF-treated groups. The P value with respect to comparison between fMLF-treated and fMLF- and MeDiHOME-treated cells was P > 0.1.
2.5 Immunoblot analysis
Cells were lysed in 36 mM HEPES, 225 mM NaCl, 13.5 mM MgCl2, 0.9% Triton X-100, 4.5 mM NaF, 4.5 mM β-glycerophosphate, 1.8 mM EGTA, 1.8 mM EDTA, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 10 μg/ml pepstatin A, 1 mM Na3VO4, 0.5 mM PMSF, passed through a 25 gauge needle ten times, and incubated on ice for 30 min. Samples were analysed by gel electrophoresis and transferred to a PVDF membrane (Immobilon-P, Millipore; Billerica, MA, USA). Membrane blocking, antibody hybridizations and detection were as described below.
2.6 Non-equilibrium pH gradient electrophoresis
Cells (9×106) were lysed in 80 μl 8.5 M urea, 4% CHAPS, 2% Biolyte 3/10 ampholytes, and 1 mg/ml dithiothreitol by passage through a 25 gauge needle 10 times and incubation for 50 min at room temperature. Lysates were clarified by centrifugation and the protein concentrations of the supernatants evaluated by a modified Bradford assay (Zor and Selinger 1996). Non-equilibrium pH gradient electrophoresis (NEPHGE), a variant of isoelectric focusing, of equal masses of protein was conducted using Phastgel DryIEF gels (Pharmacia) as previously described (Ferullo and Nespoulous 1991). After electrophoresis, proteins were transferred to PVDF (Immobilon-P, Millipore) by capillary action. In brief, the gel was placed on wet Whatman 3 MM paper, overlaid with wet PVDF, two wet pieces of Whatman 3 MM paper, two dry pieces of Whatman 3 MM paper, and 0.5 in of paper towels cut to the size of the gel. A weight was placed on the assembly and, after 1 h at room temperature, the membrane was removed. After blocking non-specific binding sites by incubation in 5% non-fat dry milk (in 10 mM TrisHCl, pH 7.5, 2.5 mM EDTA, 50 mM NaCl, 0.1% Tween-20) the membrane was hybridized with primary antibody, washed, hybridized with horseradish peroxidase-conjugated anti-mouse antibody (Sigma), and analysed by enhanced chemiluminescence (Amersham-Pharmacia). Anti-p47phox antibody was purchased from Transduction Laboratories.
3. Results
3.1 EpOMEs weakly activate the respiratory burst
A number of polyunsaturated fatty acids stimulate the neutrophil respiratory burst (Badwey et al 1981). Linoleic acid is a potent stimulus of this response; indeed, the response of neutrophils treated with linoleic acid exceeds that observed with arachidonic acid treatment (Li et al 1996). Given recent work indicating that the epoxy derivatives of linoleic acid, 9(10)- and 12(13)-EpOME, stimulate neutrophil chemotaxis (Totani et al 2000), we evaluated whether the EpOMEs modulate neutrophil activation.
HL-60 cells are a human promyelocytic cell line widely used as a system to model human neutrophils (review in Collins 1987). These cells can be induced to differentiate to a neutrophil-like state by agents such as dimethyl sulphoxide or dibutyryl cyclic AMP (Chaplinski and Niedel 1982). To evaluate whether 9(10)- or 12(13)-EpOME stimulates the neutrophil respiratory burst, we incubated HL-60 cells with an initial concentration of 500 μM dibutyryl cyclic AMP for approximately 4 days. The resulting population of differentiated, neutrophil-like cells was stimulated with an equimolar mixture of 9(10)- and 12(13)-EpOME and activation of the respiratory burst monitored by evaluating WST-1 reduction. At concentrations exceeding 10 μM, EpOMEs induced the respiratory burst. However the intensity of the EpOME-induced respiratory burst was less than that induced by linoleic acid (figure 2), suggesting that the EpOMEs are a relatively weak neutrophil stimulant. When the potency of the dihydroxy sEH metabolites of the EpOMEs (a mixture of 9,10- and 12,13-DiHOME) was evaluated, a dose–response trend was not observed (data not shown), suggesting that the DiHOMEs are substantially less active than the EpOMEs with respect to respiratory burst stimulation.
Figure 2.
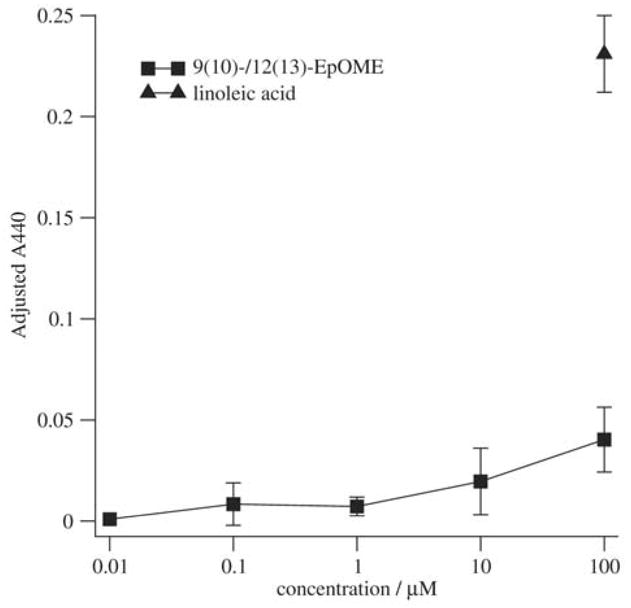
9(10)-/12(13)-EpOME stimulates the neutrophil respiratory burst. Granulocytic HL-60 cells were treated with the indicated concentration of 9(10)-/12(13)-EpOME or linoleic acid, and cotreated with 7.5 μM EGTA as indicated. WST-1 reduction was evaluated as described in Materials and methods. Adjusted A440 values reflect the extent of WST-1 reduction which was inhibited by 200 U/ml superoxide dismutase. Error bars represent population standard error of two to three independent experiments. Analysis of the EpOME dose–response curve by linear regression from 10 nM to 200 μM yielded a statistically significant (P = 0.01) dose–response.
3.2 Methyl 9,10-/12,13-DiHOME inhibits the neutrophil respiratory burst
Neutrophil-like HL-60 cells, when treated with the phorbol ester PMA, exhibit a strong respiratory burst. To determine whether 9(10)- or 12(13)-EpOME, or related members of the linoleate metabolic pathway, modulate the respiratory burst when it is induced by another stimulus, we evaluated the PMA-induced respiratory burst of human granulocytic cells directly after treatment with methyl linoleate, an equimolar mixture of methyl 9(10)- and 12(13)-EpOME, or an equimolar mixture of methyl 9,10- and 12,13-DiHOME. Using a qualitative microscopic assay of nitroblue tetrazolium-reducing activity, we found that doses of methyl 9,10-/12,13-DiHOME paralleling EpOME concentrations reported in severe burn patients (Kosaka et al 1994) potently suppressed the phorbol ester-induced respiratory burst in granulocytic HL-60 cells. A 20 μM concentration did not produce a similar inhibitory effect (figure 3). Given previous work suggesting that 9(10)-/12(13)-EpOME represents a protoxin of its epoxide hydrolase metabolite, 9,10-/12,13-DiHOME, in at least some systems (Moghaddam et al 1997), we also examined whether methyl 9(10)-/12(13)-EpOME inhibits the neutrophil respiratory burst. Unlike methyl 9,10-/12,13-DiHOME, inhibition of the respiratory burst was not observed with methyl 9(10)-/12(13)-EpOME, nor with the parent ester, methyl linoleate (figure 3).
Figure 3.
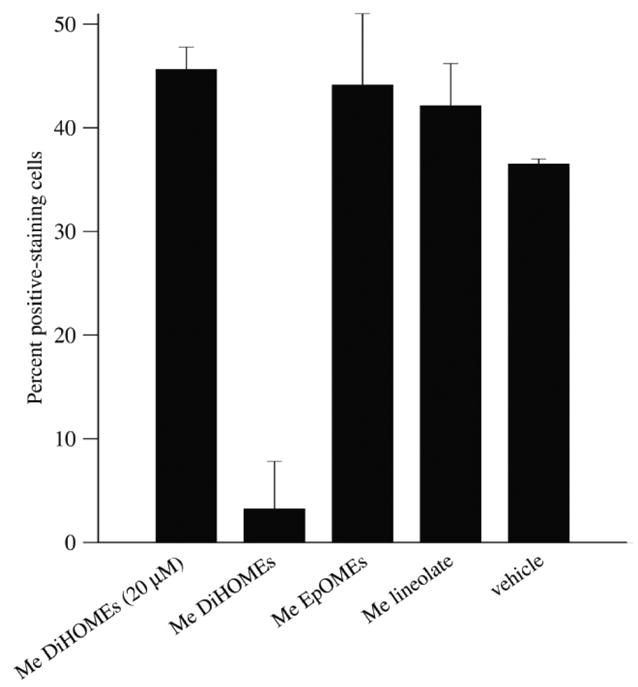
Methyl 9,10-/12,13-DiHOME (Me DiHOMEs) inhibit the neutrophil respiratory burst. Granulocytic HL-60 cells were treated with 10 nM PMA in conjunction with 200 μM of the indicated compound (unless otherwise indicated) or with vehicle. Nitroblue tetrazolium reduction was evaluated as described in Materials and methods. Error bars represent population standard error of two to three independent experiments. The vehicle (no PMA stimulation) control value was typically 0–2%. The P values compared to vehicle were P < 0.01 (Me DiHOMEs, 20 μM), P < 0.01 (Me DiHOMEs, 200 μM), P = 0.075 (Me EpOMEs), and P < 0.05 (Me linoleate).
Under appropriate conditions, lucigenin-dependent chemiluminescence provides a relatively specific measure of superoxide generated by the respiratory burst (Li et al 1998). To confirm that phorbol ester-induced nitroblue tetrazolium reduction represented respiratory burst-associated superoxide generation, we quantified the capacity of methyl 9,10-/12,13-DiHOME to inhibit the PMA-induced respiratory burst by monitoring lucigenin-dependent chemiluminescence. PMA induced significant superoxide production by granulocytic cells, as reflected in increased lucigenin-dependent chemiluminescence. In contrast, lucigenin-dependent chemiluminescence was markedly diminished in cells cotreated with PMA and methyl 9,10-/12,13-DiHOME (figure 4), which was consistent with results obtained using the nitroblue tetrazolium assay (figure 3). This observation suggests that methyl 9,10-/12,13-DiHOME inhibits superoxide production during activation of the respiratory burst. We noted that the ethanol vehicle alone (at 0.2% v/v) detectably enhanced PMA-induced lucigenin-dependent chemiluminescence in this assay (data not shown), consistent with previous reports (Raddassi and Murray 2000). Thus, vehicle controls are particularly appropriate when using this assay system.
Figure 4.
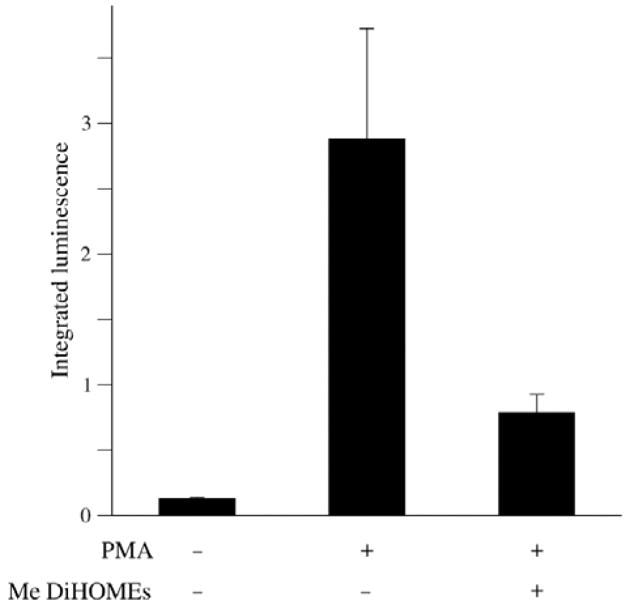
Methyl 9,10-/12,13-DiHOME inhibits PMA-induced lucigenin-dependent chemiluminescence. Granulocytic HL-60 cells were stimulated as indicated with 10 nM PMA or the PMA vehicle (−) and cotreated with 200 μM of methyl 9,10-/12,13-DiHOME or with the corresponding vehicle (−). Integrated (30 min) lucigenin-dependent chemiluminescence (expressed in arbitrary units) was evaluated as described in Materials and methods. Error bars represent the population standard error of two independent experiments. The P values compared to cells treated only with PMA were P < 0.01 (vehicle/vehicle) and P = 0.01 (treatment with PMA and Me DiHOMEs).
3.3 Structural determinants of DiHOME-mediated respiratory burst inhibition
Since a mixture of methyl 9,10- and 11,12-DiHOME inhibited the neutrophil respiratory burst, we evaluated whether this activity was specific to either of the DiHOME regioisomers. Using a quantitative assay for the neutrophil respiratory burst, we compared respiratory burst inhibition in cells cotreated with 10 nM PMA and either methyl 9,10-DiHOME or methyl 11,12-DiHOME. With each isomer, inhibition was observed at 20 μM and, in both cases, inhibition exceeded 50% at 200 μM (figure 5). Furthermore, inhibition observed with 200 μM of either isomer was indistinguishable from that observed with treatment with a 200 μM equimolar mixture of the isomers (figure 5), suggesting that both isomers have similar potency with respect to respiratory burst inhibition.
Figure 5.
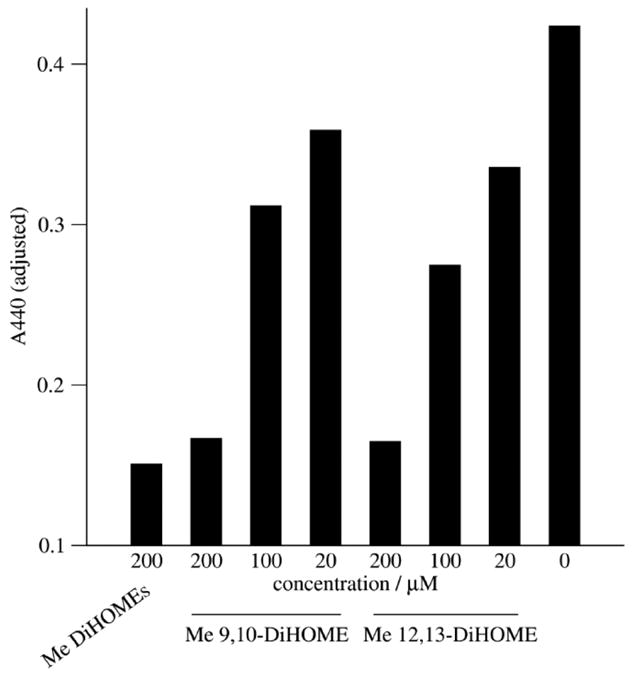
Methyl 9,10-DiHOME and methyl 12,13-DiHOME inhibit the respiratory burst at similar concentrations. Granulocytic HL-60 cells were treated with 10 nM PMA in conjunction with the indicated concentration of the compound mentioned or with vehicle. WST-1 reduction was evaluated as described in Materials and methods; adjusted A440 represents absorbance at 440 nM after subtracting absorbance at 440 nm of cells cotreated with 10 nM PMA and 200 U/ml superoxide dismutase.
Upon neutrophil activation, some esterified fatty acids are released as fatty acids into the extracellular milieu (Kerkhoff et al 1999). To determine whether 9,10-/12,13-DiHOME is active as a fatty acid, we evaluated the capacity of 9,10-/12,13-DiHOME to inhibit the respiratory burst. In contrast to the corresponding methyl esters, 9,10-/12,13-DiHOME did not inhibit the respiratory burst at 200 μM (figure 6A).
Figure 6.
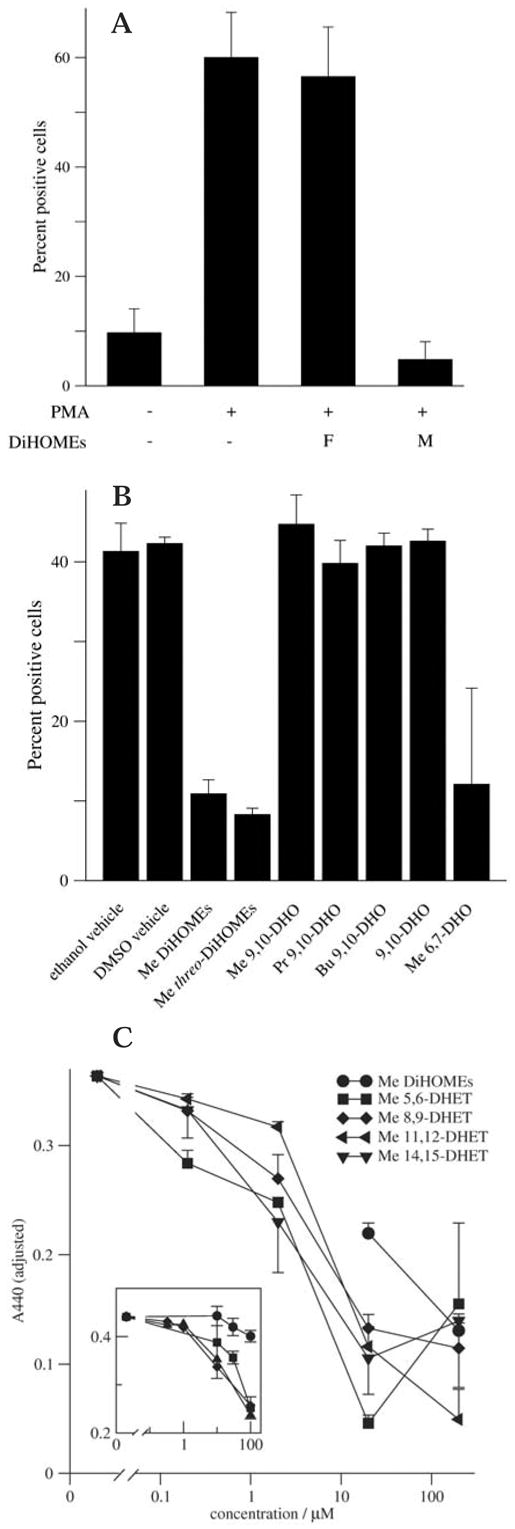
(A) Methyl 9,10-/12,13-DiHOME specifically inhibits the neutrophil respiratory burst. Granulocytic HL-60 cells were treated with 20 nM PMA and/or with 200 μM of either the fatty acid (F) or methyl ester (M) of 9,10-/12,13-DiHOME (DiHOMEs). The respiratory burst was assessed by microscopic evaluation of nitroblue tetrazolium reduction. Error bars represent standard deviation. Results are representative of two independent experiments. (B) Methyl stearate diols do not inhibit the neutrophil respiratory burst. Granulocytic HL-60 cells were treated with 20 nM PMA in conjunction with 200 μM of the indicated lipid. Nitroblue tetrazolium reduction was assessed as described in Materials and methods. DHO represents dihydroxyoctadecanoic acid or (for esters) dihydroxyoctadecanoate. DiHOMEs represent a mixture of the indicated derivatives of 12,13-dihydroxyocta-9-enoate and 9,10-dihydroxyocta-12-enoate. Me (methyl), Pr (propyl), and Bu (butyl) refer to the corresponding esters. Error bars represent the population standard error of two independent experiments. The vehicle control value was typically 0–2%. (C) Methyl DHETs inhibit the respiratory burst. Granulocytic HL-60 cells were treated with the indicated concentration of methyl 5,6-, 8,9-, 11,12-, or 14,15-DHET and with 10 nM PMA. WST-1 reduction was evaluated as described in Materials and methods. Adjusted A440 values represent WST-1 reduction inhibited by 200 U/ml superoxide dismutase and scaled based on the value observed with 10 nM PMA (this value varied substantially (from 0.181 to 0.584) between experiments since granulocytic HL-60 cells gradually lose responsiveness to PMA with passaging). Error bars represent population standard error of three to four independent experiments. Within each experiment, variability of replicate samples (10 nM PMA) was less than 5%. Inset: Granulocytic HL-60 cells were treated with 20 nM PMA and evaluated for respiratory burst activation as described above. Symbols represent methyl 5,6,14, 15-tetrahydroxyeicosadienoate(circles),8,9,14,15-tetrahydroxyeico-sadienoate (squares), a mixture of methyl 9-, 11-, and 12-hydroxyeicosatetraenoates (triangles), and 8-hydroxyeicosatetraenoate (diamonds). Error bars represent the population standard error of three to four independent experiments.
To better characterize the specificity of DiHOME-mediated inhibition of the respiratory burst, we evaluated the inhibitory activity of several structurally related epoxide and diol esters. Most failed to inhibit the respiratory burst in the concentration range at which 9,10-/12,13-DiHOME exhibited inhibitory activity. Three exceptions were a mixture of the threo analogues (at the diol position) of the DiHOMEs, the methyl ester of 6,7-dihydroxystearate, and a mixture of DHET methyl esters (figure 6B and data not shown). Given that DHETs and their EET precursors appear to play roles in regulating a variety of biological processes, we turned to a quantitative assay of superoxide release to better characterize the potencies of the methyl DHETs. We found that the potencies of the methyl DHETs generally exceeded that of the methyl DiHOMEs by roughly ten-fold (figure 6C). While the 11,12- and 5,6- DHETs produced the greatest extent of inhibition, the 5,6- and 14,15-DHETs were most potent with respect to dose, yielding a 50% inhibitory effect at approximately 4 μM.
Given the inhibitory potential of the methyl DHETs, we evaluated the inhibitory potency of several n-ol arachidonate derivatives to determine whether the number of hydroxy groups corresponded to potency. The methyl ester analogues of sEH metabolites of the DiEpEDEs (diepoxyeicosadienoates), the methyl tetrahydroxyeicosadienoates, exhibited similar or weaker inhibitory potencies relative to methyl DiHOMEs. In contrast, both methyl 8-hydroxyeicosatetraenoate (8-HETE) and a mixture of methyl 9-, 11-, and 12-HETEs exhibited only slightly less activity than the methyl DHETs (approximately 50% inhibition at 10 μM and no significant inhibition at or less than 1 μM; figure 6C, inset).
Together, the data suggest that specific structural determinants – particularly (i) the location and number of the hydroxy groups, and the existence and relative location of adjacent double bonds, and (ii) the presence of an ester at the fatty acid terminus – are significant determinants of the inhibitory potential of both 18- and 20-carbon lipid diols.
3.4 DiHOMEs do not inhibit all neutrophil activation endpoints
Given the capacity of the methyl esters of the DiHOMEs to inhibit the respiratory burst, it was of interest to investigate the means by which methyl 9,10-/12,13-DiHOME inhibits the respiratory burst. To address this, we turned to another neutrophil stimulus, the chemotactic peptide N-formyl-methionyl-leucyl-phenylalanine (fMLF). HL-60 cells differentiated with dibutyryl cyclic AMP exhibit a maximal response to fMLF at approximately 1 μM (Xie et al 1991). We observed that granulocytic HL-60 cells stimulated with fMLF exhibited a slightly less marked respiratory burst response than that elicited by PMA stimulation. Cells cotreated with 1 μM fMLF and 200 μM methyl 9,10-/12,13-DiHOME displayed substantially less nitroblue tetrazolium-reducing activity than those stimulated with 1 μM fMLF and vehicle, indicating that methyl 9,10-/12,13-DiHOME also inhibits the fMLF-induced respiratory burst (figure 7A).
Neutrophils contain a variety of cytoplasmic granules; upon neutrophil activation, some fuse with phagosomes while others release their contents into the extracellular space. Upon neutrophil activation by fMLF, a population of neutrophil granules containing β-glucuronidase undergoes exocytosis. To determine whether activation endpoints other than the respiratory burst are also inhibited by the DiHOMEs, we evaluated whether methyl 9,10-/12,13-DiHOME inhibits fMLF-induced β-glucuronidase release from granulocytic cells. Irrespective of cotreatment with 9,10-/12,13-DiHOME or vehicle, fMLF induced a similar release of β-glucuronidase activity (figure 7B). This observation suggests that methyl 9,10-/12,13-DiHOME specifically inhibits the respiratory burst, rather than acting as an upstream inhibitor of multiple aspects of fMLF-induced HL-60 activation.
3.5 PMA-induced p47phox phosphorylation is not inhibited by methyl 9,10-/12,13-DiHOME
Neutrophil activation is characterized by a wide variety of signal transduction events. Assembly of the multiprotein NADPH oxidase complex is required for superoxide production and is preceded by the phosphorylation of p47phox and translocation of p47phox to the membrane where it assembles with cytochrome b558 (a heterodimer of gp91 and p22phox), and the accessory proteins gp61phox and Rac1/2 (review in Babior 1999). Phosphorylation of p47phox is required for NADPH oxidase activation (Faust et al 1995; Inanami et al 1998; Johnson et al 1998) and can be detected by the appearance of a series of lower-pI p47phox bands in NEPHGE gels (Rotrosen and Leto 1990; Nauseef et al 1990; Okamura et al 1988; Zhou et al 1997). To determine whether methyl 9,10-/12,13-DiHOME acts upstream of this signal transduction event, we evaluated p47phox phosphorylation in PMA-stimulated granulocytic cells by NEPHGE of cell lysates and subsequent immunoblotting for p47phox.
We first assessed the specificity of a commercially available p47phox antibody by immunoblotting p47phox immunoprecipitates of cell lysates derived from cells known to express p47phox. Only a single band (apart from antibody heavy and light chains) was detected in p47phox immunoprecipitates (figure 8A).
Figure 8.
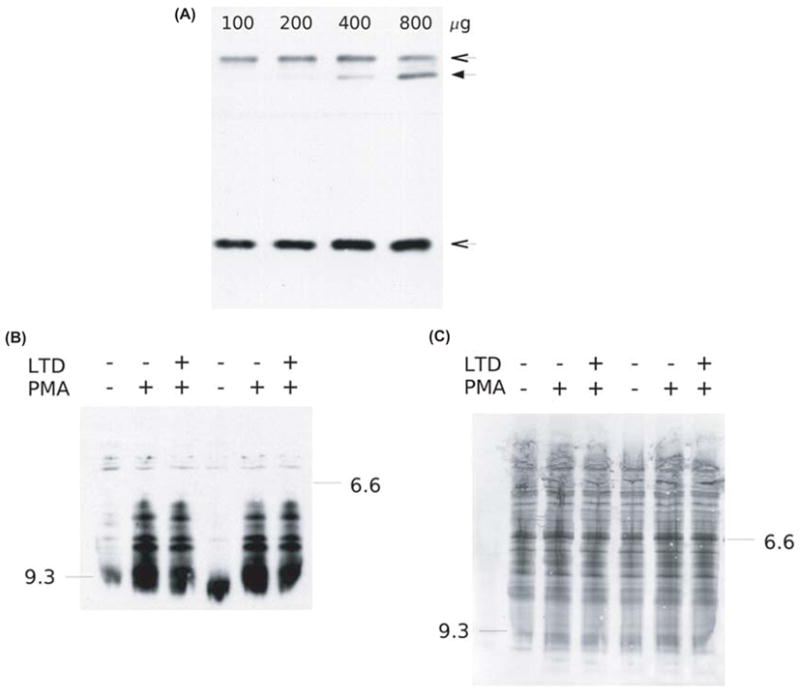
Methyl 9,10-/12,13-DiHOME does not inhibit PMA-induced p47phox phosphorylation. (A) HL-60 cells were lysed and p47phox immunoprecipitated from the indicated amount of cell lysate protein using anti-p47phox primary antibody and protein A-Sepharose beads. Protein was eluted with Laemmli sample buffer and subjected to SDS-PAGE and subsequent immunoblotting for p47phox as described in Materials and methods. Line arrows indicate the positions of the heavy and light antibody chains; the closed arrow indicates the band corresponding to p47phox. (B) Granulocytic HL-60 cells were treated with 20 nM PMA and with 200 μM methyl 9,10-/12,13-DiHOME as indicated. After 14 min, cells were harvested. Cell lysates were subjected to NEPHGE (a variant of isoelectric focusing gel electrophoresis); the gel was immunoblotted for p47phox as described in Materials and methods. pI standard locations are indicated at the margins of the image. Data from two representative experiments are shown. (C) India ink staining of the immunoblot membrane shown in C was used to verify that equal masses of protein were loaded for each sample.
To evaluate the influence of methyl 9,10-/12,13-DiHOME on PMA-induced p47phox phosphorylation, granulocytic cells were stimulated with PMA and cotreated with methyl 9,10-/12,13-DiHOME or vehicle. The corresponding cell lysates were then subjected to NEPHGE and the gel immunoblotted using an anti-p47phox antibody. We observed that, in resting granulocytic cells, a predominant p47phox band comigrated with an IEF standard of 9.3 (figure 8B), which was consistent with previous reports. In contrast, in cells activated with PMA, irrespective of methyl 9,10-/12,13-DiHOME treatment, a series of p47phox bands migrating between IEF standards of 6.6 and 9.3 was readily evident (figure 8B), suggesting that methyl 9,10-/12,13-DiHOME does not inhibit PMA-induced p47phox phosphorylation. India ink staining of the immunoblot membrane verified that these results were not due to variation between samples in the mass of total protein subjected to electrophoresis (figure 8C).
4. Discussion
Polyunsaturated fatty acids such as linoleic and arachidonic acids stimulate the neutrophil respiratory burst (Badwey et al 1984, 1981). Although we observed a stimulatory effect upon the respiratory burst by 9(10)-/12(13)-EpOME, the effect was much less pronounced than that observed with linoleic acid or PMA treatment (figure 2), suggesting that 9(10)-/12(13)-EpOME is a relatively weak stimulant of the respiratory burst. The concentrations of 9(10)-/12(13)-EpOME at which the respiratory burst was stimulated (20–200 μM) are similar to those reported in severe physiological disorders such as ARDS (Hayakawa et al 1990; Ozawa et al 1988a; Kosaka et al 1994) and to those reported to trigger superoxide production in pulmonary epithelial cells (Okamura et al 2002).
In contrast, an equimolar mixture of methyl 9,10- and 12,13-DiHOME strongly inhibited the respiratory burst at similar concentrations (figures 2 and 4). While we did not observe regioisomeric specificity with respect to potency (figure 5), we found that the esterified state of the carboxyl group is a critical determinant of the inhibitory potency of 9,10-/12,13-DiHOME (figure 6D). In contrast, the capacity of unsaturated fatty acids to activate neutrophil NADPH oxidase is dependent on the presence of the charged carboxyl group (Steinbeck et al 1991). One possibility is that the methyl ester itself represents the physiologically relevant compound. However, while human leukocytes express a fatty acid ester synthase activity (Wright et al 1987) and fatty acid methyl esters have been detected in a physiological context (Schmidt et al 1996), no studies have demonstrated that methyl esters of the DiHOMEs are synthesized in vivo in a physiological context. In contrast, when the methyl ester is administered to Sf-21 cells, the methyl moiety is cleaved and only the free DiHOMEs are detected (Greene et al 2000b). This suggests that the efficacy of methyl 9,10-/12,13-DiHOME, but not the corresponding acid, in respiratory burst inhibition is attributable to the ability of the uncharged ester to readily translocate across the cytoplasmic membrane. If intracellular esterases then cleave the ester, producing DiHOMEs, then the ester form simply represents a prodrug of 9,10-/12,13-DiHOME. Alternatively, methyl 9,10-/12,13-DiHOME may mimic an endogenous membrane constituent, such as a linoleate-derived phospholipid, produced during neutrophil activation.
The mechanism by which the DiHOMEs inhibit the respiratory burst is distinct from that of other respiratory burst inhibitors such as nitrolinoleate, cyclosporin H, lipoxin A4, or 15-HETE, which inhibit multiple aspects (both superoxide production and degranulation) of neutrophil activation (Coles et al 2002; Wenzel-Seifert et al 1991; Smith et al 1993; Gewirtz et al 1999; Hachicha et al 1999). The inhibitory effect of the methyl DiHOMEs is rapid, and does not require a preincubation step. Furthermore, the inhibitory effect is specific; inhibition of the respiratory burst is not accompanied by inhibition of other neutrophil activation endpoints such as β-glucuronidase release (figure 7B). Given the additional observation that the methyl DiHOMEs inhibit activation in response to stimuli with distinct mechanisms of action, fMLF and PMA, it seems probable that the methyl DiHOMEs inhibit an activity intimately associated with the NADPH oxidase. The methyl DiHOMEs may stimulate consumption of an NADPH oxidase substrate such as NADPH or may induce physicochemical alterations in the NADPH oxidase membrane microenvironment which modulates the activity of the NADPH oxidase. At 50–100 μM, arachidonic acid induces an interaction between p47phox and p22phox, an interaction likely to play an important role in NADPH oxidase activation (Sumimoto et al 1994; Hata et al 1998; Kerkhoff et al 2005).
An alternative possibility is that the methyl DiHOMEs target a protein kinase C activity upstream of the NADPH oxidase. Unsaturated fatty acids are well-established modulators of protein kinase C activity (McPhail et al 1984; O’Flaherty and Nishihira 1987; Shinomura et al 1991). PMA-induced respiratory burst activation requires protein kinase C and this activation step has been implicated in p47phox phosphorylation (Allard et al 1999; Reeves et al 1999; Korchak et al 1998). However, PMA-induced phosphorylation of p47phox is observed irrespective of cotreatment with methyl 9,10-/12,13-DiHOME (figure 8). This suggests that methyl 9,10-/12,13-DiHOME inhibits the respiratory burst at a point independent of, or downstream from, p47phox phosphorylation. Additional phosphorylation events have been postulated to inhibit p47phox (DeLeo et al 1999; Lopes et al 2004) and, although its relationship to NADPH oxidase activation remains unclear, p67phox phosphorylation is also observed during NADPH oxidase activation (review in Quinn and Gauss 2004). Further studies should evaluate whether inhibition is associated specifically with late phosphorylation events on p47phox or with the phosphorylation state of other components of the NADPH oxidase.
While widely used as a neutrophil model, granulocytic HL-60 cells generally lack certain characteristics typically observed in primary human neutrophils (review in Collins 1987). Given this, the relevance of our observation to physiological contexts such as ARDS awaits evaluation of the activity of the DiHOMEs in primary human neutrophils.
In summary, EpOMEs weakly activate the respiratory burst; however, the esterified form of DiHOMEs strongly inhibits the respiratory burst at mid-micromolar concentrations. DHET esters exhibit an approximately 10-fold greater inhibitory potency and significantly inhibit the respiratory burst even at high nanomolar concentrations. The inhibitory activity of the DiHOMEs and DHETs with respect to the respiratory burst may represent an inhibitory feedback signal. Possibly, in some contexts, activation of the respiratory burst proceeds to such an extent that substantial superoxide-, hydroxyl radical-, or hypochlorite-induced damage to endogenous cell membrane lipid components accumulates. In such a scenario, micromolar concentrations of epoxide-, diol-, or chlorohydrin-containing esterified lipids might be realized in the immediate proximity of the NADPH oxidase. Such localized membrane concentrations are realistic; in resting platelets, EETs are observed exclusively in the phospholipid fraction – once stimulated, EETs are de-esterified, producing cytoplasmic concentrations estimated at 1 μM (Zhu et al 1995). Given this, it is clear that localized concentrations of esterified EETs in the resting platelet might well exceed 1 μM. Interestingly, EETs influence neutrophil adherence in the low micromolar range (Pratt et al 2002). Since both arachidonate and linoleate are present in relatively high abundance in their esterified forms in neutrophil phospholipids (Yano et al 1983; Johnson et al 1999), it does not seem unreasonable to propose that either esterified DiHOMEs and/or esterified DHETs represent inhibitory signals with respect to the neutrophil NADPH oxidase activation, nor to speculate that other similar membrane-associated systems such as the plant NADPH oxidase or the mitochondrial respiratory chain are regulated in a similar fashion.
Acknowledgments
We thank Dr Robert Rice (University of California, Davis) for use of laboratory equipment, Qihong Huang for critical review of this manuscript, and John Newman for assistance with development of HPLC and GC/MS methods. This work was supported by NIEHS Grant R01 ES02710, NIH R01 HL 59699-06A1, the NIEHS Superfund Basic Research Program P42 ES04699, and NIEHS Center P30 ES05707 (to B D H) from the National Institutes of Health, and by National Institutes of Health Training Grant HL07013 (to D A T).
Abbreviations used
- AMP
adenosine monophosphate
- ARDS
acute respiratory distress syndrome
- ATCC
American Type Culture Collection
- CHAPS
3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonic acid
- DHET
dihydroxyeicosatetraenoic acid
- DiEpEDEs
diepoxyeicosadienoates
- DiHOME
dihydroxyoctadecenoic acid
- EDTA
ethylenediamine tetra-acetic acid
- EET
epoxyicosatetraenoic acid
- EGTA
ethylene glycol tetraacetic acid
- EpETEs
epoxyeicosatrienoates
- EpOME
epoxyoctadecenoic acid
- fMLF
N-formyl-methionyl-leucyl-phenylalanine
- GC/MS
gas chromatography/mass spectroscopy
- HBSS
Hank’s buffered salt solution
- 15-HETE
hydroxyeicosatetraenoic acid
- 8-HETE
methyl 8-hydroxyeicosatetraenoate
- HEPES
N-(2-hydroxyethyl) piperazine-N’-(2-ethanesulphonic acid)
- HL-60
human leukaemia cell line
- HPLC
high performance liquid chromatography
- IEF
isoelectric focusing
- NADPH
nictonamide adenine dinucleotide
- NEPHGE
non-equilibrium pH gradient electrophoresis
- NMR
nuclear magnetic resonance
- PAF
platelet-activating factor
- PBS
phosphate buffered saline
- PMA
phorbolmyristate acetate
- PVDF
polyvinylidene fluoride
- sEH
soluble epoxide hydrolase
- TLC
thin layer chromatography
- UV
ultra-violet
- WST-1
2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulphophenyl)-2H-tetrazolium monosodium salt
Footnotes
Corresponding editor: ANURADHA LOHIA
References
- Allard B, Long E, Block E, Zhao X. Dependence of superoxide anion production on extracellular and intracellular calcium ions and protein kinase C in PMA-stimulated bovine neutrophils. Can J Vet Res. 1999;63:13–17. [PMC free article] [PubMed] [Google Scholar]
- Babior BM. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- Badwey JA, Curnutte JT, Karnovsky ML. cis-Polyunsaturated fatty acids induce high levels of superoxide production by human neutrophils. J Biol Chem. 1981;256:12640–12643. [PubMed] [Google Scholar]
- Badwey JA, Curnutte JT, Robinson JM, Berde CB, Karnovsky MJ, Karnovsky ML. Effects of free fatty acids on release of superoxide and on change of shape by human neutrophils reversibility by albumin. J Biol Chem. 1984;259:7870–7877. [PubMed] [Google Scholar]
- Chaplinski T, Niedel J. Cyclic nucleotide-induced maturation of human promyelocytic leukemia cells. J Clin Invest. 1982;70:953–964. doi: 10.1172/JCI110707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles B, Bloodsworth A, Clark SR, Lewis MJ, Cross AR, Freeman BA, O’Donnell VB. Nitrolinoleate inhibits superoxide generation, degranulation, and integrin expression by human neutrophils: novel antiinflammatory properties of nitric oxide-derived reactive species in vascular cells. Circ Res. 2002;91:375–381. doi: 10.1161/01.res.0000032114.68919.ef. [DOI] [PubMed] [Google Scholar]
- Collins S. The HL-60 promyelocytic leukemia cell line: proliferation, differentiation, and cellular oncogene expression. Blood. 1987;70:1233–1244. [PubMed] [Google Scholar]
- DeLeo FR, Allen LA, Apicella M, Nauseef WM. NADPH oxidase activation and assembly during phagocytosis. J Immunol. 1999;163:6732–6740. [PubMed] [Google Scholar]
- Falck J, Lumin S, Blair I, Dishman E, Martin M, Waxman D, Guengerich F, Capdevila J. Cytochrome P-450-dependent oxidation of arachidonic acid to 16-, 17-, and 18-hydroxyeicosatetraenoic acids. J Biol Chem. 1990;265:10244–10249. [PubMed] [Google Scholar]
- Faust LR, el Benna J, Babior BM, Chanock SJ. The phosphorylation targets of p47phox, a subunit of the respiratory burst oxidase. Functions of the individual target serines as evaluated by site-directed mutagenesis. J Clin Invest. 1995;96:1499–1505. doi: 10.1172/JCI118187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferullo JM, Nespoulous L. Two-dimensional electrophoresis of plant proteins with phastsystem using nonequilibrium pH gradient separation. Anal Biochem. 1991;198:131–133. doi: 10.1016/0003-2697(91)90516-v. [DOI] [PubMed] [Google Scholar]
- Gewirtz AT, Fokin VV, Petasis NA, Serhan CN, Madara JL. LXA4, aspirin-triggered 15-epi-LXA4, and their analogs selectively downregulate PMN azurophilic degranulation. Am J Physiol. 1999;276:C988–C994. doi: 10.1152/ajpcell.1999.276.4.C988. [DOI] [PubMed] [Google Scholar]
- Greene J, Newman J, Williamson K, Hammock B. Toxicity of epoxy fatty acids and related compounds to cells expressing human soluble epoxide hydrolase. Chem Res Toxicol. 2000a;13:217–226. doi: 10.1021/tx990162c. [DOI] [PubMed] [Google Scholar]
- Greene J, Williamson K, Newman J, Morisseau C, Hammock B. Metabolism of monoepoxides of methyl linoleate: bioactivation and detoxification. Arch Biochem Biophys. 2000b;376:420–432. doi: 10.1006/abbi.2000.1753. [DOI] [PubMed] [Google Scholar]
- Hachicha M, Pouliot M, Petasis NA, Serhan CN. Lipoxin (LX)A4 and aspirin-triggered 15-epi-LXA4 inhibit tumor necrosis factor 1-alpha-initiated neutrophil responses and trafficking: regulators of a cytokine-chemokine axis. J Exp Med. 1999;189:1923–1930. doi: 10.1084/jem.189.12.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton MB, Kettle AJ, Winterbourn CC. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood. 1998;92:3007–3017. [PubMed] [Google Scholar]
- Hardy S, Ferrante A, Poulos A, Robinson B, Johnson D, Murray A. Effect of exogenous fatty acids with greater than 22 carbon atoms (very long chain fatty acids) on superoxide production by human neutrophils. J Immunol. 1994;153:1754–1761. [PubMed] [Google Scholar]
- Harrell MD, Stimers JR. Differential effects of linoleic acid metabolites on cardiac sodium current. J Pharmacol Exp Ther. 2002;303:347–355. doi: 10.1124/jpet.102.038166. [DOI] [PubMed] [Google Scholar]
- Hata K, Ito T, Takeshige K, Sumimoto H. Anionic amphiphile-independent activation of the phagocyte NADPH oxidase in a cell-free system by p47phox and p67phox, both in C terminally truncated forms. Implication for regulatory Src homology 3 domain-mediated interactions. J Biol Chem. 1998;273:4232–4236. doi: 10.1074/jbc.273.7.4232. [DOI] [PubMed] [Google Scholar]
- Hayakawa M, Kosaka K, Sugiyama S, Yokoo K, Aoyama H, Izawa Y, Ozawa T. Proposal of leukotoxin, 9,10-epoxy-12-octadecenoate, as a burn toxin. Biochem Int. 1990;21:573–579. [PubMed] [Google Scholar]
- Hayakawa M, Sugiyama S, Takamura T, Yokoo K, Iwata M, Suzuki K, Taki F, Takahashi S, Ozawa T. Neutrophils biosynthesize leukotoxin, 9, 10-epoxy-12-octadecenoate. Biochem Biophys Res Commun. 1986;137:424–430. doi: 10.1016/0006-291x(86)91227-1. [DOI] [PubMed] [Google Scholar]
- Hu J, Taki F, Sugiyama S, Asai J, Izawa Y, Satake T, Ozawa T. Neutrophil-derived epoxide, 9,10-epoxy-12-octadecenoate, induces pulmonary edema. Lung. 1988;166:327–337. doi: 10.1007/BF02714065. [DOI] [PubMed] [Google Scholar]
- Inanami O, Johnson JL, McAdara JK, Benna JE, Faust LR, Newburger PE, Babior BM. Activation of the leukocyte NADPH oxidase by phorbol ester requires the phosphorylation of p47PHOX on serine 303 or 304. J Biol Chem. 1998;273:9539–9543. doi: 10.1074/jbc.273.16.9539. [DOI] [PubMed] [Google Scholar]
- Ishizaki T, Ozawa T, Voelkel N. Leukotoxins and the lung. Pulm Pharmacol Ther. 1999;12:145–155. doi: 10.1006/pupt.1999.0179. [DOI] [PubMed] [Google Scholar]
- Ishizaki T, Shigemori K, Nakai T, Miyabo S, Ozawa T, Chang S, Voelkel N. Leukotoxin, 9,10-epoxy-12-octadecenoate causes edematous lung injury via activation of vascular nitric oxide synthase. Am J Physiol. 1995;269:L65–L70. doi: 10.1152/ajplung.1995.269.1.L65. [DOI] [PubMed] [Google Scholar]
- Johnson JL, Park JW, Benna JE, Faust LP, Inanami O, Babior BM. Activation of p47(PHOX), a cytosolic subunit of the leukocyte NADPH oxidase. Phosphorylation of ser-359 or ser-370 precedes phosphorylation at other sites and is required for activity. J Biol Chem. 1998;273:35147–35152. doi: 10.1074/jbc.273.52.35147. [DOI] [PubMed] [Google Scholar]
- Johnson MM, Vaughn B, Triggiani M, Swan DD, Fonteh AN, Chilton FH. Role of arachidonyl triglycerides within lipid bodies in eicosanoid formation by human polymorphonuclear cells. Am J Respir Cell Mol Biol. 1999;21:253–258. doi: 10.1165/ajrcmb.21.2.3489. [DOI] [PubMed] [Google Scholar]
- Kerkhoff C, Klempt M, Kaever V, Sorg C. The two calcium-binding proteins, S100A8 and S100A9, are involved in the metabolism of arachidonic acid in human neutrophils. J Biol Chem. 1999;274:32672–32679. doi: 10.1074/jbc.274.46.32672. [DOI] [PubMed] [Google Scholar]
- Kerkhoff C, Nacken W, Benedyk M, Dagher MC, Sopalla C, Doussiere J. The arachidonic acid-binding protein S100A8/A9 promotes NADPH oxidase activation by interaction with p67phox and rac-2. FASEB J. 2005;19:467–469. doi: 10.1096/fj.04-2377fje. [DOI] [PubMed] [Google Scholar]
- Korchak HM, Rossi MW, Kilpatrick LE. Selective role for beta-protein kinase C in signaling for O−2 generation but not degranulation or adherence in differentiated HL60 cells. J Biol Chem. 1998;273:27292–27299. doi: 10.1074/jbc.273.42.27292. [DOI] [PubMed] [Google Scholar]
- Kosaka K, Suzuki K, Hayakawa M, Sugiyama S, Ozawa T. Leukotoxin, a linoleate epoxide: its implication in the late death of patients with extensive burns. Mol Cell Biochem. 1994;139:141–148. doi: 10.1007/BF01081737. [DOI] [PubMed] [Google Scholar]
- Li Y, Ferrante A, Poulos A, Harvey D. Neutrophil oxygen radical generation. Synergistic responses to tumor necrosis factor and mono/polyunsaturated fatty acids. J Clin Invest. 1996;97:1605–1609. doi: 10.1172/JCI118585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhu H, Kuppusamy P, Roubaud V, Zweier J, Trush M. Validation of lucigenin (bis-N-methylacridinium) as a chemilumigenic probe for detecting superoxide anion radical production by enzymatic and cellular systems. J Biol Chem. 1998;273:2015–2023. doi: 10.1074/jbc.273.4.2015. [DOI] [PubMed] [Google Scholar]
- Lopes LR, Dagher MC, Gutierrez A, Young B, Bouin AP, Fuchs A, Babior BM. Phosphorylated p40PHOX as a negative regulator of NADPH oxidase. Biochemistry. 2004;43:3723–3730. doi: 10.1021/bi035636s. [DOI] [PubMed] [Google Scholar]
- Markaverich BM, Crowley JR, Alejandro MA, Shoulars K, Casajuna N, Mani S, Reyna A, Sharp J. Leukotoxin diols from ground corncob bedding disrupt estrous cyclicity in rats and stimulate MCF-7 breast cancer cell proliferation. Environ Health Perspect. 2005;113:1698–1704. doi: 10.1289/ehp.8231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhail LC, Clayton CC, Snyderman R. A potential second messenger role for unsaturated fatty acids: activation of Ca2+-dependent protein kinase. Science. 1984;224:622–625. doi: 10.1126/science.6231726. [DOI] [PubMed] [Google Scholar]
- Moghaddam M, Grant D, Cheek J, Greene J, Williamson K, Hammock B. Bioactivation of leukotoxins to their toxic diols by epoxide hydrolase. Nat Med. 1997;3:562–566. doi: 10.1038/nm0597-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran J, Weise R, Schnellmann R, Freeman J, Grant D. Cytotoxicity of linoleic acid diols to renal proximal tubular cells. Toxicol Appl Pharmacol. 1997;146:53–59. doi: 10.1006/taap.1997.8197. [DOI] [PubMed] [Google Scholar]
- Nauseef WM, Volpp BD, Clark RA. Immunochemical and electrophoretic analyses of phosphorylated native and recombinant neutrophil oxidase component p47-phox. Blood. 1990;76:2622–2629. [PubMed] [Google Scholar]
- Newman JW, Watanabe T, Hammock BD. The simultaneous quantification of cytochrome P450 dependent linoleate and arachidonate metabolites in urine by HPLC-MS/MS. J Lipid Res. 2002;43:1563–1578. doi: 10.1194/jlr.d200018-jlr200. [DOI] [PubMed] [Google Scholar]
- O’Flaherty JT, Nishihira J. 5-Hydroxyeicosatetraenoate promotes Ca2+ and protein kinase C mobilization in neutrophils. Biochem Biophys Res Commun. 1987;148:575–581. doi: 10.1016/0006-291x(87)90915-6. [DOI] [PubMed] [Google Scholar]
- Okamura N, Malawista SE, Roberts RL, Rosen H, Ochs HD, Babior BM, Curnutte JT. Phosphorylation of the oxidase-related 48k phosphoprotein family in the unusual autosomal cytochrome-negative and X-linked cytochrome-positive types of chronic granulomatous disease. J Biol Chem. 1988;263:6777–6782. [PubMed] [Google Scholar]
- Okamura S, Ameshima S, Demura Y, Ishizaki T, Matsukawa S, Miyamori I. Leukotoxin-activated human pulmonary artery endothelial cell produces nitric oxide and superoxide anion. Pulm Pharmacol Ther. 2002;15:25–33. doi: 10.1006/pupt.2001.0322. [DOI] [PubMed] [Google Scholar]
- Ozawa T, Sugiyama S, Hayakawa M, Satake T, Taki F, Iwata M, Taki K. Existence of leukotoxin 9,10-epoxy-12-octadecenoate in lung lavages from rats breathing pure oxygen and from patients with the adult respiratory distress syndrome. Am Rev Respir Dis. 1988a;137:535–540. doi: 10.1164/ajrccm/137.3.535. [DOI] [PubMed] [Google Scholar]
- Ozawa T, Sugiyama S, Hayakawa M, Taki F, Hanaki Y. Neutrophil microsomes biosynthesize linoleate epoxide (9,10-epoxy-12-octadecenoate), a biological active substance. Biochem Biophys Res Commun. 1988b;152:1310–1318. doi: 10.1016/s0006-291x(88)80428-5. [DOI] [PubMed] [Google Scholar]
- Pratt P, Rosolowsky M, Campbell W. Effects of epoxyeicosatrienoic acids on polymorphonuclear leukocyte function. Life Sci. 2002;70:2521–2533. doi: 10.1016/s0024-3205(02)01533-3. [DOI] [PubMed] [Google Scholar]
- Quinn MT, Gauss KA. Structure and regulation of the neutrophil respiratory burst oxidase: comparison with nonphagocyte oxidases. J Leukoc Biol. 2004;76:760–781. doi: 10.1189/jlb.0404216. [DOI] [PubMed] [Google Scholar]
- Raddassi K, Murray JJ. Ethanol increases superoxide anion production stimulated with 4β-phorbol 12-myristate 13-acetate in human polymorphonuclear leukocytes: involvement of protein kinase C. Eur J Biochem. 2000;267:720–727. doi: 10.1046/j.1432-1327.2000.01048.x. [DOI] [PubMed] [Google Scholar]
- Reeves EP, Dekker LV, Forbes LV, Wientjes FB, Grogan A, Pappin DJ, Segal AW. Direct interaction between p47phox and protein kinase C: evidence for targeting of protein kinase C by p47phox in neutrophils. Biochem J. 1999;344(Pt 3):859–866. [PMC free article] [PubMed] [Google Scholar]
- Rotrosen D, Leto TL. Phosphorylation of neutrophil 47-kDa cytosolic oxidase factor. Translocation to membrane is associated with distinct phosphorylation events. J Biol Chem. 1990;265:19910–19915. [PubMed] [Google Scholar]
- Schmidt A, Vogel RL, Witherup KM, Rutledge SJ, Pitzenberger SM, Adam M, Rodan GA. Identification of fatty acid methyl ester as naturally occurring transcriptional regulators of the members of the peroxisome proliferator-activated receptor family. Lipids. 1996;31:1115–1124. doi: 10.1007/BF02524285. [DOI] [PubMed] [Google Scholar]
- Segal AW. How neutrophils kill microbes. Annu Rev Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevanian A, Mead J, Stein R. Epoxides as products of lipid autoxidation in rat lungs. Lipids. 1979;14:634–643. doi: 10.1007/BF02533449. [DOI] [PubMed] [Google Scholar]
- Shinomura T, Asaoka Y, Oka M, Yoshida K, Nishizuka Y. Synergistic action of diacylglycerol and unsaturated fatty acid for protein kinase C activation: its possible implications. Proc Natl Acad Sci USA. 1991;88:5149–5153. doi: 10.1073/pnas.88.12.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegfried M, Aoki N, Lefer A, Elisseou E, Zipkin R. Direct cardiovascular actions of two metabolites of linoleic acid. Life Sci. 1990;46:427–433. doi: 10.1016/0024-3205(90)90086-7. [DOI] [PubMed] [Google Scholar]
- Sisemore M, Zheng J, Yang J, Thompson D, Plopper C, Cortopassi G, Hammock B. Cellular characterization of leukotoxin diol-induced mitochondrial dysfunction. Arch Biochem Biophys. 2001;392:32–37. doi: 10.1006/abbi.2001.2434. [DOI] [PubMed] [Google Scholar]
- Smith KR, Pinkerton KE, Watanabe T, Pedersen TL, Ma SJ, Hammock BD. Attenuation of tobacco smoke-induced lung inflammation by treatment with a soluble epoxide hydrolase inhibitor. Proc Natl Acad Sci USA. 2005;102:2186–2191. doi: 10.1073/pnas.0409591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Justen JM, Nidy EG, Sam LM, Bleasdale JE. Transmembrane signaling in human polymorphonuclear neutrophils: 15(S)-hydroxy-(5Z,8Z,11Z,13E)-eicosatetraenoic acid modulates receptor agonist-triggered cell activation. Proc Natl Acad Sci USA. 1993;90:7270–7274. doi: 10.1073/pnas.90.15.7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbeck MJ, Robinson JM, Karnovsky MJ. Activation of the neutrophil NADPH-oxidase by free fatty acids requires the ionized carboxyl group and partitioning into membrane lipid. J Leukoc Biol. 1991;49:360–368. doi: 10.1002/jlb.49.4.360. [DOI] [PubMed] [Google Scholar]
- Sugiyama S, Hayakawa M, Nagai S, Ajioka M, Ozawa T. Leukotoxin, 9, 10-epoxy-12-octadecenoate, causes cardiac failure in dogs. Life Sci. 1987;40:225–231. doi: 10.1016/0024-3205(87)90336-5. [DOI] [PubMed] [Google Scholar]
- Sumimoto H, Kage Y, Nunoi H, Sasaki H, Nose T, Fukumaki Y, Ohno M, Minakami S, Takeshige K. Role of Src homology 3 domains in assembly and activation of the phagocyte NADPH oxidase. Proc Natl Acad Sci USA. 1994;91:5345–5349. doi: 10.1073/pnas.91.12.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AS, Berridge MV. Superoxide produced by activated neutrophils efficiently reduces the tetrazolium salt, WST-1 to produce a soluble formazan: a simple colorimetric assay for measuring respiratory burst activation and for screening anti-inflammatory agents. J Immunol Methods. 2000;238:59–68. doi: 10.1016/s0022-1759(00)00156-3. [DOI] [PubMed] [Google Scholar]
- Tiberghien F, Didier A, Bohbot A, Loor F. The multiscreen filtration system to measure chemoattractant-induced release of leukocyte granule enzymes by differentiated HL-60 cells or normal human monocytes. J Immunol Methods. 1999;223:63–75. doi: 10.1016/s0022-1759(98)00202-6. [DOI] [PubMed] [Google Scholar]
- Totani Y, Saito Y, Ishizaki T, Sasaki F, Ameshima S, Miyamori I. Leukotoxin and its diol induce neutrophil chemotaxis through signal transduction different from that of fMLP. Eur Respir J. 2000;15:75–79. doi: 10.1183/09031936.00.15107500. [DOI] [PubMed] [Google Scholar]
- Viswanathan S, Hammock BD, Newman JW, Meerarani P, Toborek M, Hennig B. Involvement of CYP 2C9 in mediating the proinflammatory effects of linoleic acid in vascular endothelial cells. J Am Coll Nutr. 2003;22:502–510. doi: 10.1080/07315724.2003.10719328. [DOI] [PubMed] [Google Scholar]
- Wenzel-Seifert K, Grunbaum L, Seifert R. Differential inhibition of human neutrophil activation by cyclosporins A, D, and H. Cyclosporin H is a potent and effective inhibitor of formyl peptide-induced superoxide formation. J Immunol. 1991;147:1940–1946. [PubMed] [Google Scholar]
- Wright M, Bieser KJ, Kinnunen PM, Lange LG. Nonoxidative ethanol metabolism in human leukocytes: detection of fatty acid ethyl ester synthase activity. Biochem Biophys Res Commun. 1987;142:979–985. doi: 10.1016/0006-291x(87)91510-5. [DOI] [PubMed] [Google Scholar]
- Xie MS, Jacobs LS, Dubyak GR. Regulation of phospholipase D and primary granule secretion by P2-purinergic- and chemotactic peptide-receptor agonists is induced during granulocytic differentiation of HL-60 cells. J Clin Invest. 1991;88:45–54. doi: 10.1172/JCI115303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano K, Hattori H, Imai A, Nozawa Y. Modification of positional distribution of fatty acids in phosphatidylinositol of rabbit neutrophils stimulated with formylmethionyl-leucyl-phenylalanine. Biochim Biophys Acta. 1983;752:137–144. doi: 10.1016/0005-2760(83)90241-2. [DOI] [PubMed] [Google Scholar]
- Yao Y, Redl H, Bahrami S, Schlag G. The inflammatory basis of trauma/shock-associated multiple organ failure. Inflamm Res. 1998;47:201–210. doi: 10.1007/s000110050318. [DOI] [PubMed] [Google Scholar]
- Zhang W, Nagao M, Takatori T, Iwadate K, Itakura Y, Yamada Y, Iwase H, Oono T. Immunohistochemical dynamics of leukotoxin (9,10-epoxy-12-octadecenoic acid) in lungs of rats. Int J Legal Med. 1995;107:174–178. doi: 10.1007/BF01428400. [DOI] [PubMed] [Google Scholar]
- Zheng J, Plopper CG, Lakritz J, Storms DH, Hammock BD. Leukotoxin-diol: a putative toxic mediator involved in acute respiratory distress syndrome. Am J Respir Cell Mol Biol. 2001;25:434–438. doi: 10.1165/ajrcmb.25.4.4104. [DOI] [PubMed] [Google Scholar]
- Zhou H, Duncan RF, Robison TW, Gao L, Forman HJ. Ca2+-dependent p47phox translocation in hydroperoxide modulation of the alveolar macrophage respiratory burst. Am J Physiol. 1997;273:L1042–L1047. doi: 10.1152/ajplung.1997.273.5.L1042. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Schieber EB, McGiff JC, Balazy M. Identification of arachidonate P-450 metabolites in human platelet phospholipids. Hypertension. 1995;25:854–859. doi: 10.1161/01.hyp.25.4.854. [DOI] [PubMed] [Google Scholar]
- Zor T, Selinger Z. Linearization of the Bradford protein assay increases its sensitivity: theoretical and experimental studies. Anal Biochem. 1996;236:302–308. doi: 10.1006/abio.1996.0171. [DOI] [PubMed] [Google Scholar]
- Zurek G, Gee SJ, Hammock BD. Development of an enzyme immunoassay for linoleic acid diols in urine. Anal Chim Acta. 2002;466:247–256. [Google Scholar]


