Abstract
Neurotrophic factors, including basic fibroblast growth factor (FGF-2) and brain derived neurotrophic factor (BDNF) are known to be affected by exposure to stressful experiences. Here, we examine the effects of behaviorally controllable (escapable tailshock, ES) or uncontrollable (inescapable tailshock, IS) stress on the expression of FGF-2 and BDNF mRNA in subregions of the medial prefrontal cortex (mPFC) and the hippocampal formation (HF) of male Sprague-Dawley rats. ES rats were placed in Plexiglas boxes equipped with a free spinning wheel & IS rats were placed in identical boxes with the wheels fixed. ES & IS rats were yoked such that they received the same tailshocks, but the ES rat could terminate each shock for both rats. No stress controls (NS) remained in their home cages. Rats were sacrificed 0, 2, 24, or 72 hr after termination of the stress session. In situ hybridization was performed to measure FGF-2 and BDNF mRNA in the mPFC and HF. In the mPFC, ES produced a significant increase in FGF-2 mRNA expression in 0 and 2 hr post-stress. In the HF, ES produced a greater increase in FGF-2 mRNA expression than IS and NS only in CA2. ES also produced an increase in BDNF mRNA expression in the anterior cingulate at 0 hr post-stress. No effects of stressor controllability on BDNF were observed in the HF, although both ES and IS decreased BDNF mRNA in the DG. FGF-2 in the mPFC may be involved in emotional regulation (“coping”) during stressful experiences.
INTRODUCTION
A perceived lack of control over adverse experiences has been proposed to be a predisposing factor in the development of stress-related disorders such as post-traumatic stress disorder (PTSD)22, 39 and depression41. Work with animal models has revealed that behavioral control over a stressor can confer resilience to many of the negative effects of stressor exposure 25. The influence of stressor controllability has been studied in animals by comparing the effects of escapable shocks (ES) with exactly identical yoked inescapable shocks (IS) 23. IS produces a constellation of behavioral changes that do not follow ES, a phenomenon called learned helplessness 25. Electric shocks are used as the stressor because it is difficult to manipulate the behavioral control that an organism has over other stressors in a manner such that the animals with and without control are exposed to physically identical events. Inhibition of the mPFC during exposure to ES or IS completely blocks the protective effects of control over the stressor on subsequent behaviors that are known to be differentially affected by IS and ES 1, suggesting that the mPFC mediates the protective effects of control. The hippocampal formation (HF) is also differentially affected by IS, compared to ES. We have observed that IS (but not ES) reduces neurogenesis in the HF, and that ES (but not IS) increases protein levels of the neurotrophic factor basic fibroblast growth factor (FGF-2) in the HF 7. Moreover, uncontrollable swim stress reduces LTP in the CA1 region of the HF to a greater extent than does controllable swim stress 18.
Neurotrophic factors, including both FGF-2 and BDNF, have been implicated in stress related mood disorders such as depression 10, 36, and FGF-2 mRNA is decreased in the HF of patients with major depressive disorder (MDD) 14. FGF-2 27 and BDNF 38 are known to be sensitive to acute stressor exposure, in particular in the hippocampus. For example, FGF-2 mRNA levels increase in the HF, but not the PFC, in response to acute restraint stress 27. Hippocampal BDNF has been proposed to be involved in mediating the effects of IS and learned helplessness 37, 40.
Less is known about how stress influences neurotrophic factor expression in the mPFC, or how neurotropic factors in the mPFC might be influenced by the controllability of the stressor. We 6 have previously observed that in male (but not female) rats BDNF mRNA expression is increased in the mPFC after IS. BDNF mRNA expression is also known to increase in the mPFC in response to restraint 28 and immobilization stress 20, but acute restraint stress does not alter FGF-2 mRNA levels in the PFC 13. However, the role of FGF-2 in the PFC is of particular clinical interest because patients with major depressive disorder exhibit decreased FGF-2 mRNA in both the dorsolateral PFC and the anterior cingulate, and this decrease is attenuated by antidepressant treatment 11. The mPFC is known to mediate the impact of stressor controllability on both brain and behavior 1, yet no studies to date have examined the impact of stressor controllability on the expression of neurotrophins in the mPFC.
To investigate the effects on controllable and uncontrollable stressors on expression of FGF-2 and BDNF mRNA in subdivisions of the HF and mPFC, we exposed rats to yoked IS or ES or to no-stress control treatment (NS). Rats were sacrificed 0, 2, 24, or 72 hr after the end of the stressor session. Using in situ hybridization we examined BDNF and FGF-2 mRNA expression in the anterior cingulate (AC), prelimbic (PL), and infralimbic (IL) regions of the mPFC and in the CA1, CA2, and dentate gyrus (DG) subfields of the HF. Despite the interest in BDNF and FGF-2, there are no prior studies that have directly compared the effects of physically equated controllable and uncontrollable stressors on these mRNAs.
METHODS
Subjects
Subjects were 75 male Sprague-Dawley rats (Harlan Labs, Madison WI) weighing 275–325 g. and housed 2 per cage on a 12 hr light/dark cycle (on at 0700 and off at 1900). Experiments were conducted between 0800–1200 hr. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Colorado at Boulder; approvals can be provided upon request. All efforts were taken to minimize the number of animals used and to minimize suffering.
Wheel-turn escape/yoked inescapable shock procedure
Each rat was placed in a Plexiglas box (14 × 11 × 17 cm) with a wheel mounted in the front and a Plexiglas rod extending from the back. The rat’s tails were taped to the Plexiglas rod and affixed with copper electrodes. Rats received shocks in yoked pairs (ES and IS). The treatment consisted of 80 trials with an average inter-trial interval of 60 s. Shocks began simultaneously for both rats in a pair and terminated for both whenever the ES rat met a response criterion. Initially the shock was terminated by a one-quarter turn of the wheel. The response requirement was increased by one-quarter turn when each of 3 consecutive trials was completed in less than 5 s. Subsequent latencies under 5 s increased the requirement by 50% up to a maximum of 4 full turns. If the requirement was not reached in less than 30 s, the shock was terminated and the requirement reduced to a single one-quarter turn. This procedure was used to insure that the ES animals learned an operant response. Any rat (along with its yoked partner) that did not learn the response within 10 trials was eliminated from the experiment. Shock intensity was 1.0 mA for the first 27 trials, 1.3 mA for the second 27 trials, and 1.6 mA for the last 26 trials. We have previously used this procedure 1, 7 to insure that ES rats do not habituate to the shocks and so continue to turn the wheel. Using this procedure, no rats failed to adequately learn the escape response. Nonshocked control (NS) rats remained undisturbed in the colony.
Tissue collection
Rats were sacrificed 0, 2, 24, or 72 hr following the stress session, or at equivalent times for the NS controls. Rats in the 2, 24, and 72 hr groups were returned to their home cages before sacrifice. All animals were assigned to groups as cage-mate pairs such that, upon removal from or return to the home cage, no rat was left isolated. Rats were sacrificed by rapid decapitation. Trunk blood was collected in heparinized tubes and placed in wet ice. Brains were immediately removed, rapidly frozen in dry ice chilled isopentane, and placed in a −80°C freezer for later analysis. Brain sections (10 μm) were taken using a −20°C cryostat and thaw mounted on poly-l-lysine coated slides. Brain sections were returned to the −80°C freezer after thaw-mounting.
In situ hybridization
Sections were fixed in a buffered 4% paraformaldehyde solution for 1 h at room temperature. Slides were washed in 2X saline sodium citrate (SSC) and acetylated in 0.1 M triethanolamine containing 0.25% acetic anhydride for 10 min to minimize nonspecific hybridization by reducing positive charge on tissue and polylysine coated slides. Slides were then washed again in distilled water, dehydrated in a series of graded ethyl alcohol concentrations, and air dried. 35S-UTP/35S-CTP labelled cRNA probes were generated for FGF-2 or BDNF mRNA from cDNA subclones in transcription vectors using standard in vitro transcription methodology. FGF-2 and BDNF cDNA was kindly provided by Dr. Huda Akil (The University of Michigan School of Medicine, Ann Arbor, MI, USA) and Dr. James. Herman, (University of Cincinnati Medical Center, Cincinnati, OH, USA), respectively.
To generate 35S-labeled complementary RNA to FGF-2 OR BDNF mRNA, 1 μg of linearized plasmid DNA; 1× T3 transcription buffer (Promega); 125 μC of 35S-UTP; 4 μl of H2O; 12.5 mM dithiothreitol (DTT); 150 μM GTP, CTP, and ATP; 20 U of RNase inhibitor; and 6 U T3 polymerase in a total volume of 25 μl were incubated for ~2 h at 37 °C. To isolate the complete complementary RNA from single nucleotides, a Sephadex G50-50 column was used. The 35S-labeled probe was diluted in hybridization buffer to yield an approximate concentration of 1×106 cpm/65 μl. The hybridization buffer consisted of 50% formamide, 10% dextran sulfate, 2× SSC, 50 mM sodium phosphate buffer (pH=7.4), 1× Denhardt's solution, and 0.1 mg/ml yeast tRNA. The radiolabeled probe/hybridization mixture (65 μl) was applied to each slide, and sections were coverslipped. Slides were placed in covered plastic boxes lined with filter paper moistened with 50% formamide/50% H2O and incubated for 12–16 h at 55 °C. Coverslips were floated off in 2× SSC, and slides were rinsed three times in 2× SSC. Slides were incubated in RNase A (200 μg/ml) for 60 min at 37 °C, followed by successive washes in 2×, 1×, 0.5×, and 0.1× SSC for 2–3 min each, with an additional incubation in 0.1× SSC for 60 min at 70 °C. Slides were rinsed in distilled H2O, dehydrated in alcohols, and exposed to BIOMAX MR X-ray film (Kodak, Rochester, NY, USA) for approximately 8 days.
Image analysis
Semi-quantitative analyses were performed for FGF-2 and BDNF mRNA expression. Images from X-ray films were scanned and digitized using Scion Image (NIH Image, http://rsb.info.nih.gov/nih-image/). See Figures 1 and 2 for example autoradiographs of sections labeled for FGF-2 mRNA (Fig 1) and BDNF mRNA (Fig 2) in the mPFC and HF.
Figure 1.
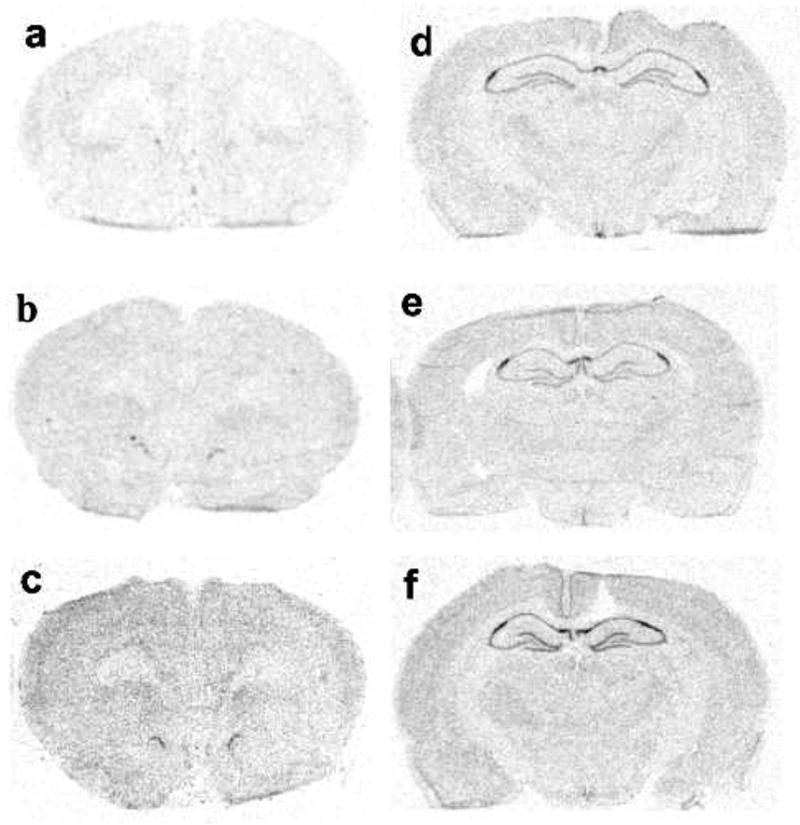
Representative autoradiographs of FGF-2 mRNA in the mPFC (a–c) and in the hippocampus (d–f). FGF-2 mRNA in no stress controls (a, d) and 2 hr after inescapable (b, e) or escapable stress (c, f).
Figure 2.
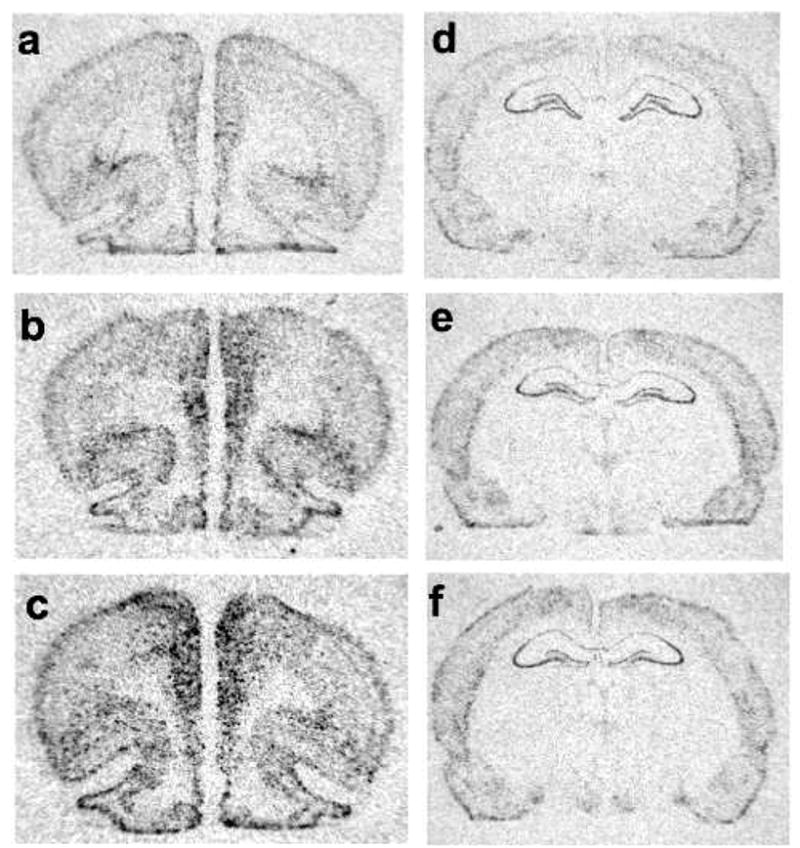
Representative autoradiographs of BDNF mRNA in the mPFC (a–c) and in the hippocampus (d–f). FGF-2 mRNA in NS controls (a, d) and 2 hr after IS (b, e) or ES (c, f).
Each region of interest was analyzed using a rectangle that was the same size for each slice, but differed in size for the various regions (in pixels: 35 × 35 for each mPFC region, 45 × 20 for CA2, 70 × 20 for CA1, and 50 × 35 for DG). Signal pixels of a region of interest were defined as having a gray value of 3.5 S.D. above the mean gray value of a cell-poor area close to the region of interest. The number of pixels and the average gray values above the set background were then computed for each region of interest and multiplied, giving an integrated densitometric measurement. An average of 6–8 measurements were made for each region of interest (DG, CA1, and CA2 regions of the hippocampus; and AC, PL, and IL regions of the mPFC), and these values were further averaged to obtain a single integrated density value per region for each rat. The specificity of the probe was confirmed in a control experiment by using a sense probe. No specific hybridization was observed in the sense-treated sections (data not shown).
Statistical analysis
Mean integrated density for each subregion was normalized as a percent of NS controls. Separate one-way ANOVAs were performed on FGF-2 and BDNF integrated density (percent control) within each subregion on HC and mPFC. Fisher’s LSD post-hoc tests were performed to determine differences between groups. Alpha was set at .05.
RESULTS
FGF-2 mRNA
In the mPFC, there were time-, region-, and stressor- dependent increases in FGF-2 mRNA expression after IS and ES (Fig 3). However, ES produced more rapid and robust increases than did IS. In AC (Fig 3a), there was a significant effect of group on FGF-2 expression, F (8, 66) = 4.23, p < .001. ES rats expressed more FGF-2 mRNA than did NS at 0 and 2 hr, and ES also produced a larger increase in FGF-2 mRNA than did IS at 2 hr, p < .05. There were no significant differences between IS and NS in AC. Thus, ES but not IS increased FGF-2 mRNA in AC. In PL (Fig 3b), there was also a significant effect of group on FGF-2 expression, F (8, 66) = 4.99, p < .001. ES rats expressed more FGF-2 mRNA than NS at 0 and 2 hr, and FGF-2 mRNA in IS rats was higher than NS only at 2 hr, p < .05. In addition, FGF-2 mRNA in ES subjects was higher than in IS subjects at 2 hr, p < .05. In IL (Fig 3c), there was also a significant effect of group, F (8, 66) = 4.04, p < .001. ES rats expressed more FGF-2 mRNA than did NS subjects at 0 and 2 hr, and IS rats expressed more FGF-2 mRNA than did NS only at 2 hr in IL, p < .05. In addition, ES subjects expressed more FGF-2 mRNA than IS at 0 hr in IL, p < .05. Thus, FGF-2 mRNA increased more rapidly and to a higher level after ES than after IS in IL and PL.
Figure 3.
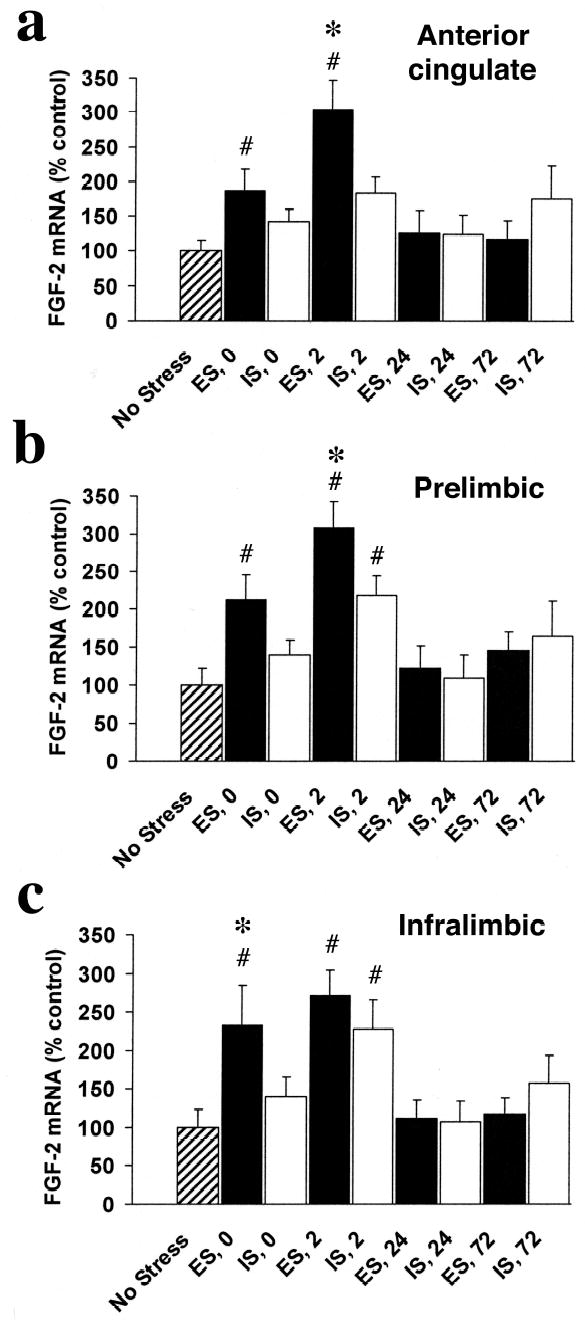
FGF-2 mRNA integrated density (percent of NS control) in subregions of the mPFC in rats exposed to escapable (ES) or inescapable stress (IS) and no stress controls (NS) at 0, 2, 24, and 72 hr after termination of the stress session in the anterior cingulate (a), prelimbic (b), and infralimbic (c) regions. FGF-2 mRNA expression increases in a time- and region- dependent manner in the mPFC after ES or IS. ES produced a faster (increased at 0 hr in all regions compared to NS) and greater increase of FGF-2 mRNA in subregions of the mPFC than did IS.
# greater than NS, p < .05
* greater than IS, p < .05
In the hippocampus, there were also time-, region-, and stressor- dependent increases in FGF-2 mRNA expression after IS and ES (Fig. 4). In CA1 (Fig 4a), there was a significant effect of group, F (8, 66) = 5.56, p < .001. ES led to more FGF-2 mRNA than did NS at 2 and 24 hr, and IS led to more FGF-2 mRNA than NS only at 24 hr. There were no significant differences between ES and IS in CA1, though there was a trend at 2 hr (p=.15). In CA2 (Fig 4b), there was also a significant effect of group, F (8, 66) = 10.60, p < .001. ES led to more FGF-2 mRNA than did NS at 2, 24, and 72 hr, and IS produced more FGF-2 mRNA than NS at 24 and 72 hr. In addition, ES induced more FGF-2 mRNA than IS at 2 hr, p < .05. In DG (Fig 4c), there was a significant effect of group, F (8, 66) = 3.96, p < .001. ES produced more FGF-2 mRNA than NS at 2 and 24 hr, and IS also led to more FGF-2 mRNA than NS at 2 and 24 hr. There were no significant differences between ES and IS in DG, though there was a trend at 2 hr (p=.15).
Figure 4.
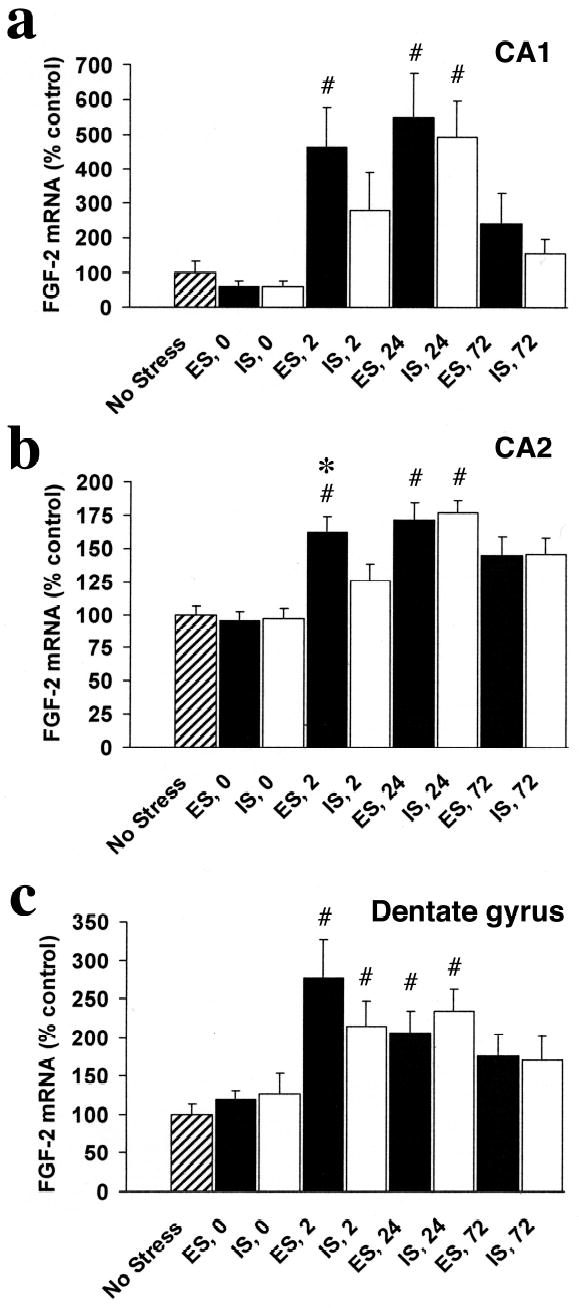
FGF-2 mRNA integrated density (percent of NS control) in subregions of the hippocampal formation (HF) in rats exposed to escapable (ES) or inescapable stress (IS) and no stress controls (NS) at 0, 2, 24, and 72 hr after termination of the stress session in the CA1 (a), CA2 (b), and dentate gyrus (c). FGF-2 mRNA expression increases in a time-, stressor-, and region- dependent manner in the hippocampus after ES or IS. ES produced a faster (increased at 0 hr in all regions compared to NS) and a greater increase FGF-2 in the CA2 subfield than did IS.
# greater than NS, p < .05
* greater than IS, p < .05
BDNF mRNA
In the mPFC, there were time-, region-, and stressor- dependent increases in BDNF mRNA expression after IS and ES (Fig 5). In AC (Fig 5a), there was a significant effect of group on BDNF expression, F (8, 66) = 5.81, p < .001. Both IS and ES led to more BDNF mRNA than NS at 0 and 2 hr. However, only the differences between ES and NS were statistically significant. In addition, ES expressed more BDNF mRNA than IS at 0 hr, p < .05. In PL (Fig 5b), there was also a significant effect of group on BDNF expression, F (8, 66) = 15.51, p < .001. Both IS and ES led to more BDNF mRNA than NS at 0 and 2 hr (p < .05). There were no significant differences between ES and IS in PL. In IL (Fig 5c), there was a significant effect of group on BDNF mRNA expression, F (8, 66) = 11.82, p < .001. Both IS and ES led to more BDNF mRNA than NS at 0 and 2 hr, p < .05. There were no significant differences between IS and ES in IL.
Figure 5.

BDNF mRNA integrated density (percent of NS control) in subregions of the mPFC in rats exposed to escapable (ES) or inescapable stress (IS) and no stress controls (NS) at 0, 2, 24, and 72 hr after termination of the stress session in the anterior cingulate (a), prelimbic (b), and infralimbic (c) regions. BDNF mRNA expression increases in a time- and region- dependent manner in the mPFC after ES or IS.
# greater than NS, p < .05
* greater than IS, p < .05
In the HF, there were time-dependent decreases in BDNF mRNA expression after both IS and ES, but only in the DG (Fig 6). There was no significant effect of group on BDNF mRNA in either CA1 (Fig 6a), or CA2 (Fig 6b). In the DG (Fig 6c), there was a significant effect of group on BDNF expression, F (8, 66) = 3.74, p < .001. Both ES and IS led to less BDNF mRNA relative to NS at 0, 2, and 24 hr, all p < .05. There were no significant differences between IS and ES in the DG.
Figure 6.
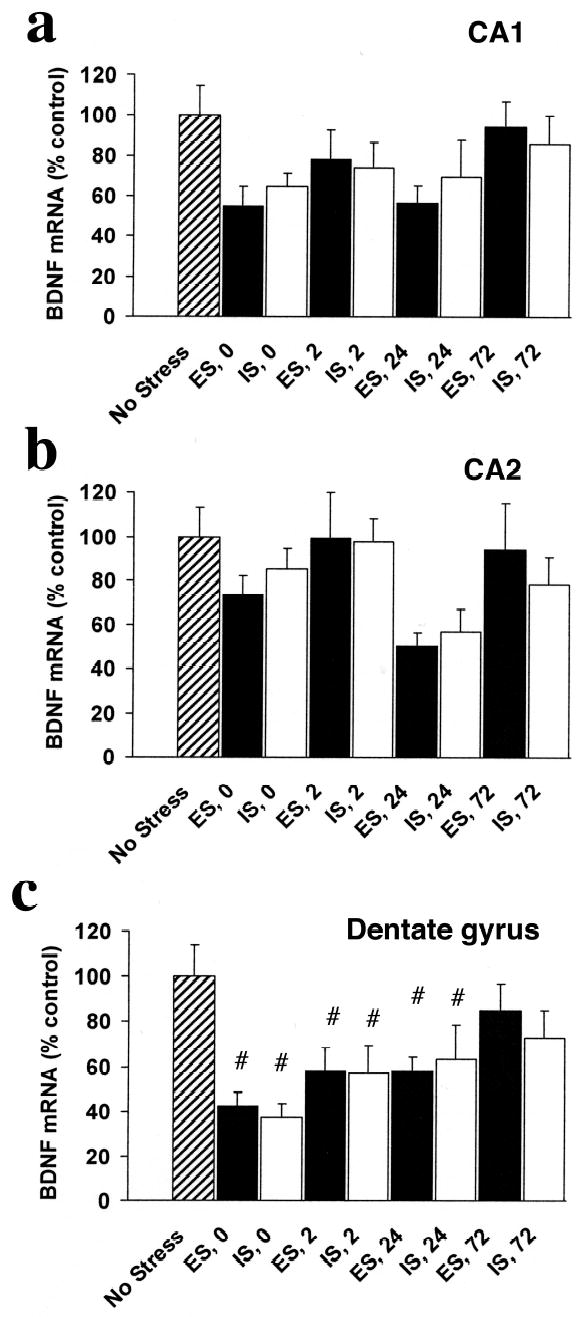
BDNF mRNA integrated density (percent of NS control) in subregions of the hippocampal formation (HF) in rats exposed to escapable (ES) or inescapable stress (IS) and no stress controls (NS) at 0, 2, 24, and 72 hr after termination of the stress session in the CA1 (a), CA2 (b), and dentate gyrus (c). BDNF mRNA expression decreased in the dentate gyrus after both ES and IS.
# less than NS, p < .05
DISCUSSION
The effects of controllable (ES) and uncontrollable (IS) tailshock on FGF-2 and BDNF mRNA expression in the HF and mPFC were examined with the use of in situ hybridization. The results indicate that IS and ES result in differential changes in BDNF and FGF-2 mRNA expression in the HF and mPFC that vary by region, subfield, and time post-stressor. Stressor controllability effects on neurotrophic factor mRNA were greater in the mPFC than in the HC. In the mPFC both IS and ES tended to increase, rather than decrease, BDNF and FGF-2 mRNA. Importantly, these increases occurred more rapidly and were larger after ES than after IS. Moreover, the impact of stressor controllability on neurotrophic factor gene expression in the PFC were more pronounced for FGF-2 than BDNF, with increases observed in all subregions at either 0 hr (IL) or 2 hr (AC and PL) post-ES. Increased BDNF was observed in the AC at 0 hr post-ES. Increases in FGF-2 mRNA were also observed in the HF, especially in the CA2 subfield, at 2 hr-post-ES, while no controllability effects were observed for BDNF mRNA in the HF. However, robust decreases in BDNF mRNA were observed in the DG in both groups from 0 hr to 24 hr post-stress.
It has been reported that exposure to an acute stressor, restraint, increases FGF-2 mRNA expression in brain regions including the HF 28. The present results are the first to report whether the behavioral controllability of the stressor modulates the impact of the stressor on FGF-2 mRNA expression in the mPFC. Although it is known that the stress-inducible adrenal hormone corticosterone can regulate neurotrophic factor expression 9, 16, 27, it is unlikely that the changes in FGF-2 reported here are related to corticosterone because IS and ES identical to that used here are known to produce equal increases in plasma corticosterone levels 24. FGF-2 mRNA expression increased preferentially after exposure to ES in all subregions of the mPFC soon after termination of the stressor. This increase was approximately 250% of control levels in the IL at 0 hr post-ES and 300% of control levels in the AC and PL regions at 2 hr post-ES. Although FGF-2 mRNA levels increased relative to NS controls post-IS, this increase was observed only in the PL and IL, and only 2 hr post-stress. In contrast, FGF-2 mRNA was increased in all subregions of the mPFC at both 0 and 2 hr post-ES, relative to NS control levels. Thus, ES produces not only a greater increase in FGF-2 mRNA expression than does IS, but also a more rapid increase in its expression. FGF-2 has a wide variety of neurotrophic and neuroprotective functions including facilitation of neurogenesis 19, angiogenesis43, axonal branching 2, and synaptogenesis21 and may therefore contribute to the adaptation to stressor exposure conferred by the ability to control the stressor by a behavioral response.
Recent work in our laboratory has highlighted the importance of the mPFC in mediating the protective effects of behavioral control on many of the consequences of exposure to a traumatic stressor. It is clear that activation of the mPFC is necessary for the benefits of behavioral control, because temporary inactivation of the ventral mPFC with muscimol blocks the behavioral and cellular effects provided by control over the stressor 1. Conversely, temporary activation of the mPFC during IS blocks the behavioral and neurochemical consequences of IS 31. Thus, activation of the mPFC during stressor exposure is central to the differential effects of IS and ES. Activation of the mPFC can act to inhibit the amygdala 32 and the dorsal raphe nucleus 1 and thus eliminate the sensitization to subsequent stressors produced by IS. Furthermore, lesions of the mPFC block instrumental contingency learning, which involves learning about control over performance by motivational states 3, as well as memory for previously extinguished conditioned fear 33. It is important to note that we have observed that inactivation of the mPFC does not affect the wheel turn escape learning per se 1, and others have shown that the periaquiductal gray mediates escape learning 15.
Control not only blunts the impact of the controllable stressor itself, but also blocks the effects of a subsequent uncontrollable stressor 42. This protective effects of ES on reactivity to stress is persistent, lasting at least one week after stressor exposure 31, suggesting that experience-dependent plasticity may be occurring as a result of exposure to ES. In support of this idea, protein synthesis in the mPFC is necessary for the protective effects of ES 31. One possibility is that mPFC activation by ES leads to structural plasticity via FGF-2 induction. FGF-2 is well known to mediate several types of structural plasticity, including synaptogenesis and neurite branching35. However, additional studies will be needed to directly test the possible role of mPFC FGF-2 in the protective effects of ES.
Expression of mRNA for BDNF, a well-known mediator of experience-dependent neuronal plasticity 5, was also increased in the mPFC in response to ES, but only in the AC, and only at 0 hr post-ES. We have shown that activation of the PL and IL regions of the mPFC are necessary for the effects of stressor controllability 1. Because BDNF mRNA expression was not differentially affected by IS and ES in either of these regions, it seems unlikely that BDNF is involved in mediating these effects. BDNF mRNA expression was increased after both IS and ES in the PL and IL for at least 2 hr post-stress. We have previously observed that after IS, BDNF mRNA expression increases in the mPFC and returns to control levels by 60 min post-stress in male rats6. Moreover, in the previous study BDNF mRNA levels increased significantly in the AC (about 225%) after IS while in the present study while in the present study BDNF increases did not reach significance in this group although there was a strong trend (about 205% increase, p = .07). These discrepancies are likely due to differences in the tailshock procedure used in the two studies. In the present study, 80 trials of yoked, incrementing intensity tailshock were administered, while in the previous study, 100 trials of inescapable tailshock (5 sec, 1 mA each) were administered while rats were restrained in tubes. Thus, the two experiments can not be directly compared.
In the HF, ES produced a significant increase in FGF-2 mRNA expression in CA2, but only weakly and non-significantly elevated FGF-2 mRNA in the other subfields, at 2 hr post-stress. We have previously observed that ES produces an increase in nuclear expression of FGF-2 protein in DG and CA1, but not CA2, of the HF, while IS does not 7. Several mechanisms can be responsible for differential expression patterns of FGF-2 mRNA vs nuclear mRNA protein. FGF-2 occurs in several isoforms that are translated from a common mRNA, thus the FGF-2 mRNA expression reported in the present study potentially represents transcription of all FGF-2 isoforms. However, FGF-2 protein expression in the nucleus can vary depending on the specific isoform. An 18 kDa isoform is located primarily in the cytosol 30, and there are several higher molecular weight isoforms that contain a nuclear localization sequence and thus are observed in the nucleus 34. The antibody used in Bland et al. 7 labels all of the FGF-2 isoforms. However, only nuclear signal was measured in that study so it is likely that only the high molecular weight isoforms were quantified. Iranslocation of the FGF-2 protein into the nucleus can vary depending on the cellular environment 17. For example, FGF-2 can be transported to the nucleus during phase G1 of the cell cycle 8, suggesting the possibility that the FGF-2 expression reported in Bland et al.7 was related to precursor cell proliferation. Finally, FGF-2 protein can be secreted 26, thus FGF-2 protein may exist in the extracellular space where it would not be detected using immunohistochemistry. It should be noted that increased FGF-2 mRNA was not detected post-ES (relative to IS) in a study using PCR on whole hippocampi12. This difference is likely due to the ability to accurately measure each HF subfield (and specific cell layers) when in situ hybridization is used.
Hippocampal BDNF has been proposed to play a part in learned helplessness 40, and infusion of BDNF into the HF decreases learned helplessness behaviors such as escape deficits in the shuttle box test 37. However, in that study learned helplessness was tested in the same environment in which exposure to the original stressor (inescapable footshocks) occurred 37. Thus it is possible that the BDNF microinjections, which were delivered surgically 2 days after the initial stressor, interfered with the memory for the context, rather than interfering with learned helplessness. BDNF mRNA levels decreased in the dentate gyrus from 0 to 24 hr after both ES and IS. This stress-induced decrease in BDNF mRNA expression in the DG is consistent with decreases in BDNF mRNA expression in the DG produced by various acute stressors that have been reported by our laboratory 4 and others 29, 38. Thus, stressor controllability did not modulate the BDNF reduction produced by the stressor.
In summary, the present results indicate that the neurotrophic factors FGF-2 and BDNF in the mPFC and HF are differentially regulated by controllable and uncontrollable stressors. FGF-2 is more responsive to behavioral control of a stressor than is BDNF, because expression of mRNA for FGF-2 was increased after controllable, relative to uncontrollable stress, in the mPFC as well as, to a lesser extent, the HF. This finding suggests that FGF-2 activation in the mPFC may be involved in coping with a traumatic stressor.
Acknowledgments
Supported by a NARSAD Young Investigator Award to S.T.B., and by NIH grants DA016004 (NRSA post-doc fellowhip to S.T.B), MH050479 (S.F.M) and DA1319 (S.F.M). We wish to thank Dr. Huda Akil for the generous gift of the FGF-2 plasmid and Dr. James Herman for the generous gift of the BDNF plasmid. We also thank Ashley Israel and Tiare Evans for assistance with the densitometry analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- 2.Aoyagi A, Nishikawa K, Saito H, Abe K. Characterization of basic fibroblast growth factor-mediated acceleration of axonal branching in cultured rat hippocampal neurons. Brain Res. 1994;661:117–126. doi: 10.1016/0006-8993(94)91188-6. [DOI] [PubMed] [Google Scholar]
- 3.Balleine BW, Dickinson A. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37:407–419. doi: 10.1016/s0028-3908(98)00033-1. [DOI] [PubMed] [Google Scholar]
- 4.Barrientos RM, Sprunger DB, Campeau S, Higgins EA, Watkins LR, Rudy JW, Maier SF. Brain-derived neurotrophic factor mRNA downregulation produced by social isolation is blocked by intrahippocampal interleukin-1 receptor antagonist. Neuroscience. 2003;121:847–853. doi: 10.1016/s0306-4522(03)00564-5. [DOI] [PubMed] [Google Scholar]
- 5.Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004;22:123–131. doi: 10.1080/08977190410001723308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bland ST, Schmid MJ, Der-Avakian A, Watkins LR, Spencer RL, Maier SF. Expression of c-fos and BDNF mRNA in subregions of the prefrontal cortex of male and female rats after acute uncontrollable stress. Brain Res. 2005;1051:90–99. doi: 10.1016/j.brainres.2005.05.065. [DOI] [PubMed] [Google Scholar]
- 7.Bland ST, Schmid MJ, Greenwood BN, Watkins LR, Maier SF. Behavioral control of the stressor modulates stress-induced changes in neurogenesis and fibroblast growth factor-2. Neuroreport. 2006;17:593–597. doi: 10.1097/00001756-200604240-00008. [DOI] [PubMed] [Google Scholar]
- 8.Bouche G, Gas N, Prats H, Baldin V, Tauber JP, Teissie J, Amalric F. Basic fibroblast growth factor enters the nucleolus and stimulates the transcription of ribosomal genes in ABAE cells undergoing G0----G1 transition. Proc Natl Acad Sci U S A. 1987;84:6770–6774. doi: 10.1073/pnas.84.19.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chao HM, Sakai RR, Ma LY, McEwen BS. Adrenal steroid regulation of neurotrophic factor expression in the rat hippocampus. Endocrinology. 1998;139:3112–3118. doi: 10.1210/endo.139.7.6114. [DOI] [PubMed] [Google Scholar]
- 10.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Evans SJ, Choudary PV, Neal CR, Li JZ, Vawter MP, Tomita H, Lopez JF, Thompson RC, Meng F, Stead JD, Walsh DM, Myers RM, Bunney WE, Watson SJ, Jones EG, Akil H. Dysregulation of the fibroblast growth factor system in major depression. Proc Natl Acad Sci U S A. 2004;101:15506–15511. doi: 10.1073/pnas.0406788101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frank M, Der Avakian A, Bland S, Watkins L, Maire S. Stress-induced glucocorticoids suppress the antisense molecular regulation of FGF-2 expression. Psychoneuroendocrinology. doi: 10.1016/j.psyneuen.2007.02.001. submitted. [DOI] [PubMed] [Google Scholar]
- 13.Fumagalli F, Bedogni F, Slotkin TA, Racagni G, Riva MA. Prenatal stress elicits regionally selective changes in basal FGF-2 gene expression in adulthood and alters the adult response to acute or chronic stress. Neurobiol Dis. 2005;20:731–737. doi: 10.1016/j.nbd.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Gaughran F, Payne J, Sedgwick PM, Cotter D, Berry M. Hippocampal FGF-2 and FGFR1 mRNA expression in major depression, schizophrenia and bipolar disorder. Brain Res Bull. 2006;70:221–227. doi: 10.1016/j.brainresbull.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Graeff FG, Guimaraes FS, De Andrade TG, Deakin JF. Role of 5-HT in stress, anxiety, and depression. Pharmacol Biochem Behav. 1996;54:129–141. doi: 10.1016/0091-3057(95)02135-3. [DOI] [PubMed] [Google Scholar]
- 16.Hansson AC, Cintra A, Belluardo N, Sommer W, Bhatnagar M, Bader M, Ganten D, Fuxe K. Gluco- and mineralocorticoid receptor-mediated regulation of neurotrophic factor gene expression in the dorsal hippocampus and the neocortex of the rat. Eur J Neurosci. 2000;12:2918–2934. doi: 10.1046/j.1460-9568.2000.00185.x. [DOI] [PubMed] [Google Scholar]
- 17.Johnson HM, Subramaniam PS, Olsnes S, Jans DA. Trafficking and signaling pathways of nuclear localizing protein ligands and their receptors. Bioessays. 2004;26:993–1004. doi: 10.1002/bies.20086. [DOI] [PubMed] [Google Scholar]
- 18.Kavushansky A, Vouimba RM, Cohen H, Richter-Levin G. Activity and plasticity in the CA1, the dentate gyrus, and the amygdala following controllable vs. uncontrollable water stress. Hippocampus. 2006;16:35–42. doi: 10.1002/hipo.20130. [DOI] [PubMed] [Google Scholar]
- 19.Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee Y, Duman RS, Marek GJ. The mGlu2/3 receptor agonist LY354740 suppresses immobilization stress-induced increase in rat prefrontal cortical BDNF mRNA expression. Neurosci Lett. 2006;398:328–332. doi: 10.1016/j.neulet.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 21.Li AJ, Suzuki S, Suzuki M, Mizukoshi E, Imamura T. Fibroblast growth factor-2 increases functional excitatory synapses on hippocampal neurons. Eur J Neurosci. 2002;16:1313–1324. doi: 10.1046/j.1460-9568.2002.02193.x. [DOI] [PubMed] [Google Scholar]
- 22.Livanou M, Basoglu M, Marks IM, De SP, Noshirvani H, Lovell K, Thrasher S. Beliefs, sense of control and treatment outcome in post-traumatic stress disorder. Psychol Med. 2002;32:157–165. doi: 10.1017/s0033291701004767. [DOI] [PubMed] [Google Scholar]
- 23.Maier SF. Learned helplessness and animal models of depression. Prog Neuropsychopharmacol Biol Psychiatry. 1984;8:435–446. [PubMed] [Google Scholar]
- 24.Maier SF, Ryan SM, Barksdale CM, Kalin NH. Stressor controllability and the pituitary-adrenal system. Behav Neurosci. 1986;100:669–674. doi: 10.1037//0735-7044.100.5.669. [DOI] [PubMed] [Google Scholar]
- 25.Maier SF, Seligman ME. Learned helplessness: Theory and evidence. Journal of Experimental Psychology: General. 1976;105:3–46. [Google Scholar]
- 26.Mignatti P, Morimoto T, Rifkin DB. Basic fibroblast growth factor released by single, isolated cells stimulates their migration in an autocrine manner. Proc Natl Acad Sci U S A. 1991;88:11007–11011. doi: 10.1073/pnas.88.24.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molteni R, Fumagalli F, Magnaghi V, Roceri M, Gennarelli M, Racagni G, Melcangi RC, Riva MA. Modulation of fibroblast growth factor-2 by stress and corticosteroids: from developmental events to adult brain plasticity. Brain Res Brain Res Rev. 2001;37:249–258. doi: 10.1016/s0165-0173(01)00128-x. [DOI] [PubMed] [Google Scholar]
- 28.Molteni R, Lipska BK, Weinberger DR, Racagni G, Riva MA. Developmental and stress-related changes of neurotrophic factor gene expression in an animal model of schizophrenia. Mol Psychiatry. 2001;6:285–292. doi: 10.1038/sj.mp.4000865. [DOI] [PubMed] [Google Scholar]
- 29.Murakami S, Imbe H, Morikawa Y, Kubo C, Senba E. Chronic stress, as well as acute stress, reduces BDNF mRNA expression in the rat hippocampus but less robustly. Neuroscience Research. 2005;53:129–139. doi: 10.1016/j.neures.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 30.Olsnes S, Klingenberg O, Wiedlocha A. Transport of exogenous growth factors and cytokines to the cytosol and to the nucleus. Physiol Rev. 2003;83:163–182. doi: 10.1152/physrev.00021.2002. [DOI] [PubMed] [Google Scholar]
- 31.Paul E, Amat J, Baratta M, Zarza C, Watkins LR, Maier SF. Activating the ventromedial prefrontal cortex (vmPFC) during uncontrollable stress blocks the usual consequences of uncontrollable stress. Society for Neuroscience abstract. 2006 doi: 10.1523/JNEUROSCI.3630-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Renko M, Quarto N, Morimoto T, Rifkin DB. Nuclear and cytoplasmic localization of different basic fibroblast growth factor species. J Cell Physiol. 1990;144:108–114. doi: 10.1002/jcp.1041440114. [DOI] [PubMed] [Google Scholar]
- 35.Reuss B, von Bohlen und Halbach O. Fibroblast growth factors and their receptors in the central nervous system. Cell Tissue Res. 2003;313:139–157. doi: 10.1007/s00441-003-0756-7. [DOI] [PubMed] [Google Scholar]
- 36.Riva MA, Molteni R, Bedogni F, Racagni G, Fumagalli F. Emerging role of the FGF system in psychiatric disorders. Trends Pharmacol Sci. 2005;26:228–231. doi: 10.1016/j.tips.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 37.Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995;15:1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Der Kolk BA. Clinical Implications of Neuroscience Research in PTSD. Ann N Y Acad Sci. 2006;1071:277–293. doi: 10.1196/annals.1364.022. [DOI] [PubMed] [Google Scholar]
- 40.Vollmayr B, Faust H, Lewicka S, Henn FA. Brain-derived-neurotrophic-factor (BDNF) stress response in rats bred for learned helplessness. Mol Psychiatry. 2001;6:471–474, 358. doi: 10.1038/sj.mp.4000907. [DOI] [PubMed] [Google Scholar]
- 41.Wardle J, Steptoe A, Gulis G, Sartory G, Sek H, Todorova I, Vogele C, Ziarko M. Depression, perceived control, and life satisfaction in university students from Central-Eastern and Western Europe. Int J Behav Med. 2004;11:27–36. doi: 10.1207/s15327558ijbm1101_4. [DOI] [PubMed] [Google Scholar]
- 42.Williams JL, Maier SF. Transituational immunization and therapy of learned helplessness in the rat. Journal of Experimental Psychology: Animal Behavior Processes. 1977;3:240–253. [Google Scholar]
- 43.Yukawa H, Takahashi JC, Miyatake SI, Saiki M, Matsuoka N, Akimoto M, Yanamoto H, Nagata I, Kikuchi H, Hashimoto N. Adenoviral gene transfer of basic fibroblast growth factor promotes angiogenesis in rat brain. Gene Ther. 2000;7:942–949. doi: 10.1038/sj.gt.3301182. [DOI] [PubMed] [Google Scholar]


