Abstract
Recently, it has been reported that 25-hydroxyvitamin D3-1α-hydroxylase [1α(OH)ase, CYP27B1], required to convert non-toxic 25-hyxdroxyvitamin D3 [25(OH)D3] to its active metabolite [1α,25(OH)2D3], is present in the epithelial cells of the human colon. In the present study, the potential chemoprotective role of 25(OH)D3 was evaluated for colon cancer using the HT-29, human colon cancer cell line. Colon cancer cells were treated with 25(OH)D3 (500 nM or 1 μM), 1α,25(OH)2D3 (500 nM), cholecalciferol (D3; 1 μM) or vehicle and cell number determined at days 2 and 5 post-treatment. Results showed that both 25(OH)D3 and 1α,25(OH)2D3 induced dose- and time-dependent antiproliferative effects on the HT-29 cells, with maximum inhibition noted at day 5. Western blot analyses revealed an up-regulation of VDR and 1α(OH)ase expression following 24h of treatment with 25(OH)D3, and 1α,25(OH)2D3. These results are consistent with the expression of VDR and 1α(OH)ase in samples of normal colonic tissue, aberrant crypt foci (ACFs) and colon adenocarcinomas. The VDR expression was sequentially increased from normal to precancerous lesions to well-differentiated tumors and then decreased in poorly differentiated tumors. Expression of 1α(OH)ase was equally expressed in normal, precancerous lesions and malignant human colon tissues. The increased expression of 1α(OH)ase in colon cancer cells treated with the pro-hormone and its anti-proliferative effects, suggest that 25(OH)D3 may offer possible therapeutic and chemopreventive option in colon cancer.
Keywords: colon chemoprevention, 25-hydroxyvitamin D3, VDR, CYP27B1
1. Introduction
Epidemiological studies have been instrumental in demonstrating an inverse relationship between vitamin D intake, sunlight exposure and risk for colon cancer (1). Recently, a meta-analysis conducted by Gorham et al (2) examined the dose response relationship between vitamin D intake or serum 25(OH)D3 and risk for colon cancer. Dose-gradient curves generated from eighteen of the forty-four existing observational studies demonstrated that individuals with ≥ 1000 IU/day oral vitamin D or ≥ 33 ng/ml serum 25(OH)D3 had 50% lower risk for developing colon cancer compared to reference values (2).
In spite of the extensive evidence demonstrating the anti-cancer activity of 1,25(OH)2D3 in colon cancer, its clinical use is limited by its susceptibility to cause hypercalcemia. Hence, in order to retain the efficacy but reduce its toxicity numerous analogs have been synthesized and evaluated for the prevention and treatment of colon cancer. Until recently, little attention was given to 25(OH)D3 as a potential chemopreventive agent since it was commonly believed that the enzyme 1α(OH)ase was selectively present in the kidney. The existence of 1α(OH)ase in colon epithelial cells has led to interest in the potential use of this non-toxic prohormone, 25(OH)D3, as an effective chemoprotective agent against colon cancer (3, 4, 5). The possibility of using The 25(OH)D3 as a chemopreventive and/or chemotherapeutic agent has been further strengthen by in vitro studies which have demonstrated that cells containing 1α(OH)ase are able to convert 25(OH)D3 into 1,25(OH)2D3 (6, 7). For example, Bareis et al (7) demonstrated that the Caco-2 colon cancer cells, which is a moderately differentiated colon cancer cell line, are able to produce 1,25(OH)2D3 from the pro-hormone. Here we report that normal, aberrant crypt foci (ACF) and malignant human cancer samples express VDR and 1α(OH)ase and that 25(OH)D3 is efficacious as an antiproliferative agent in human colon cancer cells.
2. Materials and Methods
2.1. Tumor Specimens and Histological Grading
Colon cancers were randomly selected from the University of Illinois at Chicago Gastrointestinal Tumor Bank. The University of Illinois at Chicago and Veterans Administration Institutional Review Boards approved use of these tissues. Differentiation was assessed as previously described (8).
2.2. Human Colon Cancer Cell lines
The HT-29, Caco-2 and SW480 cell lines were obtained from American Type Culture Collection (Manassas, VA) and maintained in RPMI 1640 media (Life Technologies, Inc., Grand Island, NY) with 10% fetal bovine serum, 2 mM L-glutamine and 1% antibiotic-antimycotic solution and kept in a 37°C humidified atmosphere of 5% CO2.
2.3. Analysis of Cell Proliferation
For determination of proliferation, HT-29 cells were seeded at a density of 2 × 104 per well in a 12-well cell culture plate and allowed to adhere overnight. After incubation with or without 25(OH)D3 for the appropriate times, cells were detached with trypsin and cell number was determined by the Coulter counter.
2.4. FACS Analysis
Colon cancer cells were seeded at a density of 5.0 × 105 in 25cm2 flasks and allowed to adhere for 24 h. Following treatment with or without 1.0 μM 25(OH)D3 for 48 h, they were harvested with trypsin and washed with PBS. The samples were then stained with propidium iodide using the detergent-trypsin method described by Vindelov (9).
2.5. Measurement of Apoptosis
Cells undergoing apoptosis were evaluated using the In Situ Cell Death Detection Kit (Roche, Indianapolis, IN). A quantitative assessment was made by determining the percentage of apoptotic cells.
2.6. Western Blot analysis
Treated and untreated cells were lysed in freshly prepared extraction buffer. Protein concentration was determined using a modified Lowry method (Bio Rad, Hercules, CA). Samples were then separated on 10% polyacrylamide gels and transferred to nitrocellulose membranes. The membranes were blocked and then incubated with appropriate primary and secondary antibodies. Anti-VDR antibody was from Neomarkers (Freemont, CA), sheep Anti-murine 25-hydroxyvitamin D3-1α-hydroxylase antibody was from The Binding Site (San Diego, CA). The chemiluminescence reaction was performed using the ECL system. Bands of interest were compared to that of actin and relative intensity ratios were calculated.
2.7. Immunofluorescence studies
SW480 cells were seeded on cover slips and allowed to adhere overnight. After incubation with or without 25(OH)D3 (1 μM) for 24 h, the cells were fixed in buffered formalin, washed with PBST (PBS containing 0.1% Tween 20), permeabilized in 0.2% Triton X-100/PBS, blocked with 1% BSA, and then incubated with anti-VDR rat monoclonal antibody (1:200) for 1 h at RT. Cells were washed and incubated with TRIC-labeled anti-rat secondary antibody for 1h. After staining nuclei with DAPI, cells were visualized using the Olympus BX51 microscope. Tissues were sectioned (4 μm thick) and processed for immunohistochemistry as previously described (10).
3. Results
3.1. Expression of 1 α(OH)ase and VDR in Human colon tissue and cancer cells
The expression patterns of VDR and 1α(OH)ase were evaluated in human colon tissues. As shown in Fig 1A-E, 1α(OH)ase showed consistently strong expression in normal, premalignant (ACF) and malignant colonic epithelial cells, irrespective of tumor cell differentiation. In contrast, minimal VDR expression was observed in normal colonic epithelial cells, with the expression localized predominantly in the nucleus (Fig 1F). Samples from ACFs revealed that the amount of VDR is significantly higher than that of adjacent normal tissue. Moreover, with malignant transformation, total cellular VDR expression increased markedly and was especially high in well-differentiated tumors (Fig 1H-I) but then decreased with tumor cell de-differentiation (Fig 1J). Furthermore, these data suggest that 1α(OH)ase (Fig 1B) and VDR (Fig 1G) expression levels in colorectal cancer pre-cancerous lesions such as ACFs and polyps may permit the pro-hormone 25(OH)D3 for use in colorectal cancer chemoprevention.
Fig 1.
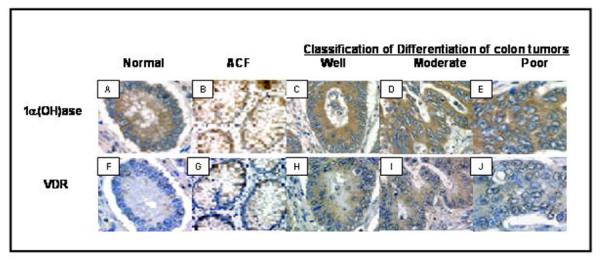
Expression of 1α-hydroxylase and VDR in human colon tissue. Similar non-nuclear levels of 1α-hydroxylase expression is noted in normal colonic epithelial cells (A), aberrant crypt foci (B), as well as, in well-differentiated tumor cells (C), moderately differentiated tumor cells (D) and poorly differentiated tumor cells (E). VDR expression levels were low in normal colonic epithelial cells (F), increased in aberrant crypt foci (G) with further increase noted in well-differentiated tumor cells (H). In contrast, moderately differentiated tumor cells (I) appear to have less VDR than the well-differentiated. VDR expression was found to be almost completely lost in poorly differentiated tumor cells (J). Magnification ×1000.
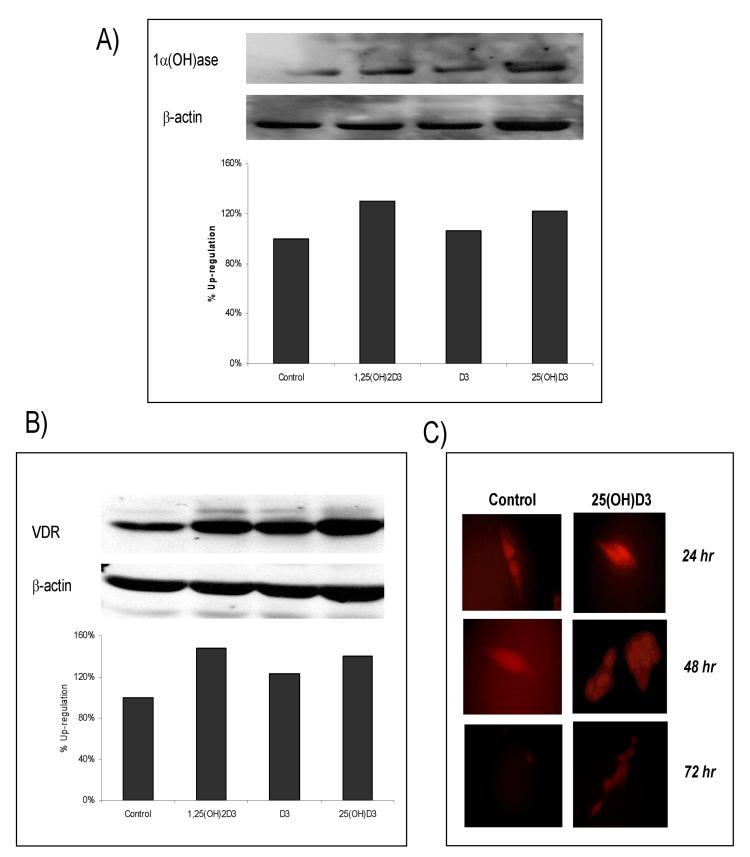
The expression profile of VDR and 1α(OH)ase in human colon tissue prompted us to investigate whether 25(OH)D3 treatment regulated the expression levels of VDR and 1α(OH)ase in the HT-29 cells. For this, HT-29 cells were treated with vehicle (ETOH), 25(OH)D3 (1 μM), 1α,25(OH)2D3 (0.5 μM; positive control], or cholecalciferol (D3, 1 μM, negative control). Following 24 h of treatment, protein lysate was collected and separated for western blot analyses. As shown in Fig 2A, both 25(OH)D3 and 1,25(OH)2D3 up-regulated the levels of expression of 1α(OH)ase by 22 and 30%, respectively. In a similar manner, VDR expression levels (Fig 2B) were significantly increased following treatment with 25(OH)D3 and 1α,25(OH)2D3 by 40 and 48%, respectively. Treatment with the cholecalicerfol (negative control) had no effect on the expression levels of 1α(OH)ase (Fig 2A) and showed only a marginal increase in the levels of VDR (Fig 2B).
Fig 2.
Expression levels of 1α-hydroxylase and VDR in colon cancer cells treated with 25-hydroxyvitamin D3. Western blot analysis showing 1α-hydroxylase (A) and VDR (B) with appropriate actin control. HT-29 cells were treated with 1α,25(OH)2D3 (0.5 μM), D3 (1 μM), 25(OH)D3 (1.0 μM) for 24 hrs. Both 1α,25(OH)2D3 and 25(OH)D3 found to up-regulate 1α-hydroxylase and VDR. (C) SW480 cells were plated and treated with control or 25(OH)3 (1.0 μM) for 24 h to 72 h. Immunofluorescence revealed an up-regulation of VDR by 24 h and show evidence that it remains elevated at 72 h post tx.
In addition to HT29 cells, we evaluated the effects of 25(OH)D3 on SW480 colon cancer cells. The SW480 cell line was selected for this study since it is one of the best characterized human colon cancer cell lines, moreover it is widely used as a model system for vitamin D compounds, and is shown to have both VDR negative [VDR(-)] and VDR positive [VDR(+)] sub-populations (i.e., VDR(+) flat, polygonal and adherent line; and VDR(-) rounded, refractile, and less adherent) (11). For these experiments, SW480 cells were plated on cover slips and treated with or without 25(OH)D3 (1 μM) for 24, 48 or 72 h. At the appropriate time points, cells were fixed and immunofluorescence was completed as described in the materials and methods section. As shown in Fig 2C, VDR expression levels were increased in the VDR(+) flat, polygonal and adherent line by 24 h post treatment with 25(OH)D3 and remained elevated 72 h after treatment. Cumulatively, these data show that 25(OH)D3 treatment significantly increases the levels of 1α(OH)ase and VDR in colon cancer cells, and moreover support the notion that 25(OH)D3 can regulate the expression of VDR and 1α(OH)ase in a similar manner as the active metabolite in vitro. These results indicate that 25(OH)D3 can exhibit similar anti-cancer activities as have been reported for 1α,25(OH)2D3.
3.2. The pro-hormone 25-hydroxyvitamin D3 exerts anti-proliferative effects on human colon cancer cells
The anti-proliferative effects of 25(OH)D3 were investigating using the HT-29 cells. For these studies, cells were plated, allowed to adhere for 24 h prior to treatment with the different vitamin D compounds. As shown in Fig 3, five day treatment of HT-29 cells with either 1α,25(OH)2D3, or 25(OH)D3 significantly inhibited the growth of the HT-29 cells. The percent growth inhibition was 14, 65, and 53% for D3 (1μM), 1α,25(OH)2D3 (0.5 μM), and 25(OH)D3 (0.5 μM), respectively. A dose-dependent effect for 25(OH)D3 treatment was also evident (53 vs. 77%, for 0.5 and 1.0 μM) for the HT-29 cells. Similar findings were found in the Caco-2 and SW480 cells (data not shown).
Fig 3.

HT-29 cells were treated with ETOH (control); cholecalciferol (negative control [1μM]); 1,25 (OH)2D3 (positive control [0.5 μM]; 25(OH)D3 at 0.5 or 1.0 μM; or 1α(OH)D5 at a concentration of 0.5 or 1.0 μM, and cell number determined 5 days post treatment. Percent growth inhibition is presented.
In order to determine the effects of 25(OH)D3 on cell cycle progression HT-29, Caco-2, and HCT116 cells were treated with 25(OH)D3 (1 μM), 1α,25(OH)2D3 (0.5 μM), or ETOH for 48 h and DNA content was analyzed by flow cytometry. Our studies failed to reveal cell cycle arrest with either 1α,25(OH)2D3 or 25(OH)D3 during the 48h treatment period. However, using the tunnel assay, we found evidence of SW480 cells undergoing apoptosis following 72 h of treatment with either 1α,25(OH)2D3 (0.5 μM) or 25(OH)D3 (1.0 μM). These results suggest that 25(OH)D3 treatment may be inducing growth inhibition in part by causing apoptosis in the colon cancer cells.
4. Discussion
Vitamin D3 is biologically inert and requires successive hydroxylations by mitochondrial P450 enzymes present in the liver and kidney to form the biologically active hormone 1α,25(OH)2D3. The final step in metabolism is the conversion of prohormone 25(OH)D3 to the active metabolite and hormone 1α,25(OH)2D3 by 1α(OH)ase. Due to the hypercalcemic nature of 1α,25(OH)2D3, many relatively non-calcemic analogs of vitamin D have been synthesized and evaluated for their efficacy as anti-cancer agents. Ideally, the prohormone 25(OH)D3, which is naturally occurring and non-toxic could be developed, however due to the traditional concept of renal hydroxylation of the prohormone, until recently enthusiasm for establishing it as a cancer chemoprotective agent had been non-significant. In recent years, the expression of 1α(OH)ase, the enzyme that catalyzes this conversion, has been reported in several extra-renal tissues, including the pancreas, prostate, and colon(12) leading to the hypothesis that 25(OH)D3, may posses chemopreventive efficacy in the organs expressing endogenous 1α(OH)ase. In the colon, it has been reported that 1α(OH)ase is present in normal, and malignant colon tumors (3, 4). Results also showed that high- to moderately differentiated colon tumors express increased 1α(OH)ase, while poorly differentiated tumors consistently have shown reduced expression or complete loss of 1α(OH)ase activity (5). More recently, we have demonstrated that 1α(OH)ase and VDR are also highly expressed in the pre-cancerous lesions (ACF) of human colon samples (10). Antiproliferative effects of vitamin D analogs in colon cancer cells have been reported (13). In this report we evaluated effects of 25(OH)D3 in several human colon cancer cell lines. Interestingly 25(OH)D3 enhanced expression of both VDR and 1α(OH)ase in these cells. It is not clear whether 25(OH)D3 directly suppresses cell proliferation of colon cancer cells in vitro or even if its conversion to 1α,25(OH)2D3 is required for its anti-proliferative actions. Nonetheless, these results provide the basis for further evaluation of 25(OH)D3 as a candidate agent for the treatment of colon cancer. Furthermore, these findings support the possible therapeutic use of 25(OH)D3 in individuals who are at high risk for developing colon cancer, including those with prior history of colonic polyps or with large numbers of ACFs.
Acknowledgements
This work was supported by NCI Mentored Career Development Award Grant K01CA103861
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Harris DM, Go VL. Vitamin D and colon carcinogenesis. J Nutr. 2004;134:3463S–3471S. doi: 10.1093/jn/134.12.3463S. [DOI] [PubMed] [Google Scholar]
- 2.Gorham ED, Garland CF, Garland FC, Grant WB, Mohr SB, Lipkin M, Newmark HL, Giovannucci E, Wei M, Holick MF. Vitamin D and prevention of colorectal cancer. J Steroid Biochem Mol Biol. 2005;97:179–194. doi: 10.1016/j.jsbmb.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 3.Cross HS, Bises G, Lechner D, Manhart T, Kállay E. The vitamin D endocrine system of the gut-its possible role in colorectal cancer prevention. J Steroid Biochem Mol Biol. 2005;97:121–128. doi: 10.1016/j.jsbmb.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Tangpricha V, Flanagan JN, Whitlatch LW, Tseng CC, Chen TC, Holt PR, Lipkin MS, Holick MF. 25-hydroxyvitamin D-1α-hydroxylase in normal and malignant colon tissue. Lancet. 2001;357:1673–1674. doi: 10.1016/S0140-6736(00)04831-5. [DOI] [PubMed] [Google Scholar]
- 5.Bises G, Kallay E, Weiland T, Wrba F, Wenzl E, Bonner E, Kriwanek S, Obrist P, Cross HS. 25-hydroxyvitamin D3-1α-hydroxylase expression in normal and malignant human colon. J Histochem Cytochem. 2004;52:985–989. doi: 10.1369/jhc.4B6271.2004. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz GG, Eads D, Rao A, Cramer SD, Willingham MC, Chen TC, Jamieson DP, Wang L, Burnstein KL, Holick MF, Koumenis C. Pancreatic cancer cells express 25-hydroxyvitamin D-1α-hydroxylase and their proliferation is inhibited by the prohormone 25-hydroxyvitamin D3. Carcinogenesis. 2004;25:1015–1026. doi: 10.1093/carcin/bgh086. [DOI] [PubMed] [Google Scholar]
- 7.Bareis P, Bises G, Bischof MG, Cross HS, Peterlik M. 25-hydroxy-vitamin D metabolism in human cancer cells during tumor progression. Biochem BioPhys Res Comm. 2001;285:1012–1027. doi: 10.1006/bbrc.2001.5289. [DOI] [PubMed] [Google Scholar]
- 8.Carroll RE, Matkowskyj KA, Chakrabarti S, McDonald TJ, Benya RV. Aberrant expression of gastrin-releasing peptide and its receptor by well differentiated colon cancers in humans. Am J Physiol. 1999;276:G655–65. doi: 10.1152/ajpgi.1999.276.3.G655. [DOI] [PubMed] [Google Scholar]
- 9.Vindelov LL, Christensen IJ, Nissen NI. A detergent-trypsin method for the preparation of nuclei for flow cytometric DNA analysis. Cytometry. 1983;3:323–7. doi: 10.1002/cyto.990030503. [DOI] [PubMed] [Google Scholar]
- 10.Matusiak D, Murillo G, Carroll RE, Mehta RG, Benya RV. Expression of vitamin D receptor and 25-hydroxyvitamin D3-1α-hydroxylase in normal and malignant human colon. Cancer Epid, Biomakers & Prev. 2005;14:2370–2376. doi: 10.1158/1055-9965.EPI-05-0257. [DOI] [PubMed] [Google Scholar]
- 11.Palmer HG, Gonzalez-Sancho JM, Espada J, Berciano MT, Puig I, Baulida J, Quintanilla M, Cano A, Garcia de Herreros A, Lafarga M, Munoz A. Vitamin D promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of β-catenin signaling. J Cell Biol. 2001;154:369–387. doi: 10.1083/jcb.200102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Townsend K, Evans KN, Campbell MJ, Colston KW, Adams JS, Hewison M. Biological actions of extra-renal 25-hydroxyvitamin D-1α-hydroxylase and implications for chemoprevention and treatment. J Steroid Biochem Mol Biol. 2005;97:103–109. doi: 10.1016/j.jsbmb.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Guyton KZ, Kensler TW, Posner GH. Vitamin D and vitamin D analogs as cancer chemopreventive agents. Nutr Rev. 2003;61:227–38. doi: 10.1301/nr.2003.jul.227-238. [DOI] [PubMed] [Google Scholar]