Abstract
Substances that alter the measurable concentration of the analyte or alter antibody binding can potentially result in immunoassay interference. Interfering, endogenous substances that are natural, polyreactive antibodies or autoantibodies (heterophiles), or human anti-animal antibodies together with other unsuspected binding proteins that are unique to the individual, can interfere with the reaction between analyte and reagent antibodies in immunoassay. Lipaemia, cross-reactivity, and exogenous interferences due to pre-analytical variation, matrix and equipment reaction also affect immunoassay. Interfering substances may lead to falsely elevated or falsely low analyte concentration in one or more assay systems depending on the site of the interference in the reaction and possibly result in discordant results for other analytes. The prevalence of interference is generally low in assays containing blocking agents that neutralise or inhibit the interference but is often higher in new, untested immunoassays. A wide range of analytes measured by immunoassay including hormones, tumour markers, drugs, cardiac troponin and microbial serology may be affected.
Interference in immunoassay may lead to the misinterpretation of a patient's results by the laboratory and the wrong course of treatment being given by the physician. Laboratories should put processes in place to detect, test and report suspected interferences. It is equally important that physicians communicate any clinical suspicion of discordance between the clinical and the laboratory data to the laboratory. The detection of interference may require the use of an alternate assay or additional measurements, before and after treatment with additional blocking reagent, or following dilution of the sample in non-immune serum. It is imperative that laboratories inform physicians of the follow-up procedure and report on the presence of any interference. The establishment of on-going laboratory-physician contact is essential to the continuing awareness of wrong patient results due to interference.
Introduction
Despite the analytical sensitivity of immunoassay and measurements often being made without the need for prior extraction, immunoassays may lack adequate specificity and accuracy.1 Specificity is dependent not only on the binding property of the antibody but also on the composition of the sample antigen and its matrix, reagent composition, and immunoassay format. Substances that alter the measurable concentration of the analyte in the sample or alter antibody binding can potentially result in assay interference. Analytical interference is defined as the effect of a substance present in the sample that alters the correct value of the result.2
When interference is present it may be analyte-dependent or -independent. Analyte-independent interferences refer to the common interferences of haemolysis, lipaemia and effects of anticoagulant and sample storage, and are independent of the analyte concentration. Analyte-dependent interferences in immunoassays refer to interaction between constituents in the sample with one or more reagent antibodies. They include compounds with chemical differences but structural similarities that cross-react with the antibody, heterophile antibodies, human anti-animal antibodies, autoanalyte antibodies, rheumatoid factors and other proteins.
Interference can lead to falsely elevated or falsely low analyte concentration depending on the site of the interference in the immunoassay reaction. The interference may result in discordant results for one or more analytes, and may be detected in one or more other assay systems for the affected analyte. The magnitude of the effect depends on the concentration of the interfering substance, but not necessarily in a directly proportional way. Interference affects a wide range of immunoassay analytes including hormones, tumour markers, drugs, cardiac troponin, and microbial serology. It may result in the misinterpretation of a patient’s results from which the wrong course of treatment is given.3 For example, human chorionic gonadotropin (hCG) assays have the potential for misdiagnosis of either pregnancy or malignancy and unnecessary treatment of non-existent trophoblastic disease.4–6 Other clinical sequelae of wrong results include unnecessary further laboratory and clinical investigations, and unnecessary drug therapy.7–13 The consequence of falsely negative results and subsequent drug overdosing of the patient is another serious clinical problem.14 It is important to recognise the potential for interference in immunoassay and to put procedures in place to identify them wherever possible.15–18
Nature of Interferences
Interfering, endogenous substances that occur in both healthy and pathological patient samples arise from properties of the specimen. The sample properties are unique to the patient and interference results from an interaction with one or more steps in the immunoassay procedure such that the measurable analyte concentration in the sample or antibody binding is altered (Table 1).19,20 Other unsuspected binding protein(s) in the individual also can cause interference in immunoassay by interfering with the reaction between analyte and assay antibodies. In reagent-excess assays in which the two-site immunometric assay (IMA) is commonly used, there is an increased likelihood of a potential cross-reactant forming a bridge between the two antibodies. During the antigen-antibody interaction conformational changes to antigens, induced by antibodies, may alter the specificity of antibodies. For these reasons there may be a higher prevalence of unpredictable cross-reaction in IMAs compared with a single-site antigen-antibody reaction in reagent-limited assays.21 Exogenous antibodies given to a patient for therapy may also compete with the assay antibody for the analyte and disturb the antigen-antibody reaction resulting in immunoassay interference, e.g., administration of Fab fragments derived from anti-digoxin antibodies (Digibind).22
Table 1.
Nature of Interferences.
|
Immunoassays are generally unaffected by sample haemolysis and icterus unlike other analytes measured by spectral or chemical means.23 However, lipaemia can interfere in some immunoassays especially those by nephelometry and turbidimetry.24 Other non-specific, exogenous interferences can arise from aberrant assay reagent or equipment interaction with patient sample, dissimilar reactions for the standard matrix and patient sample, e.g., sample-induced changes in pH and ionic strength of the reaction mixture, or from pre-analytical variables affecting the sample analyte concentration by physical masking of the antibody label.19 Potential sample carryover due to inadequate assay washing or failure to detect a sample clot can also result in over- or under-estimation of values. Samples or assay reagents contaminated with substances that interfere with measurement of the label, e.g., enzyme inhibitors, fluorophores from ophthalmic examination, or radioimaging isotopes, require removal by washing.
Cross-reacting species also result in over- or underestimation of sample analyte concentration if an immunoassay reagent contains antibodies directed toward molecules other than the antigen of interest. Cross-reaction is a problem in diagnostic immunoassays where endogenous molecules with a similar structure to the measured analyte exist or where metabolites of the analyte have common cross-reactive epitopes, and where there is administration of structurally similar medications.2 Early hCG immunoassays were cross-reactive with luteinizing hormone (LH)25 but the development of more specific antibodies has led to most of today’s assays for hCG having little or no cross-reaction with LH. However, cross-reactivity with drugs and their metabolites is still a problem for the measurement of steroids by immunoassay. For example, cortisol assays can show significant cross-reactivity with fludrocortisone derivatives and result in false-positive cortisol values in patients using these drugs.26 In immunoassays for drugs of abuse screening, false-positive interference may occur from medications or their metabolites that have similar chemical structures.27–30 For the monitoring of the transplant anti-rejection drug cyclosporin A in whole blood, required for dosage adjustment in patients after heart or liver transplantation, the concentration of the parent drug is used. Immunoassays of cyclosporin A show cross-reactivity for cyclosporin metabolites with levels up to 174% higher in individual patients compared with the HPLC reference method.31 In digoxin immunoassays also, the presence of digoxin-like immunoreactive factors that are commonly found in renal failure, liver disease and hypertension, cause interference by cross-reaction.32,33 Falsely low assay results can also occur when a cross-reacting substance is present in the sample and a wash or separation step is used in the assay protocol whereby the cross-reactant dissociates faster than the analyte.17 Steimer et al.14 initially reported a false-negative case of digoxin intoxication due to canrenone interference in a digoxin assay and have since shown that several steroid-like compounds, including spironolactone, canrenone, and their metabolites, cause suppression of digoxin results in some commercial immunoassays.34 Minimisation of the interference by cross-reacting substances that are present in a high enough concentration to cause a false-positive or false-negative result is desirable.35 Interference due to cross-reactivity is highly dependent on assay specificity, which is not the focus of this paper.
Changes to the Measurable Analyte Concentration in the Sample
Hormone Binding Proteins
Hormone binding globulins can alter the measurable analyte concentration in the sample either by their removal from or blocking of the analyte. For example, steroids can bind to sex hormone binding globulin36,37 or cortisol, to cortisol binding globulin38 and cause decreased free analyte concentration. Binding of cortisol can be minimised by denaturation of the binding protein or by the addition of blocking agent. Displacement of analyte from endogenous hormone binding proteins, e.g., free thyroxine (FT4) displaced from thyroid binding globulin by non-esterified free fatty acids (FFAs), can alter assay equilibrium and either decrease or increase the analyte concentration.39,40 These FFAs can be generated in-vitro in non-frozen samples from patients treated with heparin, secondary to the induction of heparin-induced lipase activity.41–43 Increased serum triglyceride levels can accentuate this problem.41
Pre-analytical Factors
Binding of cations present in serum, e.g., Mg2+ or Ca2+, to drugs or proteins can change antigen conformation and the measurable analyte concentration.19 Sample type can affect analyte concentration with differences in results for samples collected in lithium heparin, EDTA, and sodium fluoride/potassium oxalate or tubes without anticoagulant reported for some analytes, e.g., cardiac troponin, hormones.44,45 Inappropriate sample type and specimen processing or storage can change the properties of a sample over time and affect results. For example, adrenocorticotrophin (ACTH) is reported to be stable in EDTA plasma at 4°C for only 18 hours compared with 18 other hormones that are stable for >120 hours.45,46 Increased EDTA concentration in the sample-reagent mixture due to insufficient sample volume causes chelation of Mg2+ and Zn2+ and can affect the activity of the alkaline phosphatase enzyme label used in chemiluminescence assays. Filling of EDTA-sample tubes to ≤50% affects intact parathyroid hormone47 and ACTH measurements by the DPC Immulite assays. The physical masking of the antibody by lipids and silicone oils present in some blood collection devices or tubes, or by fibrin in plasma samples, can physically interfere with antigen-antibody binding.19,48–50 Siliconised plastic tubes caused a 30–60% decrease in ACTH immunoreactivity by the Nichols Institute radioimmunoassay (RIA) possibly by interference with formation of either the biotin-avidin complex or the antibody-antigen-antibody sandwich.48 Conversely, silicone formed a complex with C-reactive protein (CRP) that enhanced the antigen-antibody reaction in the Vitros CRP assay and falsely elevated results.51
Autoanalyte Antibodies
Autoantibodies have been described and can cause interference in both non-immunoassay and immunoassay methods for a number of analytes including macro-enzymes (creatine kinase, amylase), thyroid hormones in both free and total forms,40,52,53 thyroglobulin,52 insulin,54,55 prolactin,56 and testosterone.57 False-positive or false-negative values may arise, depending on whether the autoantibody-analyte complex partitions into the free or the bound analyte fraction. Regardless of whether a reagent-limited or reagent-excess immunoassay is used, interference from autoantibodies can occur in both formats.
Autoantibodies to thyroid hormones have been reported in patients with Hashimoto’s thyroiditis, Graves’ disease, hyperthyroidism after treatment, carcinoma, goitre and non-thyroid autoimmune conditions.52 The prevalence of thyroid hormone autoantibodies is dependent on the detection system and may be as high as 10% in patients with autoimmune disease although only a minority of samples cause interference.52,53 Their presence should be suspected when FT4 and thyroid-stimulating hormone (TSH) results appear to be discordant to the laboratory or the clinician.58 Sakata et al. reported that the prevalence of anti-T3 or anti-T4 antibodies among a healthy Japanese population was 1.8% but that concentrations of free thyroid hormones and TSH in autoantibody-positive sera were within the normal range. 59
Interference is also a problem for thyroglobulin (Tg) assays with endogenous Tg antibody autoantibodies (TgAb) a major contributor to such interference and present in up to 30% of differentiated thyroid cancer patients.60 Falsely low Tg results can occur by IMAs and falsely elevated results by RIA. This has led to the suggestion that discordance between IMA and RIA results indicates the presence of assay interference from TgAb.61
The presence of anti-prolactin autoantibodies in the form of macroprolactin (macro-PRL) can cause hyperprolactinaemia without pituitary disease and may lead to unnecessary medical or surgical procedures.56 Macro-PRL is primarily a macro-molecular complex of prolactin (PRL) and an IgG antibody directed against specific epitope(s) on the PRL molecule, and generally is regarded as biologically inactive because of its decreased bioavailability.62 Smith et al.63 report an incidence of 24% of hyperprolactinaemic sera containing macro-PRL with differences of 2.3- to 7.8-fold in PRL concentration observed in ten of these sera using nine immunoassay systems. There was no single assay that gave a normal concentration of PRL for all sera in the presence of macro-PRL. Whereas the ACS:180 and Tosoh 1200 assays have similar immunoreactivity towards monomeric PRL, reactivity is variable for macro-PRL from different individuals due to differences in the degree to which macro-PRLs contribute to total serum PRL concentration.64,65 Laboratories should know how their PRL assay reacts with macro-PRLs and ideally test for their presence in all patients with hyperprolactinaemia by gel filtration chromatography or pre-treatment with polyethylene glycol (PEG). It is important to both recognise the presence of macro-PRL and provide an estimate of the monomeric PRL concentration because some patients with macroprolactinaemia may have clinically significant, elevated monomeric PRL levels.62
Antibody Interference
Changes to Antibody Binding
Heterophile Antibodies
Heterophile antibodies consist of natural antibodies and autoantibodies that are polyreactive against heterogeneous, poorly defined antigens of different chemical composition and generally show low affinity, weak binding.66,67 Natural idiotypic antibodies are antibodies produced by an idiotype (anti-id) that can bind other antibodies. They may affect antigen binding to antibody in immunoassays by binding to the antigen and affecting analyte concentration, or by mimicking the binding of antigen due to its mirror-image structure.68 Anti-ids together with polyspecific and natural or autoimmune rheumatoid factor (RF), account for most heterophile interference in immunoassays.66,68 Interfering, endogenous antibodies are called heterophile antibodies when there is no clearly defined immunogen, and the antibody reacts with immunoglobulin from two or more species, or has RF activity.69 In the case of RF, false-positives arise by binding of RF to the Fc constant domain of antigen-antibody complexes if the detection antibody is labelled anti-human IgG. The presence of RF in serum can cause false-positives in troponin assays,9,70–73 thyroid function tests,12,74 and with the detection of HCV-specific IgM.75
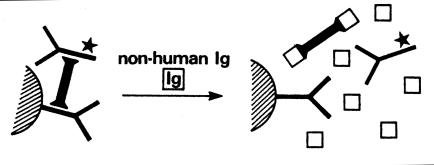
Heterophile antibodies interfere with immunoassays by a non-competitive mechanism. Heterophiles can bind to the conjugate, enzyme, or other parts of the detection system in reagent-limited assays, mainly of the non-RIA type, and cause interference in assays for steroid hormones, thyroid function tests, and digoxin.7,76–78 In conventional two-site IMAs for the measurement of non-immunoglobulin antigen, heterophile antibody or any multivalent antibody-binding substance binds animal capture antibody to the detection antibody and falsely elevates the patient value by producing an assay signal (Figure 1).79 Assays using either polyclonal or monoclonal antibodies may be affected. The same heterophile may react differently for different antibody combinations hence giving rise to a falsely elevated result in one assay but a lower result in another assay. The presence of excess non-human immunoglobulin in the assay reduces the possibility of the interfering substances binding to the capture and detection antibody by binding instead to the interfering immunoglobulin (Figure 2).79 Although manufacturers routinely add blocking agent to their assay formulations, not all heterophile interference can be blocked by non-immune globulin, including pooled globulin from several species. Heterophile antibodies may show reactivity to idiotypes that are not present in the blocking agent. Both IgG and IgM heterophile antibodies are reported to occur.8
Figure 1.
A diagrammatic representation of analyte and interfering antibody-binding substance (I) binding in a conventional two-site immunoassay. By linking the capture and signal antibodies, these multivalent substances produce an assay signal and result in a false-positive analyte value. Reproduced with the permission of Clinical Chemistry from Ref 79.
Figure 2.
A diagrammatic representation of the elimination of interference from antibody-binding substances (I) binding to the reagent antibodies in two-site immunoassays by the addition of non-human immunoglobulins (Ig), which binds to I. Reproduced with the permission of Clinical Chemistry from Ref 79.
Human Anti-animal Antibodies
Human anti-animal antibodies (HAAA) are high affinity, specific polyclonal antibodies produced against a specific animal immunogen whole immunoglobulin of IgG or IgM class.80 They show strong binding with antigen of a single chemical composition and are produced in a high titre such that they compete with the test antigen by cross-reacting with reagent antibodies of the same species to produce a false signal. HAAA are most commonly human anti-mouse antibodies (HAMA), but also include antibodies to rats, rabbits, goats, sheep, cattle, etc. and are reported to occur in 30–40% of patient samples.81 A large number of documented examples of circulating antibodies produced to animal immunoglobulins have been reported, with those against mice, rabbit and goat immunoglobulins important as these animals are used to produce commercial immunoassay reagents.80 Mouse antibody is especially prevalent in the serum of animal workers, patients on monoclonal antibody for therapy or imaging, and others exposed to mice. Interfering, endogenous antibodies should be called a specific HAAA rather than a heterophile when there is a history of medicinal treatment with animal immunoglobulin and immunoglobulin from the same species is used in the immunoassay.69 The nomenclature becomes confusing where the immunogen is not known and a heterophile antibody is recognised in mouse or other animal-specific immunoassays. The term HAMA or other HAAA is often used to refer to these heterophile antibodies.
HAMA interference has been reported for numerous analytes including cardiac marker assays,82,83 thyroid function tests,84–87 drugs,78 tumour markers.88 Two-site IMA methods are more prone to interference from antibodies to animal IgG in human serum and may cross-react with reagent antibodies especially those from the same species. Reaction with both the capture and detection antibodies forms an antigen-antibody complex that behaves immunochemically in the same way as the analyte-antibody complex resulting in false-positive results. False-negative results can occur in two-site assays also, presumably due to HAMA reacting with one of the antibodies and preventing reaction with the analyte.80 Methods that use only one mouse monoclonal antibody in IMA assays are less prone to interference from HAMA.81 The interference caused by anti-animal antibodies can be eliminated by sample pre-treatment or assay redesign.80
High-dose Hook Effect
In IMA systems where the analyte concentration range is large, antigen-antibody reactions can go into antigen excess and can result in false-negative results, e.g., ferritin, growth hormone, hCG, prolactin, Tg,89–93 and potentially lead to misdiagnosis.91 In particular, for two-site immunoassays in which both the capture and detection antibody are added simultaneously, free analyte and analyte bound to labelled antibody compete for the limited number of antibody-binding sites and in the presence of higher analyte concentration will decrease rather than increase label bound to the solid phase. The high-dose hook effect can be averted by a change of the sample antigen to reagent antibody ratio either by assay reformulation or dilution of the sample. For example, a prolactin concentration of 1500 mU/L increased to 950,000 mU/L when dilutions of the sample were reassayed.92 To detect a high dose hook effect in Tg assays, it is recommended that specimens be analysed undiluted and in 1:10 dilution.93
Other Proteins
Other proteins that affect antibody binding and can interfere in immunoassays include complement, lysozyme and paraprotein. Binding of an IgG kappa paraprotein to a TSH assay antibody may have sterically blocked the binding of TSH and led to a falsely low TSH value.94 Complement interferes by binding to the Fc fragment of immunoglobulin and can block the analyte-specific binding sites on an antibody. This inhibits the binding of labelled and unlabelled antigen.20 Lysozyme also binds to immunoglobulin and can form a bridge between solid-phase IgG and detection antibody.81
Incidence of Immunoassay Interference
The prevalence of immunoassay interference is variable and dependent on the type of antibody interference. It may vary from 0.05% for interference from heterophile antibody and HAMA,95 to ≥6%,52,66,68,96–99 depending upon the assay and the analyte. Approximately 40% of serum samples contain non-analyte antibody binding substances, with 15% interference in non-blocked assays.79,100 Ward et al. identified 7 out of 21,000 samples from a hospital population with heterophile interference and HAMA, the interference being as low as 0.03% in blocked IMAs. 95 However, the addition of blockers does not guarantee the elimination of interference. Despite the occurrence of immunoassay interference having been initially reported in the 1970s,101–103 the problem remains today with heterophile antibody interference frequently detected for new immunoassays when tested in the field. Preissner et al.99 recently reported a high heterophile antibody interference rate of >1.5% for a new commercial Tg assay.
The extent of affected immunoassays was highlighted in a multicentre survey of erroneous immunoassay results from assays of 74 analytes in ten donors conducted by 66 laboratories in seven countries.98 Approximately 6% of analyses gave false-positive results with the potential for incorrect clinical interpretation. Of these analyses, 1.8% (n = 65) of results involving 13 analytes were determined to be heterophile false-positive while another 4.2% (n = 146) of results involving 17 analytes gave false-positives of uncertain etiology that were not restored to within the reference interval by addition of heterophile blocking reagent. The blood was from donors with RF-positive illnesses, multiple sclerosis, or lupus, and had detectable RF (31 to >1000 kIU/L) and/or HAMA (3–589 μg/L). Examples of some of the affected analytes are shown in Table 2.98 Bloods from nine of the ten donors resulted in false-positive results of uncertain etiology for six of seven estradiol assay systems (58% of analyses performed) and for two of eight cortisol systems (20% of analyses). For blood from one donor, eight of the eleven tested follicle-stimulating hormone (FSH) and LH assay systems reported false-negative results. The highest percentage of heterophile false-positive results in this survey occurred for plasma myoglobin (48% of analyses performed in two of seven tested assay systems).
Table 2.
False-positive immunoassay results in a multicentre study of 74 analytes 98
| Analyte | No. of assay systems tested | Number (%) of heterophile false-positives per number of analyses performeda | Number (%) of false-positives of uncertain etiology per number of analyses performedb |
|---|---|---|---|
| Thyroglobulin Antibody | 3 | 2/19 (11%) | 4/19 (21%) |
| AFPc | 12 | 0/105 | 9/105 (8.6%) |
| β-hCG + hCG | 15 | 14/281 (5.0%) | 26/281 (9.3%) |
| CA 19-9d | 10 | 1/84 (1.2%) | 9/84 (11%) |
| Cortisol | 8 | 0/85 | 17/85 (20%) |
| Estradiol | 7 | 3/59 (5.1%) | 34/59 (58%) |
| FSH / LH | 12 | 0/136 | 9/136 (6.6%)g |
| Myoglobin | 7 | 28/59 (48%) | 0/59 |
| PTHe | 4 | 6/43 (14%) | 1/43 (2.3%) |
| TNIf | 9 | 18/156 (12%) | 9/156 (5.8%) |
| TSH | 14 | 10/249 (4.0%) | 4/249 (1.6%) |
Heterophile false-positive when immunoassay result was elevated above the reference interval and restored to within the reference interval by pre-treatment with heterophile blocking reagent, or within the reference interval but nevertheless reduced by >30% by pre-treatment with heterophile blocking reagent.
Abnormally increased results that were not restored to within the reference interval by pre-treatment with heterophile blocking reagent.
AFP, α-fetoprotein
CA 19-9, cancer antigen 19-9
PTH, parathyroid hormone
TNI, cardiac troponin I
Results were false-negative
Properties of Interfering Antibody Substances
The interfering antibody substances in immunoassay have several properties in common (Table 3). These include their uniqueness within any one individual and the ability of the concentration to change over time within the same individual due to, e.g., infection, immunisation, blood transfusion, or to remain permanent, e.g., autoimmune disease, animal handling, ingestion of animal food products. False-positive or false-negative results can arise from antibody interferences compared with quality controls, which remain unaffected. The interfering antibody substances may be assay-dependent and interfere within one or more manufacturers’ immunoassay systems, or they may interfere in a number of immunoassays for different analytes.8,98 Because the antibody source is different in different assays, it may be affected to varying extents by interfering substances.
Table 3.
Properties of interfering substances.
|
An example of an analyte that may be affected by heterophile antibody interference in one or more commercial assays is hCG. Cole and co-workers have highlighted how false-positive hCG results led to unnecessary further laboratory and clinical investigations and unnecessary surgery and/or drug therapy in choriocarcinoma or gestational trophoblastic disease.4–6 False-positive hCG concentrations were detected using most of the currently available, commercial hCG immunoassays (Table 4).6,104 Because the different hCG assays have diverse assay formats and use antibodies from different animal sources, the presence of heterophile antibodies may affect hCG tests in differing ways and produce widely varying results.
Table 4.
Examples of false-positive hCG resultsa for different assays in seven patients erroneously diagnosed with choriocarcinoma or gestational trophoblastic disease6,104
| Patient No.b | RIA (IU/L) | Abbott AxSYM (IU/L) | Bayer ACS:180 or Centaur (IU/L) | Beckman Access (IU/L) | DPC Immulite (IU/L) | Dade Behring Stratus (IU/L) | Serono MAIAclone (IU/L) |
|---|---|---|---|---|---|---|---|
| 1 | 28 | 14 | 9.7 | 30 | - | <2 | <2 |
| 2 | <2 | 68 | <2 | 4.6 | <2 | <2 | 2.0 |
| 3 | 10 | 110 | 4.5 | 6.6 | 4.2 | 2.6 | 13 |
| 4 | 11 | 175 | <2 | <2 | <2 | - | 7.8 |
| 5 | - | 139 | 32 | - | <2 | - | - |
| 6 | - | - | 12 | - | 42 | - | - |
| 7 | - | - | - | - | - | 23 | - |
-, Not measured
hCG values ≥10 IU/L are highlighted
All false-positive hCG results were identified by finding hCG immunoreactivity in serum but not in a concurrent urine sample. The addition of heterophile blocking reagent prevented false results
Interfering proteins can be low affinity polyspecific antibodies present in high concentrations or high affinity in low concentrations. Because these interfering substances vary from patient to patient, and their concentration may change from time to time in one patient, they can be difficult to detect and avoid. Covinsky et al.8 showed the multiple effects on many assays of an interfering antibody from a patient with an Escherichia coli (E. coli) septicaemia and how the interference changed with time in the patient (Table 5). Cardiac troponin I (TNI), measured by the Dade Behring Dimension-RxL IMA, increased from 8.7 μg/L on day 3, to 220 μg/L on day 7 following admission of the patient with a urinary tract infection. This was despite any evidence of cardiac damage and a TNI of <0.4 μg/L by the Dade Behring Stratus II assay (Table 5). Other assays for TSH, α-fetoprotein (AFP), cancer antigen-125 (CA-125), and hCG, measured with the Abbott AxSYM, also gave false-positive values. Incubation of the sample with the patient’s E. coli isolate largely adsorbed out the interference. An IgM λ antibody, identified on immunofixation electrophoresis and produced in response to the E. coli infection, caused the falsely increased values in the various two-site IMAs.
Table 5.
False-positive results in multiple immunometric assays due to an interference that changed over time8
| Analyte /Assay system | Untreated sample | Patient sample pre-treated with patient's E. coli isolatea |
|---|---|---|
| TNI Dade Dimension RxL (μg/L) | 235 | <2 |
| TNI Dade Stratus II (μg/L) | <0.4 | Not done |
| AFP Abbott AxSYM (μg/L) | 41 | 5 |
| CA-125 Abbott AxSYM (kU/L) | 225 | 14 |
| hCG Abbott AxSYM (IU/L) | 17 | <5 |
| TSH Abbott AxSYM (mU/L) | 14 | 1.4 |
Incubation of sample with patient's E. coli isolate for 16–18 h prior to assay
Techniques to Minimise Antibody Interferences in Immunoassay
Methods for the reduction of heterophile antibody and anti-animal interferences in immunoassays are shown in Table 6 and include ways to remove or block the interfering antibody.80,81 The interference can be removed by prior extraction of the analyte from the sample, e.g., by gel chromatography, or immunoextracted by the addition of murine or other animal species serum immobilised onto Sepharose beads, or the addition of immobilised Protein A suspension. Alternatively, precipitation with PEG 6000 can remove an anti-animal interference. Heating to 70–90°C is useful for heat-stable analytes only.
Addition of low concentrations of serum or immunoglobulin from the same species as the antibody reagents in the reaction mixture can prevent interference in some samples by neutralising or inhibiting the interference (Table 6). The blocking reagent can be included in the assay diluent or the sample can be pre-treated with additional blocker prior to assay. Determination of the exact amount of blocker sufficient to eliminate interference in all patient samples is difficult to determine in practice as the immune response to interfering antibodies is so variable between individuals. The effectiveness of the added reagent on blocking the interference depends on the species and subclass of the blocker. Non-immune serum, species-specific polyclonal IgG, anti-human IgG or polymerised mouse IgG, non-immune mouse monoclonals, or species-specific fragments of IgG [Fc, Fab, F(ab’)2] from the same species used to produce the reagent antibodies, are commonly used as blocking agents by the manufacturers of kit assays.80,81
Table 6.
Methods for reduction of interference from heterophile antibodies and human anti-animal antibodies80,81
|
Several heterophile blocking reagents (HBR), immunoglobulin inhibiting reagent (IIR), and antibody blocking tubes are commercially available.80 However, in some cases addition of one or more of these blocking agents in manufacturers’ immunoassay reagents is either insufficient or not successful in preventing interference; approximately two-thirds of the observed interferences in a multicentre study were not abolished by blocking agents.98
Detection and Testing for Interference in Suspected Samples
Processes need to be in place to make both laboratories and physicians aware of the potential for immunoassay interference, which can lead to clinical misinterpretation. The processes include on-going education, review of patient results in the clinical setting, protocols for the testing of suspected interference, and notification of interferences both to the physician and to the diagnostic manufacturer. To minimise the reporting of false-positive or false-negative results, a constant dialogue is required between physician and laboratory about unexpected immunoassay results. In general, physicians fail to provide sufficient if any clinical details on request forms and need to be educated about the limitations of assays including possible interferences, the laboratory’s need for relevant clinical notes and medication information, and the likelihood of a patient’s exposure to animals or anti-animal diagnostic or therapeutic agents when taking a clinical history. Physicians should be encouraged to communicate specifically with the laboratory about discordance between results and clinical findings. At the same time senior staff should be proactive in improving the laboratory-clinical communication link by presentation and discussion of laboratory data at local journal club meetings, etc.
If a discrepant result is suspected, there are various procedures that can be implemented to test for the interference (Table 7). These include analysis of the sample using an alternate assay that, if possible, employs antiserum that is raised to a different species and normally gives agreement between methods. If the interference is due to mouse immunoglobulin for example, alternate assays should not use monoclonal mouse antibody reagent because the assay may also be inaccurate. If a significantly different result is detected between methods there is the likelihood of interference. However, agreement between methods does not necessarily exclude interference nor does disagreement, if methods lack standardisation and clinical decision limits differ. The assumption that a result is correct because a majority of immunoassay methods give similar values was shown to be invalid in the multicentre study by Marks in which nine of eleven LH and FSH systems were in agreement but gave falsely low results for a 72-year old postmenopausal woman who was positive for RF. 98
Table 7.
Testing for interference in suspected samples.
|
Another procedure for detecting and identifying a suspected interfering antibody is measurement before and after the addition of a blocking reagent to the sample and the finding of a significantly different result (Table 7). If the suspect interference was not reduced by the addition of more blocking agent then the addition of a series of concentrations of the blocker, or a combination of blockers from different animal sources, from very low to very high, is recommended. This will avoid either too little blocking or forcing the reaction into antigen excess.95 Measurement of a series of dilutions of the sample made using manufacturer’s diluent, provided that it contains non-immune globulin, is another indication whether there is parallelism or non-parallelism with the calibrator (Table 7). For example, a lack of 100% assay recovery for dilutions of 1+1, 1+3, and 1+7 of sample compared with a calibrator may indicate heterophile antibody interference. Demers and Spencer recommend that for unexpectedly high TSH the specimen should be diluted preferably in thyrotoxic serum and remeasured to check for parallelism.105 This is equivalent to adding different concentrations of blocking agent to the sample. Ismail et al.106 noted in a survey of 5,310 sets of TSH and gonadotropin results that of 28 cases with interference in the antibody blocking studies, six demonstrated interference only by dilution and not by blocking studies. This implicates other proteins or non-heterophilic interacting, endogenous antibodies.
Testing for the presence of anti-T3 and anti-T4 autoanti-bodies is mainly performed by radioimmunoprecipitation of labelled T3 or T4 hormones.52 However, radioactive thyroid hormone tracer is no longer readily available in routine laboratories due to the use of automated, non-isotopic immunoassays.
In an example of a case of false hyperprolactinaemia, a number of these procedures were used to identify the presence of a heterophile antibody interference (Table 8).13 The use of three alternate PRL immunoassays indicated a normal PRL value, in keeping with the patient’s clinical status. The falsely elevated PRL was normalised by the use of heterophile blocking tubes and the interfering antibody was identified as a natural IgM idiotypic antibody that bound to the bioMérieux VIDAS mouse assay antibodies but not to other PRL assay antibodies or to blocking agents in the VIDAS assay.
Table 8.
Procedures to identify a false-positive prolactin result due to a heterophile antibody interference13
| Treatment procedure to patient or control serum and re-assay by VIDAS or alternate assay where indicated | Patient sample Prolactin (μg/L) | Control sample Prolactin (μg/L) | Conclusions |
|---|---|---|---|
Untreated:
|
>200
21.6 19.1 18.4 |
-
- - - |
False-positive
Consistent with patient's clinical status |
| Dilutions in VIDAS prolactin diluent (5- to 320-fold, patient; 2- to 32-fold, control) | 117–191% recovery | 83–97% recovery | Increasing recoveries for patient sample suggest interference |
| Addition of mouse, bovine or rabbit serum | No change | - | Insufficient to block interference |
| HAMA-ELISA assay (immobilised mouse IgG and mouse conjugate) | 63 μg/L | - | Weakly positive
HAMA |
| Addition of protein A-Sepharose | No change | - | Not an IgG antibody |
| Heterophile blocking tubes (Scantibodies; murine IgG directed against human IgM): | 17.4 23.3 (Elecsys) | - | Interfering antibody likely to be a natural
IgM idiotypic antibody |
| Precipitation with 25% PEG-6000 and reassay of supernatant | 76% recovery (Elecsys) | - | Negative for macroprolactin (>60% recovery) |
The use of test profiles and cumulative reports together with the patient’s clinical information and history can alert laboratories to inconsistencies between the clinical and biochemical data. Typical examples include the lack of agreement between a profile of tests, e.g., LH and FSH profiles, or discrepant results in thyroid test panels, e.g., FT4 and TSH. In patients routinely tested for thyroid function, patient samples that have FT4 and TSH results in which the concentrations together are considered discordant should be investigated for immunoassay interference. With the Centaur or ACS:180 TSH assay, the solid phase and Lite Reagent contain normal sheep serum and mouse serum, respectively, as blocking reagents to adsorb endogenous heterophile antibodies. Suspected heterophile patient samples are routinely incubated with additional blocker (50 μL of sheep serum is added to 400 μL of patient serum before assay). Examples of a suppression of apparent TSH concentration on addition of sheep immunoglobulin or non-immune sheep serum are shown in Table 9 for four patients investigated for heterophile antibodies.95 The falsely elevated TSH result for patient 1 was only detected because the clinician insisted that the patient was in a thyrotoxic state. The unusual result for patient 4 was detected when the thyroxine dosage was increased with a concomitant increase in FT4 and FT3 while the TSH remained elevated.
Table 9.
Baseline thyroid function investigations in four patients with heterophile antibodies95
| Patient No. | Centaur FT4 Reference Interval (9–23 pmol/L) | Centaur TSH Reference Interval (0.3–5.0 mU/L) | Centaur TSH Additional sheep seruma (mU/L) | Clinical data |
|---|---|---|---|---|
| 1 | 17 | 0.10 | <0.03 | Possibly thyrotoxic state |
| 2 | 27 | 26.0 | <0.03 | Thyrotoxic state |
| 3 | 17 | 11.8 | 0.6 | Receiving thyroxine |
| 4 | 13 | 24.0 | 1.2 | Possible hypothyroid |
With these patients 10–50 μL of extra sheep serum was required to eliminate the observed interference
In practice it may be difficult to identify false-positive results in some patients. The physiological condition of the patient can lead to misinterpretation of results due to differences in metabolism, receptor binding, or post-translational defects, e.g., the “sick euthyroid” patient. An immunoassay measurement may not translate to bioactivity in a patient or relate biologically. For example, in central hypothyroidism immunoreactive TSH has reduced bioactivity,105 and in hyperprolactinaemia there may be biologically inactive but immunochemically reactive macro-PRL present in serum.56 Some suspected heterophile antibodies may mimic interference, e.g., elevated hCG in renal disease, menopause, or in the blood of non-pregnant women on haemodialysis.107 In contrast, two-site IMAs that use monoclonal antibodies may result in assay hypersensitivity and a lack of recognition of all hormone isoforms including the biologically active, clinically important forms. For example, certain isoforms of LH, FSH, and ß-hCG may not be recognised by some immunoassays resulting in falsely low results.81,104
Conclusions
Despite advances in our knowledge and understanding of the mechanisms of interference in immunoassays, there is no single procedure that can rule out all interferences. Even now there are reports of newly developed assays that suffer from high levels of interference when tested in the field.98,99 It is important to recognise the potential for interference in immunoassay and to put procedures in place to identify them wherever possible. Most important is a consideration of the final clinical picture. If there is any clinical suspicion of discordance between the clinical and the laboratory data an attempt should be made to reconcile the difference. The detection of interference may require the use of an alternate assay, or measurement before and after treatment with additional blocking reagent, or following dilution of the sample in non-immune serum. If testing is inconclusive and the interference cannot be identified, the laboratory report should indicate there is a discrepancy for that analyte due to some technical inaccuracy and suggest the test be repeated using another sample. The analyte concentration should not be reported.
Interference in immunoassay is one factor that contributes to the uncertainty of medical testing. Laboratories should be aware of the potential for interference in all immunoassays and how artefactual results may cause misinterpretation of a patient’s results and a subsequent wrong diagnosis and unwarranted treatment. The recognition of such aberrant test results requires constant surveillance by both laboratory and physician.
References
- 1.Stuart MC. The immunoassay revolution. Clin Biochem Rev. 1992;13:14–21. [Google Scholar]
- 2.Kroll MH, Elin RJ. Interference with clinical laboratory analyses. Clin Chem. 1994;40:1996–2005. [PubMed] [Google Scholar]
- 3.Ismail AAA, Barth JH. Wrong biochemistry results [editorial] Br Med J. 2001;323:705–6. doi: 10.1136/bmj.323.7315.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cole LA, Rinne KM, Shahabi S, Omrani A. False-positive hCG assay results leading to unnecessary surgery and chemotherapy and needless occurrences of diabetes and coma. Clin Chem. 1999;45:313–4. [PubMed] [Google Scholar]
- 5.Rotmensch S, Cole LA. False diagnosis and needless therapy of presumed malignant disease in women with false-positive human chorionic gonadotropin concentrations. Lancet. 2000;355:712–5. doi: 10.1016/S0140-6736(00)01324-6. [DOI] [PubMed] [Google Scholar]
- 6.Cole LA. False positive hCG immunoassay results [Abstract] Clin Chem. 2002;48 (Suppl 6):A115. [Google Scholar]
- 7.Torjesen PA, Bjøro T. Antibodies against [I125] testosterone in patient's serum: A problem for the laboratory and the patient. Clin Chem. 1996;42:2047–8. [PubMed] [Google Scholar]
- 8.Covinsky M, Laterza O, Pfeifer JD, Farkas-Szallasi T, Scott MG. An IgM λ antibody to Escherichia coli produces false-positive results in multiple immunometric assays. Clin Chem. 2000;46:1157–61. [PubMed] [Google Scholar]
- 9.Krahn J, Parry DM, Leroux M, Dalton J. High percentage of false positive cardiac troponin I results in patients with rheumatoid factor. Clin Biochem. 1999;32:477–80. doi: 10.1016/s0009-9120(99)00044-2. [DOI] [PubMed] [Google Scholar]
- 10.Plebani M, Mion M, Altinier S, Girotto MA, Baldo G, Zaninotto M. False-positive troponin I attributed to a macrocomplex [letter] Clin Chem. 2002;48:677–9. [PubMed] [Google Scholar]
- 11.Bohner J, von Pape K-W, Hannes W, Stegmann T. False-negative immunoassay results for cardiac troponin I probably due to circulating troponin I autoantibodies [letter] Clin Chem. 1996;42:2046. [PubMed] [Google Scholar]
- 12.Norden AGW, Jackson RA, Norden LE, Griffin AJ, Barnes MA, Little JA. Misleading results from immunoassays of serum free thyroxine in the presence of rheumatoid factor. Clin Chem. 1997;43:957–62. [PubMed] [Google Scholar]
- 13.Sapin R, Simon C. False hyperprolactinemia corrected by the use of heterophilic antibody-blocking agent [letter] Clin Chem. 2001;47:2184–5. [PubMed] [Google Scholar]
- 14.Steimer W, Müller C, Eber B, Emmanuilidis K. Intoxication due to negative canrenone interference in digoxin drug monitoring. Lancet. 1999;354:1176–7. doi: 10.1016/S0140-6736(99)03818-0. [DOI] [PubMed] [Google Scholar]
- 15.Kricka LJ. Interferences in immunoassay - still a threat [editorial] Clin Chem. 2000;46:1037–8. [PubMed] [Google Scholar]
- 16.Leape LL. Striving for perfection [editorial] Clin Chem. 2002;48:1871–2. [PubMed] [Google Scholar]
- 17.Valdes Jr R, Jortani SA. Unexpected suppression of immunoassay results by cross-reactivity: now a demonstrated cause for concern [editorial] Clin Chem. 2002;48:405–6. [PubMed] [Google Scholar]
- 18.Panteghini M. Performance of today's cardiac troponin assays and tomorrow's [editorial] Clin Chem. 2002;48:809–10. [PubMed] [Google Scholar]
- 19.Davies C. Concepts. In: Wild D, editor. The immunoassay handbook. 2nd edition, United Kingdom: Nature Publishing Group, 2001. pp. 78–110.
- 20.Weber TH, Käpyaho KI, Tanner P. Endogenous interference in immunoassays in clinical chemistry. A review. Scand J Clin Lab Invest. 1990;50 (Suppl 201):77–82. [PubMed] [Google Scholar]
- 21.Boscato LM, Egan GM, Stuart MC. Specificity of two-site immunoassays. J Immunol Methods. 1989;117:221–9. doi: 10.1016/0022-1759(89)90144-0. [DOI] [PubMed] [Google Scholar]
- 22.Hursting MJ, Raisys VA, Opheim KE. Drug-specific Fab therapy in drug overdose. Arch Pathol Lab Med. 1987;111:693–7. [PubMed] [Google Scholar]
- 23.Jones G. Handling common laboratory interferences. Clin Biochem Rev. 2002;23:105–11. [Google Scholar]
- 24.Price C, Newman D. In: Price CP, Newman DJ, editors. Principles and Practice of Immunoassay. 2nd edition, United Kingdom: MacMillan Reference Ltd., 1997, pp. 445–480.
- 25.Thomas CMG, Segers MFG. Discordant results for choriogonadotropin: A problem caused by lutropin β-subunit interference? [letter] Clin Chem. 1985;31:159. [PubMed] [Google Scholar]
- 26.Berthod C, Rey F. Enormous cross-reactivity of hydrocortisone hemisuccinate in the "RIANEN" RIA kit for cortisol determination. Clin Chem. 1988;34:1358. [PubMed] [Google Scholar]
- 27.Gagajewski A, Davis GK, Kloss J, Poch GK, Anderson CJ, Apple FS. False-positive lysergic acid diethylamide immunoassay screen associated with fentanyl medication [letter] Clin Chem. 2002;48:205–6. [PubMed] [Google Scholar]
- 28.Lichtenwalner MR, Mencken T, Tully R, Petosa M. False-positive immunochemical screen for methadone attributable to metabolites of verapamil. Clin Chem. 1998;44:1039–41. [PubMed] [Google Scholar]
- 29.Daher R, Haidar JH, Al-Amin H. Rifampin interference with opiate immunoassays [letter] Clin Chem. 2002;48:203–4. [PubMed] [Google Scholar]
- 30.Lewis JH, Dusci L, Hackett P, Potter JM. Drugs of abuse: analytical and clinical perspective in the 1990s. Clin Biochem Rev. 1998;19:18–32. [Google Scholar]
- 31.Steimer W. Performance and specificity of monoclonal immunoassays for cyclosporine monitoring: how specific is specific? Clin Chem. 1999;45:371–81. [PubMed] [Google Scholar]
- 32.Datta P, Xu L, Malik S, et al. Effect of antibody specificity on results of selected digoxin immunoassays among various clinical groups. Clin Chem. 1996;42:373–9. [PubMed] [Google Scholar]
- 33.Azzazy HME, Duh S-H, Maturen A, et al. Multicenter study of Abbott AxSYM® digoxin II assay and comparison with 6 methods for susceptibility to digoxin-like immunoreactive factors. Clin Chem. 1997;43:1635–40. [PubMed] [Google Scholar]
- 34.Steimer W, Müller C, Eber B. Digoxin assays: frequent, substantial, and potentially dangerous interference by spironolactone, canrenone, and other steroids. Clin Chem. 2002;48:507–16. [PubMed] [Google Scholar]
- 35.Miller JJ, Valdes Jr R. Approaches to minimizing interference by cross-reacting molecules in immunoassays. Clin Chem. 1991;37:144–53. [PubMed] [Google Scholar]
- 36.Slaats EH, Kennedy JC, Kruijswijk H. Interference of sex-hormone binding globulin in the "Coat-A-Count" testosterone no-extraction radioimmunoassay. Clin Chem. 1987;33:300–2. [PubMed] [Google Scholar]
- 37.Masters AM, Hähnel R. Investigation of sex-hormone binding globulin interference in direct radioimmunoassays for testosterone and estradiol. Clin Chem. 1989;35:979–84. [PubMed] [Google Scholar]
- 38.Vining RF. Steroid radioimmunoassay. Clin Biochem Rev. 1981;2:39–49. [Google Scholar]
- 39.Nelson JC, Wilcox RB. Analytical performance of free and total thyroxine assays. Clin Chem. 1996;42:146–54. [PubMed] [Google Scholar]
- 40.Symons RG. Interference with the laboratory assessment of thyroid function. Clin Biochem Rev. 1989;10:44–9. [Google Scholar]
- 41.Mendel CM, Frost PH, Kunitake ST, Cavalieri RR. Mechanism of the heparin-induced increase in the concentration of free thyroxine in plasma. J Clin Endocrinol Metab. 1987;65:1259–64. doi: 10.1210/jcem-65-6-1259. [DOI] [PubMed] [Google Scholar]
- 42.Liewendahl K, Tikanoja S, Mähönen H, Helenius T, Vällmäki M, Tallgren LG. Concentrations of iodothyronines in serum of patients with chronic renal failure and other nonthyroidal illnesses: role of free fatty acids. Clin Chem. 1987;33:1382–6. [PubMed] [Google Scholar]
- 43.Laji K, Rhidha B, John R, Lazarus J, Davies JS. Abnormal serum free thyroid hormone levels due to heparin administration. Q J Med. 2001;94:471–3. doi: 10.1093/qjmed/94.9.471. [DOI] [PubMed] [Google Scholar]
- 44.Armstrong GP, Barker AN, Patel H, Hart HH. Reference interval for troponin I on the ACS:Centaur assay: a recommendation based on the recent redefinition of myocardial infarction. Clin Chem. 2002;48:198–9. [PubMed] [Google Scholar]
- 45.Evans MJ, Livesey JH, Ellis MJ, Yandle TG. Effect of anticoagulants and storage temperatures on stability of plasma and serum hormones. Clin Biochem. 2001;34:107–12. doi: 10.1016/s0009-9120(01)00196-5. [DOI] [PubMed] [Google Scholar]
- 46.Ellis MJ, Livesey JH, Evans MJ. Hormone stability in human whole blood. Clin Biochem. 2003;36:109–12. doi: 10.1016/s0009-9120(02)00440-x. [DOI] [PubMed] [Google Scholar]
- 47.Glendenning P, Musk AA, Taranto M, Vasikaran SD. Preanalytical factors in the measurement of intact parathyroid hormone with the DPC IMMULITE assay. Clin Chem. 2002;48:566–7. [PubMed] [Google Scholar]
- 48.Galligan J, Ward G, Jacobi J, McMaugh C. Preanalytical variation in samples collected for assay of adrenocorticotrophin. Clin Biochem Rev. 1996;17:100. [Google Scholar]
- 49.Kilinc AS, Düzoylum A, Uncugil CF, Yücel D. Falsely increased free triidothyronine in sera stored in serum separator tubes [letter] Clin Chem. 2002;48:2296–7. [PubMed] [Google Scholar]
- 50.Wickus GG, Mordan RJ, Mathews EA. Interference in the Allégro immunoassay system when blood is collected in silicone-coated tubes [letter] Clin Chem. 1992;38:2347–8. [PubMed] [Google Scholar]
- 51.Chang C-Y, Lu J-Y, Chien T-I, et al. Interference caused by the contents of serum separator tubes in the Vitros CRP assay. Ann Clin Biochem. 2003;40:249–51. doi: 10.1258/000456303321610556. [DOI] [PubMed] [Google Scholar]
- 52.Després N, Grant AM. Antibody interference in thyroid assays: a potential for clinical misinformation. Clin Chem. 1998;44:440–54. [PubMed] [Google Scholar]
- 53.Sakata S, Nakamura S, Miura K. Autoantibodies against thyroid hormones or iodothyronine. Ann Intern Med. 1985;103:579–89. doi: 10.7326/0003-4819-103-4-579. [DOI] [PubMed] [Google Scholar]
- 54.Sapin R. Anti-insulin antibodies in insulin immunometric assays: a still possible pitfall. Eur J Clin Chem Clin Biochem. 1997;35:365–7. doi: 10.1515/cclm.1997.35.5.365. [DOI] [PubMed] [Google Scholar]
- 55.Casesnoves A, Mauri M, Dominguez JR, Alfayate R, Pico AM. Influence of anti-insulin antibodies on insulin immunoassays in the autoimmune insulin syndrome. Ann Clin Biochem. 1998;35:768–74. doi: 10.1177/000456329803500610. [DOI] [PubMed] [Google Scholar]
- 56.Glezer A, d'Alva CB, Salgado LR, et al. Pitfalls in pituitary diagnosis: peculiarities of three cases. Clin Endocrinol. 2002;57:135–9. doi: 10.1046/j.1365-2265.2002.01567.x. [DOI] [PubMed] [Google Scholar]
- 57.Kuwahara A, Kamada M, Irahara M, Naka O, Yamashita T, Aono T. Autoantibody against testosterone in a woman with hypergonadotropic hypogonadism. J Clin Endocrinol Metab. 1998;83:14–16. doi: 10.1210/jcem.83.1.4510. [DOI] [PubMed] [Google Scholar]
- 58.Caballero A, Corcoy R, Negredo E, Rodriguez-Espinosa J. Autoantibodies against thyroid hormones can lead to an erroneous diagnosis and potentially harmful treatment. Ann Clin Biochem. 1998;35:152–3. doi: 10.1177/000456329803500125. [DOI] [PubMed] [Google Scholar]
- 59.Sakata S, Matsuda M, Ogawa T, et al. Prevalence of thyroid hormone autoantibodies in healthy subjects. Clin Endocrinol. 1994;41:365–70. doi: 10.1111/j.1365-2265.1994.tb02558.x. [DOI] [PubMed] [Google Scholar]
- 60.Hjiyiannakis P, Mundy J, Harmer C. Thyroglobulin antibodies in differentiated thyroid cancer. Clin Oncol. 1999;11:240–4. doi: 10.1053/clon.1999.9056. [DOI] [PubMed] [Google Scholar]
- 61.Spencer CA, Takeuchi M, Kazarosyan M, et al. Serum thyroglobulin autoantibodies: prevalence, influence on serum thyroglobulin measurement, and prognostic significance in patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab. 1998;83:1121–7. doi: 10.1210/jcem.83.4.4683. [DOI] [PubMed] [Google Scholar]
- 62.Fahie-Wilson MN, Ahlquist JA. Hyperprolactinaemia due to macroprolactins: some progress but still a problem [commentary] Clin Endocrinol. 2003;58:683–5. doi: 10.1046/j.1365-2265.2003.01787.x. [DOI] [PubMed] [Google Scholar]
- 63.Smith TP, Suliman AM, Fahie-Wilson MN, McKenna TJ. Gross variability in the detection of prolactin in sera containing big big prolactin (macroprolactin) by commercial immunoassays. J Clin Endocrinol Metab. 2002;87:5410–5. doi: 10.1210/jc.2001-011943. [DOI] [PubMed] [Google Scholar]
- 64.Ward G, McMaugh C, Vinning A, Merrit T, Hickman P. Macroprolactin immunoreactivity -differences in recognition of macroprolactin by the ACS:180 and Tosoh immunoassay systems. Clin Biochem Rev. 1998;19:82. [Google Scholar]
- 65.Prazeres S, Santos MA, Ferreira HG, Sobrinho LG. A practical method for the detection of macroprolactinaemia using ultrafiltration. Clin Endocrinol. 2003;58:686–90. doi: 10.1046/j.1365-2265.2003.01721.x. [DOI] [PubMed] [Google Scholar]
- 66.Levinson SS, Miller JJ. Towards a better understanding of heterophile (and the like) antibody interference with modern immunoassays. Clin Chim Acta. 2002;325:1–15. doi: 10.1016/s0009-8981(02)00275-9. [DOI] [PubMed] [Google Scholar]
- 67.Bouvet J-P, Quan CP, Dighiero G. Polyreactivity is not an artefact [letter] J Immunol Methods. 2001;254:199–201. doi: 10.1016/s0022-1759(01)00362-3. [DOI] [PubMed] [Google Scholar]
- 68.Ismail AAA, Walker PL, Cawood ML, Barth JH. Interference in immunoassay is an underestimated problem. Ann Clin Biochem. 2002;39:366–73. doi: 10.1258/000456302760042128. [DOI] [PubMed] [Google Scholar]
- 69.Kaplan IV, Levinson SS. When is a heterophile antibody not a heterophile antibody? When it is an antibody against a specific immunogen [opinion] Clin Chem. 1999;45:616–8. [PubMed] [Google Scholar]
- 70.Dasgupta A, Banerjee SK, Datta P. False-positive troponin I in the MEIA due to the presence of rheumatoid factors in serum. Am J Clin Pathol. 1999;112:753–6. doi: 10.1093/ajcp/112.6.753. [DOI] [PubMed] [Google Scholar]
- 71.Banerjee S, Linder MW, Singer I. False-positive troponin I in a patient with acute cholecystitis and positive rheumatoid factor assay. Cardiology. 2001;95:170–1. doi: 10.1159/000047367. [DOI] [PubMed] [Google Scholar]
- 72.Fitzmaurice TF, Brown C, Rifai N, Wu AHB, Yeo K-TJ. False increase of cardiac troponin I with heterophilic antibodies. Clin Chem. 1998;44:2212–4. [PubMed] [Google Scholar]
- 73.Yeo K-TJ, Storm CA, Li Y, et al. Performance of the enhanced Abbott AxSYM cardiac troponin I reagent in patients with heterophilic antibodies. Clin Chim Acta. 2000;292:13–23. doi: 10.1016/s0009-8981(99)00260-0. [DOI] [PubMed] [Google Scholar]
- 74.Martel J, Després N, Ahnadi CE, et al. Comparative multicentre study of a panel of thyroid tests using different automated immunoassay platforms and specimens at high risk of antibody interference. Clin Chem Lab Med. 2000;38:785–93. doi: 10.1515/CCLM.2000.112. [DOI] [PubMed] [Google Scholar]
- 75.Stevenson DL, Harris AG, Neal KR, Irving WL on behalf of Trent HCV Study Group. The presence of rheumatoid factor in sera from anti-HCV positive blood donors interferes with the detection of HCV-specific IgM. J Hepatol. 1996. pp. 621–6. [DOI] [PubMed]
- 76.Check JH, Ubelacker L, Lauer CC. Falsely elevated steroidal assay levels related to heterophile antibodies against various animal species. Gynecol Obstet Invest. 1995;40:139–40. doi: 10.1159/000292323. [DOI] [PubMed] [Google Scholar]
- 77.Fiad TM, Duffy J, McKenna TJ. Multiple spuriously abnormal thyroid function indices due to heterophilic antibodies. Clin Endocrinol. 1994;41:391–5. doi: 10.1111/j.1365-2265.1994.tb02563.x. [DOI] [PubMed] [Google Scholar]
- 78.Liendo C, Ghali JK, Graves SW. A new interference in some digoxin assays: Anti-murine heterophilic antibodies. Clin Pharmacol Ther. 1996;60:593–8. doi: 10.1016/S0009-9236(96)90157-5. [DOI] [PubMed] [Google Scholar]
- 79.Boscato LM, Stuart MC. Incidence and specificity of interference in two-site immunoassays. Clin Chem. 1986;32:1491–5. [PubMed] [Google Scholar]
- 80.Kricka LJ. Human anti-animal antibody interferences in immunological assays. Clin Chem. 1999;45:942–56. [PubMed] [Google Scholar]
- 81.Selby C. Interference in immunoassay. Ann Clin Biochem. 1999;36:704–21. doi: 10.1177/000456329903600603. [DOI] [PubMed] [Google Scholar]
- 82.Sosolik RC, Hitchcock CL, Becker WJ. Heterophilic antibodies produce spuriously elevated concentrations of the MB isoenzyme of creatine kinase in a selected patient population. Am J Clin Pathol. 1997;107:506–10. doi: 10.1093/ajcp/107.5.506. [DOI] [PubMed] [Google Scholar]
- 83.White GH, Tideman PA. Heterophilic antibody interference with CARDIAC T quantitative rapid assay [letter] Clin Chem. 2002;48:201–3. [PubMed] [Google Scholar]
- 84.Zweig MH, Csako G, Spero M. Escape from blockade of interfering heterophile antibodies in a two-site immunoradiometric assay for thyrotropin. Clin Chem. 1988;34:2589–91. [PubMed] [Google Scholar]
- 85.Frost SJ, Hine KR, Firth GB, Wheatley T. Falsely lowered FT4 and raised TSH concentrations in a patient with hyperthyroidism and human anti-mouse monoclonal antibodies. Ann Clin Biochem. 1998;35:317–20. doi: 10.1177/000456329803500220. [DOI] [PubMed] [Google Scholar]
- 86.Flourié F, Parant F, Pénès MC, Alcaraz-Galvain D. Falsely elevated thyroid-stimulating hormone concentrations attributable to interference from human anti-mouse antibodies [letter] Clin Chem. 2002;48:2289. [PubMed] [Google Scholar]
- 87.Howanitz PJ, Howanitz JH, Lamberson HV, Ennis KM. Incidence and mechanism of spurious increases in serum thyrotropin. Clin Chem. 1982;28:427–31. [PubMed] [Google Scholar]
- 88.Boerman OC, Segers MFG, Poels LG, Kenemans P, Thomas CMG. Heterophilic antibodies in human sera causing falsely increased results in the CA 125 immunofluorometric assay. Clin Chem. 1990;36:888–91. [PubMed] [Google Scholar]
- 89.Ryall RG, Story CJ, Turner DR. Reappraisal of the causes of the "hook effect" in two-site immunoradiometric assays. Anal Biochem. 1982;127:308–15. doi: 10.1016/0003-2697(82)90178-6. [DOI] [PubMed] [Google Scholar]
- 90.Ohashi S, Kaji H, Abe H, Chihara K. "Paradoxical" GH suppression by secretagogues in acromegaly? Horm Metab Res. 1993;25:393–4. doi: 10.1055/s-2007-1002128. [DOI] [PubMed] [Google Scholar]
- 91.Sturgeon CM, McAllister EJ. Analysis of hCG: clinical applications and assay requirements. Ann Clin Biochem. 1998;35:460–91. doi: 10.1177/000456329803500402. [DOI] [PubMed] [Google Scholar]
- 92.St-Jean E, Blain F, Comtois R. High prolactin levels may be missed by immunoradiometric assay in patients with macroprolactinomas. Clin Endocrinol. 1996;44:305–9. doi: 10.1046/j.1365-2265.1996.663486.x. [DOI] [PubMed] [Google Scholar]
- 93.Demers LM, Spencer CA (Eds). Laboratory support for the diagnosis and monitoring of thyroid disease: Thyroglobulin (Tg) measurement. Thyroid 2003;13:57–67.
- 94.Luzzi VI, Scott MG, Gronowski AM. Negative thyrotropin assay interference associated with an IgGk paraprotein [letter] Clin Chem. 2003;49:709–10. doi: 10.1373/49.4.709. [DOI] [PubMed] [Google Scholar]
- 95.Ward G, McKinnon L, Badrick T, Hickman PE. Heterophilic antibodies remain a problem for the immunoassay laboratory. Am J Clin Pathol. 1997;108:417–21. doi: 10.1093/ajcp/108.4.417. [DOI] [PubMed] [Google Scholar]
- 96.Kuroki M, Matsumoto Y, Arakawa F, et al. Reducing interference from heterophilic antibodies in a two-site immunoassay for carcinoembyronic antigen (CEA) by using a human/mouse chimeric antibody to CEA as the tracer. J Immunol Methods. 1995;180:81–91. doi: 10.1016/0022-1759(94)00301-c. [DOI] [PubMed] [Google Scholar]
- 97.Bjerner J, Nustad K, Norum LF, Hauge Olsen K, Børmer OP. Immunometric assay interference: incidence and prevention. Clin Chem. 2002;48:613–21. [PubMed] [Google Scholar]
- 98.Marks V. False-positive immunoassay results: a multicenter survey of erroneous immunoassay results from assays of 74 analytes in 10 donors from 66 laboratories in seven countries. Clin Chem. 2002;48:2008–16. [PubMed] [Google Scholar]
- 99.Preissner CM, O'Kane DJ, Singh RJ, Morris JC, Grebe SKG. Phantoms in the assay tube: heterophile antibody interferences in serum thyroglobulin assays. J Clin Endocrinol Metab. 2003;88:3069–74. doi: 10.1210/jc.2003-030122. [DOI] [PubMed] [Google Scholar]
- 100.Boscato LM, Stuart MC. Heterophilic antibodies: a problem for all immunoassays. Clin Chem. 1988;34:27–33. [PubMed] [Google Scholar]
- 101.Sgouris JT. Limitations of the radioimmunoassay for Hepatitis B antigen [letter] N Engl J Med. 1973;288:160–1. doi: 10.1056/NEJM197301182880317. [DOI] [PubMed] [Google Scholar]
- 102.Prince AM, Brotman B, Jass D, Ikram H. Specificity of the direct solid-phase radioimmunoassay for detection of the Hepatitis-B antigen. Lancet. 1973;i:1346–50. doi: 10.1016/s0140-6736(73)91674-7. [DOI] [PubMed] [Google Scholar]
- 103.Hunter WM, Budd PS. Circulating antibodies to ovine and bovine immunoglobulin in healthy subjects: a hazard for immunoassays [letter] Lancet. 1980;ii:1136–7. doi: 10.1016/s0140-6736(80)92565-9. [DOI] [PubMed] [Google Scholar]
- 104.Cole LA, Shahabi S, Butler SA, et al. Utility of commonly used commercial human chorionic gonadotropin immunoassays in the diagnosis and management of trophoblastic diseases. Clin Chem. 2001;47:308–15. [PubMed] [Google Scholar]
- 105.Demers LM, Spencer CA (Eds). Laboratory support for the diagnosis and monitoring of thyroid disease: Thyrotropin/thyroid stimulating hormone (TSH) measurement. Thyroid 2003;13:33–44.
- 106.Ismail AAA, Walker PL, Barth JH, Lewandowski KC, Jones R, Burr WA. Wrong biochemistry results: two case reports and observational study in 5310 patients on potentially misleading thyroid-stimulating hormone and gonadotropin immunoassay results. Clin Chem. 2002;48:2023–9. [PubMed] [Google Scholar]
- 107.Schwarz A, Post K-G, Keller F, Molzahn M. Value of human chorionic gonadotropin measurements in blood as a pregnancy test in women on maintenance hemodialysis. Nephron. 1985;39:341–3. doi: 10.1159/000183402. [DOI] [PubMed] [Google Scholar]