Abstract
The sweat test has been used for more than 50 years for the diagnosis of cystic fibrosis (CF) and remains an important diagnostic test in the genomic era. The currently used reference intervals for sweat electrolytes are applied to all patients regardless of age or sex. We performed a systematic review to summarise the studies with published reference values of sweat electrolyte concentrations for the diagnosis of CF. The MEDLINE (from 1950), EMBASE (from 1980) and PubMed (from 1950) databases were searched for English language studies. An abstract was also found by hand-searching. The search generated 1136 articles that matched the search key terms. Of these, 17 studies that contained data on sweat electrolyte concentrations were included in the analysis. Among these, seven studies did not perform the sweat test in accordance with current international and Australian guidelines. Of the ten remaining studies, four reported both the sweat sodium and chloride concentrations and six reported sweat chloride concentration only. A major limitation of these studies was the subject selection. Most recruited patients with various medical conditions including respiratory diseases or undefined recruitment criteria, whilst some did not report the subjects’ age and some had small subject numbers. Only one study performed mutation analysis to determine carrier status. No study used appropriate statistical analysis to develop a sweat chloride reference interval. The literature review yielded no studies that reliably developed reference intervals for sweat electrolyte concentrations. The limitations of the studies highlight the need for reliable age-related reference intervals for sweat electrolyte concentrations in healthy subjects.
Introduction
CF is the most common autosomal recessive disorder among Caucasians in Australia with an incidence of 1 in 2500.1 The characteristic clinical features of CF are suppurative lung disease, pancreatic insufficiency, neonatal bowel obstruction (meconium ileus), multifocal biliary cirrhosis, absent vas deferens and high sweat electrolytes.2 Prior to the discovery of the CF transmembrane conductance regulator (CFTR) gene and the mutations responsible for CF, the diagnosis was based on clinical features and the measurement of elevated sweat electrolyte concentrations.2 The CFTR gene encodes a cAMP-regulated chloride channel which regulates chloride transport at the apical membrane of epithelial surfaces such as the airways, pancreatic ducts, biliary tree and sweat duct.2 In the sweat ducts, CFTR regulates chloride reabsorption and this forms the basis of the sweat test. Since the discovery of the CFTR gene in 1989, it has been possible to use gene mutation analysis as an alternative to sweat testing for the diagnosis of CF.3–5 However, more than 1200 mutations and polymorphisms have been identified6 and it is currently not feasible to routinely test for more than 20–30 of them. Some of the mutations are associated with a mild phenotype and this has increased the complexity of the diagnosis of CF. The sweat test is a measure of CFTR function and for this reason remains a valuable test for the diagnosis of CF, even in the genomic era.7
Analysis of sweat electrolytes has been used for the diagnosis of CF for more than 50 years.8,9 The first standardised methodology for sweat collection was introduced by Gibson and Cooke in 1959.9 Their method described the stimulation of muscarinic receptors by the application of pilocarpine to a local area of skin by iontophoresis and the subsequent collection of the sweat onto filter paper. A later advance on this technique was the development of the Wescor Macroduct collection system (www.wescor.com, Helena Laboratories, Mt Waverley, Victoria) in 1983.10–12 The collection and analysis of the sweat sample is recognised as being technically demanding and subject to a number of pre-analytical and analytical limitations.13–16 Recently international and Australian guidelines for the performance of the sweat test have been published with the common aim of further standardising both the collection and analytical components of the sweat test to improve accuracy.13–15 However, these guidelines do not discuss the limitations of the individual studies upon which the recommended reference intervals are based.
Currently the universally accepted reference intervals for sweat chloride concentrations are: >60 mmol/L is considered diagnostic of CF; 40–60 mmol/L borderline; and <40 mmol/L normal.13–15 Sweat sodium may be measured in addition to chloride for quality control purposes, since there is usually little difference between sweat sodium and chloride concentrations.13–15 These reference intervals are applied to all patients regardless of age or sex. The aim of this review is to critically evaluate the published studies that include reference intervals for sweat electrolyte concentrations.
Methodology
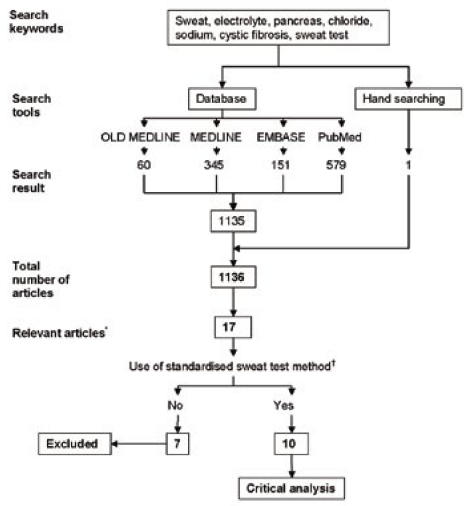
We performed a systematic review of all English language studies that included published reference values for sweat chloride and/or sodium concentrations in subjects with and without CF. The following databases were searched, OLD MEDLINE (1950 to 1965) and MEDLINE (1966 to 8 November 2006), EMBASE (1980 to 8 November 2006) and PubMed (1950 to 8 November 2006). The key words for the database search were sweat, electrolyte, pancreas, chloride, sodium, cystic fibrosis and sweat test (Figure). The titles and abstracts of the articles were screened (AM). We retrieved the full paper of studies that contained sweat electrolyte concentrations in non-CF and CF subjects. Reference lists of these articles were also searched for missing papers. The Australian and UK guidelines for the performance of the sweat test were also reviewed for additional data.15,17 In addition one abstract was found by hand searching and the author of this abstract was contacted to provide missing information. The relevant papers were reviewed by all three authors and information was collected on criteria which included subject selection (age, medical history, sample size and use of CFTR gene mutation analysis); sweat test methodology (sweat stimulation, collection and analysis), and the reporting of the sweat chloride and sodium concentrations. For this review we used the criteria described in 1994 by Solberg who defines an adequate sample size as one “consisting of at least 40 subjects.”18
Figure. Diagrammatic representation of the search methodology and inclusion criteria for the systematic review.
*These articles were considered relevant as they contained sweat electrolyte concentrations in non-CF and CF subjects.
†The standardised sweat test method refers to the method described in the international and Australian guidelines for the performance of the sweat test in relation to stimulation by pilocarpine iontophoresis and sweat chloride measurement by an accepted technique.
Results
A total of 1135 articles matched the search terms for the respective databases: 60 articles in OLD MEDLINE, 345 in MEDLINE, 151 in EMBASE and 579 in the PubMed database (Figure). From these 1135 articles, 16 articles were found to be relevant.8,9,19–32 An additional three studies which only presented data for the CF population were identified but did not fit the inclusion criteria of electrolyte concentrations on subjects with and without CF and thus were excluded.33–35 One abstract, which was presented at the International Congress of Clinical Chemistry in 1993, was also included.36 In total there were 17 studies included in this review.
The sweat test methodology used in seven of the 17 identified studies8,19–23,25 did not meet the accepted criteria for the performance of the sweat test as determined by the recently published national and international guidelines.13–15 These seven studies include four that did not use pilocarpine iontophoresis to stimulate the sweat glands8, 19–21 and three that did not report the mean sweat chloride concentration.22,23,25 The details of these studies are presented in Table 1.
Table 1.
Review of the studies that did not comply with the guidelines for the performance of sweat testing.
| Study | Sample size (n) | Selection criteria* | Age range (years) | Sweat test method |
Sweat electrolyte concentrations (mmol/L) |
Study limitations† | |
|---|---|---|---|---|---|---|---|
| Chloride (Cl−) | Sodium (Na+) | ||||||
| New York Study (di Sant’ Agnese et al, 1953)8 | Total: n = 109 |
Stimulation: mild thermal stimulus while lying on a bed at 90 °F(32 °C), 50% humidity
Collection: 1–2 hours, mid-abdominal area on a gauze patch Analysis: Sodium – flame photometer Chloride – Keys |
- Performed before the development of the standardised sweat test by Gibson and Cooke. The method used to stimulate and collect the sweat is currently not accepted.
- Sweat sodium and chloride concentrations >150 mmol/L are physiologically impossible. |
||||
| 50 | 1. CF | 2–17 | Mean: 106
SD: NR Range: 60–160 |
Mean: 133
SD: NR Range: 80–190 |
|||
| 59 | 2. Non-CF symptomatic | 1.5–64 | Mean: 32
SD: NR Range: 4–80 |
Mean: 59
SD: NR Range 1–120 |
|||
| London Study (Webb et al, 1957)21 | Total: n = 32 |
Stimulation: patient placed in a plastic bag covered with 3–4 blankets
Collection: 1–2 hours, filter paper Analysis: Sodium – flame photometer Chloride – titration with mercuric nitrate |
- Performed before the development of the standardised sweat test by Gibson and Cooke. The method used to stimulate and collect the sweat is currently not accepted. | ||||
| 12 | 1. CF | 0.9–6 | Mean: 127
SD: ± 23 Range: 68–148 |
Mean: 125
SD: ± 24 Range: 72–157 |
|||
| 20 | 2. non-CF symptomatic | 0.3 6 | Mean: 23
SD: ± 10 Range: 9–40 |
Mean: 27
SD: ± 12 Range: 4–52 |
|||
| Melbourne Study - RCH (Anderson and Freeman, 1958)20 | Total: n = 157 |
Stimulation: intradermal injection of mecholyl hydrochloride
Collection: 1 hour, from forearm on gauze pad (3x3 inch) Analysis: Sodium – flame photometer Chloride – titration with mercuric nitrate |
- Performed before the development of the standardised sweat test by Gibson and Cooke. The method used to stimulate and collect the sweat is currently not accepted.
- Sweat sodium and chloride concentrations >150 mmol/L are physiologically impossible. |
||||
| 44 | 1. CF | 0–15 | Mean: 118
SD: NR Range: 27–186 |
Mean: 95
SD: NR Range 25–176 |
|||
| 30 | 2. Non-CF symptomatic | Exact age | Mean: 37
SD: NR Range: 11–66 |
Mean: 26
SD: NR Range 9–39 |
|||
| 21 | 3. Asymptomatic siblings | Not stated | Mean: 40
SD: NR Range: 16–64 |
Mean: 34
SD: NR Range: 15–68 |
|||
| 20 | 4. Non-CF asymptomatic | Not stated | Mean: 52
SD: NR Range: 19–82 |
Mean: 56
SD: NR Range: 27–87 |
|||
| 42 | 5. Parents of CF | Not stated | Mean: 54
SD: NR Range: 10–84 |
Mean: 58
SD: NR Range: 11–98 |
|||
| Melbourne Study(Anderson and Freeman, 1960)19 | Total: n = 608 |
Stimulation: intradermal injection of mecholyl hydrochloride
Collection: 1 hour, from forearm on gauze pad (3x3 inch) Analysis: sodium – flame photometer chloride – titration with mercuric nitrate |
Mean: NR
SD: NR Range: NR |
Mean: NR
SD: NR Range: NR |
- The method used to stimulate and collect the sweat is currently not accepted.
- The results were tabulated with the actual number of subjects showing various sodium levels. Thus, mean sodium and chloride concentrations in each group were not reported. |
||
| 100 | 1.CF | <15 | |||||
| 100 | 2. Chronic chest disease | <15 | |||||
| 61 | 3. Asymptomatic sibling of CF patients | <15 | |||||
| 100 | 4. Asymptomatic parents of CF patients | >20 | |||||
| 100 | 5. Non-CF symptomatic | <15 | |||||
| 147 | 6. Non-CF asymptomatic | 15 | |||||
| Brompton Study (Hodson et al, 1983)22 | Total: n = 50 |
Stimulation: pilocarpine iontophoresis
Collection: filter paper Analysis: sodium – flame photometry |
- Only reported sweat sodium concentrations. | ||||
| 25 | 1. CF | 15–36 | Mean: NR
SD: NR Range: NR |
Mean: 103.04
SD: + 31.8 Range: NR |
|||
| 25 | 2. non-CF asymptomatic | 15–36 | Mean: NR
SD: NR Range: NR |
Mean: 43.2
SD: + 35.3 Range: NR |
|||
| Copenhagen Study (Nielson and Flensborg, 1987)23 | Total: n = 2293 |
Stimulation: pilocarpine iontophoresis
Collection: 30 minutes, on filter paper Analysis: sodium – flame photometry |
- Only sweat sodium measured.
- Mean sodium concentrations not reported. The concentrations were presented graphically. |
||||
| 203 | 1. CF | Not stated | Mean: NR
SD: NR Range: NR |
Mean: NR
SD: NR Range: NR |
|||
| 2090 | 2. Non-CF symptomatic | 0–30 | Mean: NR
SD: NR Range: NR |
Mean: NR
SD: NR Range: NR |
|||
| Edinburgh Study (Kirk and Westwood, 1989)25 | Total: n = 571 |
Stimulation: pilocarpine iontophoresis
Collection: 60 minutes (did not specify the collection material) Analysis: sodium – flame photometry |
- The numbers of subjects recruited and included in the analysis was hard to understand.
- Only reported sweat sodium concentrations. |
||||
| 39 | 1. CF | ≥ 2 weeks | Mean: NR
SD: NR Range: NR |
Mean: NR
SD: NR Range: 54–160 |
|||
| 532 | 2. non-CF symptomatic | <12 | Mean: NR
SD: NR Range: NR |
Na+ = 32.5 ± 15.3 (SD) | |||
List of abbreviations:
n = number of subjects;
NR = not reported;
RCH = The Royal Children’s Hospital;
SD = standard deviation
CF = Subjects that were diagnosed with CF based on clinical features and/or sweat test.
non-CF asym
selection criteria for their ‘control’ group are assumed to have no known disease are also included in this group.
non-CF symptomatic: Subjects that were not diagnosed with CF but had other known disease.
Of the remaining 10 studies that used standard pilocarpine iontophoresis in the performance of the sweat test, four studies included results of both sweat chloride and sodium concentrations24,26,27,36 while six studies only reported sweat chloride concentrations.9,28–32 Of the 10 studies, six reported mean and standard deviation sweat electrolyte concentrations.26,29–32,36 The other four reported mean concentrations without other statistical parameters.9,24,27,28 The details of these studies are summarised in Table 2.
Table 2.
Review of the studies that comply with international and Australian guidelines for the performance of sweat testing.
| Study | Sample size (n) | Selection criteria* | Age range (years) | Sweat test method | Sweat electrolyte concentrations (mmol/L) | Study limitations† | |
|---|---|---|---|---|---|---|---|
| Chloride (Cl−) | Sodium (Na+) | ||||||
| Hopkins Study (Gibson and Cooke, 1959)9 | Total: n = 89 |
Stimulation: pilocarpine iontophoresis
Collection: in two different medium: 15–90 minutes on gauze squares (3x3inch) and 30 minutes on filter-paper disks (2.5cm) Analysis: chloride–polarographic method |
- Not a healthy control group, thus possible that these subjects had a condition that affects sweat electrolytes or had a mild CF phenotype.
- No mention of subjects’ age. - Relatives have a higher risk of being a carrier. - This study was conducted prior to the discovery of the CFTR gene. |
||||
| 25 | 1.CF: history of excessive appetite, steatorrhoea and recurrent pulmonary disease | Not stated |
3x 3 inch gauze squares: n=14: Mean: 110.1 SD: NR Range: 84–136 2.5cm filter-paper disks n=11: Mean: 94.8 SD: NR Range: 80–122 |
Mean: NR
SD: NR Range: NR |
|||
| 47 | 2. Non-CF: 3 apparently healthy, 20 had asthma, 8 recurrent bronchitis, 3 hepatitis, 2 congenital heart disease, 1 each had non-tropical sprue, osteomyelitis, pyelonephritis, bronchiectasis, hypertension | Not stated |
3x 3 inch gauze squares: n=8: Mean: 45.4 SD: NR Range: 11–60 2.5 cm filter-paper disks n=39: Mean: 21.1 SD: NR Range: 7–49 |
Mean: NR
SD: NR Range: NR |
|||
| 17 | 3. Asymptomatic relatives of CF | Not stated |
3x 3 inch gauze squares: n=12: Mean: 33.9 SD: NR Range: 12–57 2.5cm filter-paper disks n=5: Mean: 32.5 SD: NR Range 16–47 |
Mean: NR
SD: NR Range: NR |
|||
| Washington Study (Andrews et al, 1962)28 | Total: n = 66 |
Stimulation: pilocarpine iontophoresis
Collection: 30 minutes on filter paper Analysis: chloride–coulometric |
- Small number of subjects in each group.
- Siblings have 2/3 risk of being a carrier. - This study was conducted prior to the discovery of the CFTR gene. - It is also possible that the control group had a condition that affects sweat electrolytes or had a mild CF phenotype. |
||||
| 6 | 1. CF | 1–4.5 | Mean: 110
SD: NR Range: 84-154 |
Mean: NR
SD: NR Range: NR |
|||
| 6 | 2. Asymptomatic siblings of patients with CF | 0.3-6 | Mean: 34.8
SD: NR Range: 19-53 |
||||
| 27 | 3a. non-CF symptomatic: allergic children with rhinitis, eczema, asthma without emphysema, nasal eosinophilia | 0.7-10 | Mean: 19.7
SD: NR Range: 9-35 |
||||
| 11 | 3b. non-CF symptomatic: children with asthma, evidence of emphysema | 1-10 | Mean: 26.5
SD: NR Range: 7-60 |
||||
| 16 | 4. non-CF asymptomatic: no history of allergic or pulmonary disease | 0.4–9 | Mean: 16.7
SD: NR Range: 5–33 |
||||
| Colorado Study (Gharib et al, 1965)32 | Total: n = 255 |
Stimulation: pilocarpine iontophoresis
Collection: 30 minutes on filter paper Analysis: sodium – flame photometry chloride – titration with mercuric nitrate |
- Not a ‘healthy’ control group. Group 2b includes patients with infections, siblings of CF patients (higher risk of being a carrier) and various other medical conditions. It is possible that some of these subjects had a condition that affects sweat electrolytes or had a mild CF phenotype.- The study was conducted prior to the discovery of the CFTR gene.- Subjects’ age not mentioned in Group 2b. | ||||
| 100 | 1. CF: M:F – 46:54 | <5–15 | Mean: 109
SD: ± 17 Range: 60–186 |
Mean: NR
SD: NR Range: NR |
|||
| 100 | 2a. non-CF symptomatic: patients with allergic bronchial asthma (M:F – 65:35) | <5–15 | Mean: 12.3
SD: ± 7 Range: 2.7–39 |
||||
| 55 | 2b. non-CF symptomatic: patients with frequent respiratory tract infections, chronic diarrhoea, family history of CF, failure to thrive, sepsis, rectal prolapse (M:F – 30:25) | Not stated | Mean: 14.7
SD: ± 6 Range: 4–33 |
||||
| Minneapolis Study (Warwick and Hansen, 1978)30 | Total: n = 100 |
Stimulation: pilocarpine iontophoresis by two methods
A. Orion ‘ionto-block’ method B. ‘Gibson and Cooke’ method Collection: 5 minutes on parafilm (10x10cm) Analysis: chloride–potentiometer with chloride selective electrode |
- Orion electrode has been proven to give inaccurate results due to insufficient collection time and should not be used.38 - Recruitment criteria not defined. - It is possible that the asymptomatic subjects had a condition that affects sweat electrolytes or had a CF- related condition. - This study was conducted prior to the discovery of the CFTR gene. - No mention of subjects’ age. |
||||
| 50 | 1. CF | Not stated |
Orion method Mean: 100.2 SD: ± 25.4 Range: NR Gibson-Cooke Mean: 103.7 SD: ± 21.6 Range: NR |
Mean: NR
SD: NR Range: NR |
|||
| 50 | 2. non-CF asymptomatic | Not stated |
Orion method Mean: 11.4 SD: ± 6.9 Range: NR Gibson-Cooke Mean: 9.3 SD: ± 5.8 Range: NR |
||||
| Boston Study (Shwachman et al, 1981)26 | Total: n = 504 |
Stimulation: pilocarpine iontophoresis
Collection: 30 minutes on gauze pad Analysis: chloride–titration with mercuric nitrate |
- Not a ‘healthy’ control group. Controls were referred for clinical reasons and were later found not to have CF. It is possible that some subjects had a condition that affects sweat electrolytes or had a mild CF phenotype.
- This study was conducted prior to the discovery of the CFTR gene. |
||||
| 252 | 1. CF: n = 252 | Not stated | Mean: 115.3
SD: ± 12.1 Range: 78.6-148.2 |
Mean: 111.2
SD: ± 12.0 Range: 75.4-144.6 |
|||
| 252 | 2. non-CF asymptomatic: children referred for clinical reasons but were excluded of CF | Not stated | Mean: 28.0
SD: ± 6.0 Range: 7.7-43.4 |
Mean: 28.2
SD: ± 6.1 Range: 15.9-45.9 |
|||
| Ohio Study (Davis et al, 1982)29 | Total: n = 200 |
Stimulation: pilocarpine iontophoresis
Collection: 30 minutes on filter paper Analysis: chloride–coulombmetric titration |
- Not a ‘healthy’ control group.
- Recruitment criteria not defined. - This study was conducted prior to the discovery of the CFTR gene. - No separate data for 21 healthy controls. - Exact age of the subjects was not mentioned. |
||||
| 13 | 1. CF: hospitalised CF patients with chronic pulmonary disease | 22.5 ± 2 (SD) | Mean: 100.1
SD: ± 7.2 Range: NR |
Mean: NR
SD: NR Range: NR |
|||
| 21 | 2a. non-CF asymptomatic: healthy volunteers | 53.5 ± 14.7 (SD) | Mean: 25.7
SD: ± 15.5 Range: NR |
||||
| 166 | 2b. non-CF symptomatic: patients with various pulmonary disorders such as asthma, allergies, chronic obstructive pulmonary disease | ||||||
| Birmingham Study (Hall et al, 1990)27 | Total: n = 60 |
Stimulation: pilocarpine iontophoresis
Collection: on filter paper and the time was reduced to 20 minutes (or less if the paper was visibly wet) Analysis: sodium–flame photometry chloride–potentiometric |
- It is possible that the asymptomatic subjects had a condition that affects sweat electrolytes or had a mild CF phenotype.
- No CFTR gene mutation analysis to exclude carriers or mild CF. - Small number of subjects. |
||||
| 20 | 1.CF: diagnosed in childhood by clinical features and positive sweat test (M:F–12:8) | 18-31 | Mean: 106
SD: NR Range: 81-122 |
Mean: 108
SD: NR Range: 84-137 |
|||
| 20 | 2. non-CF symptomatic: patients undergoing investigation for chest disease other than CF (M:F–13:7) | 19-40 | Mean: 33
SD: NR Range 13-52 |
Mean: 52
SD: NR Range 29-77 |
|||
| 20 | 3. non-CF asymptomatic: normal adults with no history of chest or digestive disease (M:F–7:13) | 18-39 | Mean: 31
SD: NR Range: 14-48 |
Mean: 50
SD: NR Range 29-72 |
|||
| Edinburgh Study (Kirk et al, 1992)24 | Total: n = 233 |
Stimulation: pilocarpine iontophoresis
Collection: by two methods:- 30 minutes on filter paper - Wescor Macroduct Analysis: sodium–flame photometry chloride–colorimetric |
- Combined the asymptomatic and symptomatic controls into non-CF group.
- Recruitment criteria for Group 2b not defined. - Small number of asymptomatic subjects. - Group 2a had 97 subjects referred for clinical reasons, therefore, it is possible that some subjects had a condition that affects sweat electrolytes, or had a mild CF phenotype. - CFTR gene mutation analysis was performed on the CF group, but not on the non-CF group to exclude carriers or mild CF. |
||||
| 121 | 1. CF: patients attending the CF clinics (M:F–66:55) | 0-40 | n = 112:
Mean: 100 SD: NR Range 70-132 n=9: Cl− = 51-69 SD: NR Range: NR |
n = 112:
Mean: 103 SD: NR Range: 70-132 (The nine patients with equivocal Cl− results had Na+ results of 70- 102 mmol/L) |
|||
| 97 | 2a. non-CF symptomatic: referred for the sweat test for clinical reasons | 0-40 | n = 112:
Mean: 22.5 SD: NR Range: 3-53 (Of 112 non-CF subjects, 2 had sweat chloride > 50 mmol/L) |
Mean: 40.3
SD: NR Range: 5-81 |
|||
| 15 | 2b. non-CF asymptomatic | ||||||
| Monash Medical Centre (Melbourne) Study (Doery and Huang, 1993) (Abstract)36 | Total: n = 250 |
Stimulation: pilocarpine iontophoresis
Collection: 45–60 minutes on filter paper Analysis: sodium–flame photometry chloride – colorimetry |
- Small number of CF patients recruited.
- Not a healthy control group. The non-CF group included patients referred for sweat tests. It is possible that these subjects had a condition that affects sweat electrolytes or had a mild CF phenotype. - Small number of non-CF patients in age group 10+ years. - This study was conducted after the discovery of the CFTR gene, but no mutation analysis was performed to exclude carriers. |
||||
| 9 | 1. CF | 0–4 | Mean: 97.8
SD: ± 17 Range: 73–124 |
Mean: 78
SD: ± 16 Range: 52–104 |
|||
| 125 | 2a-d. non-CF symptomatic: subjects with chest symptoms – cough, wheeze and recurrent upper respiratory tract infection | 0–1 | Mean: 13.5
SD: ± 5.6 Range: 3–38 |
Mean: 14.6
SD: ± 5.7 Range: 4–30 |
|||
| 80 | 1–5 | Mean: 16.1
SD: ± 6.0 Range: 7–39 |
Mean: 17.6
SD: ± 7.3 Range: 3–43 |
||||
| 25 | 5–10 | Mean: 17
SD: ± 7.6 Range: 7–36 |
Mean: 20.4
SD: ± 7.5 Range: 8–40 |
||||
| 11 | 10+ | Mean: 23.7
SD: ± 10.5 Range: 10–41 |
Mean: 32.3
SD: ± 13.2 Range: 10–53 |
||||
| Wisconsin Study (Farrell and Koscik, 1996)31 | Total: n = 707 |
Stimulation: pilocarpine iontophoresis
Collection: 30 minutes on filter paper Analysis: chloride –digital chloridometer |
- Exact age not mentioned.
- The sweat chloride concentrations for infants and carriers were non- parametric, yet the concentrations are reported as mean and SD. - Not a healthy control group, as the non-CF group included infants with a positive NBS test result, and infants referred for sweat tests due to clinical reasons and thus to rule out CF e.g. recurrent respiratory symptoms. - It is possible that some infants may be carriers for other mutations, although the chances are very low. |
||||
| 61 | 1a. CF: ΔF508/ΔF508 homozygote infants | 10.5 ± 9.9 (weeks) | Mean: 100.0
SD: ± 9.2 Range: NR |
Mean: NR
SD: NR Range: NR |
|||
| 47 | 1b. CF: ΔF508 compound heterozygote infants | 9.3 ± 9.3 (weeks) | Mean: 97.6
SD: ± 15.3 Range: NR |
||||
| 7 | 1c. CF: infants with non-ΔF508 mutations | 16.5 ± 17.3 (weeks) | Mean: 99.6
SD: ± 14.1 Range: NR |
||||
| 128 | 2. heterozygote carriers | 8.8 ± 5 (weeks) | Mean: 14.9
SD: ± 8.4 Range: NR |
||||
| 184 | 3a. non-CF symptomatic: normal (no mutation) | 9.3 ± 5.3 (weeks) | Mean: 10.6
SD: ± 5.2 Range: NR |
||||
| 280 | 3b. non-CF symptomatic: normal (no gene mutation analysis performed) | 21.2 ± (13.1) | Mean: 11.6
SD: ± 5.3 Range: NR |
||||
List of abbreviations: CFTR = cystic fibrosis transmembrane conductance regulator; ΔF508 = deletion of phenylalanine at position 508; M:F = Male:Female; n = number of subjects; NBS = Newborn screening; NR = not reported; RCH = The Royal Children’s Hospital; SD = standard deviation.
CF = Subjects that were diagnosed with CF based on clinical features and/or sweat test and/or mutation analysis.
non-CF asym
selection criteria for their ‘control’ group are assumed to have no known disease are also included in this group.
non-CF symptomatic: Subjects that were not diagnosed with CF but had other known disease.
Study limitations: This section includes the critical interpretation of the study by the reviewers.
Of the 10 studies that used approved sweat testing methodology, four did not report the age of the subjects,9,26,29, 30 three studies were on subjects ≤15 years of age,28,31,36 one included adults 18 years of age and over27 and one included a wide age range of subjects.24 The age of the non-CF subjects in one of the papers was not clear.32 Of the 10 studies, three did not mention the recruitment criteria of the non-CF subjects.24, 29, 30 There were small numbers of non-CF subjects recruited in three studies,27,28,36 while five recruited only a small number of CF subjects.9, 27–29, 36 Only one study performed CFTR gene mutation analysis to determine the carrier status of the non-CF group.31
Discussion
The interpretation of sweat electrolyte concentrations has been used for the diagnosis of CF for over 50 years.8,9 As can happen in science, the details of the original experiments used to generate the data can be forgotten. The aim of this review was to understand the basis of the commonly accepted reference intervals by examining all the published studies on sweat electrolyte concentrations and to critically examine their validity in relation to the diagnosis of CF now that accepted methodology has been agreed on. To this effect we found 17 English language published studies generating reference intervals between 1950 and 1996.
From the 17 published studies, seven were deemed unacceptable, as they either did not use the currently accepted pilocarpine iontophoresis sweat test method or only reported sodium concentration.8,19–23,25 Four of these studies were conducted before the development of the standardised pilocarpine iontophoresis sweat stimulation method13–15 using inappropriate stimulation by heat,8 placing the patient in a plastic bag,21 or intradermal injection of the mecholyl hydrochloride.19,20 While the former two methods were simple,8, 21 they had major limitations such as the variability in the collection time (1–2 hours) required to obtain a sufficient sweat sample which raises the concern about evaporation and contamination while collecting the sweat. Other issues included infants becoming hyperpyrexic while placed in a plastic bag and there was a report of fatal heat stroke following this technique.9 In two studies sweat was stimulated by injection of mecholyl hydrochloride and the sweat was collected over the point of injection. This method did not become accepted as it was relatively painful for the subjects.19,20
It has been well documented that sweat chloride is most directly related to the abnormal function of CFTR and shows greater discrimination for the diagnosis of CF than sweat sodium.13–15 For this reason, measuring sweat sodium alone is not recommended for the diagnosis of CF. Of the seven unacceptable studies, two analysed sweat sodium concentrations alone.22,25 In two other studies, sweat electrolyte reference intervals could not be identified as the mean sweat electrolyte concentrations were not stated.19,23 In one of these two studies, the results were tabulated with the number of subjects showing various sweat sodium levels19 and in another study the sweat sodium concentrations were graphically presented.23
There were 10 studies that used the standardised pilocarpine iontophoresis method for sweat collection.9,24,26–32,36 However each of these studies suffered from significant limitations, including subject selection, non-specification of the age of the sample population, lack of CFTR gene mutation analysis, small sample size, and incorrect statistical methods employed to develop reference intervals. In some cases the reasons for these methodological flaws were historical, such as limited knowledge about the broader CF phenotype and inability to perform CFTR gene mutation analysis (pre 1989). In other examples, however, the investigators used samples of convenience that included patients referred for sweat tests or parents and siblings of CF patients, most of whom were likely to be carriers. Many of the studies employed inappropriate statistics, the commonest error being the use of mean values from non-parametric data. Due to the variations between the 10 studies, the sweat electrolyte concentrations generated from each of the studies were not comparable to each other and so we could not perform meta-analysis.
A principal limitation of the ten studies that did use the standardised methodology was the lack of a ‘healthy’ control group.9,24,26–29,32 Subjects were recruited from hospital wards and clinics and also included subjects with a variety of medical conditions including respiratory illnesses that could have been CF.9,27–29,32,36 Alternatively subjects were generated by referrals for a sweat test for clinical reasons9,24,26,32 or from infants that had a positive newborn screening test result.31 In three of the 10 studies the recruitment of non-CF control groups was not defined24,29,30,36 and in another five studies the non-CF subjects’ age was not mentioned.9,26,29,30,32 As our understanding of CF has developed, it is possible that phenotypes that were once not thought to be consistent with CF have now been included in the expanded clinical spectrum of CF. It is also possible that some of these non-CF subjects with diseases other than CF could have conditions that cause an elevation of sweat electrolyte concentrations.
Among these 10 studies, six were undertaken before the discovery of the CFTR gene in 1989.9,26,28–30,32 However, even at that time the investigators would have been aware of the inheritance of CF. From the four studies that were undertaken after the availability of CFTR gene mutation analysis, only one study performed the CFTR gene mutation analysis (for the most common mutation ΔF508) to differentiate carriers from the non-CF control groups.31 No studies of subjects that included adolescents or adults performed CFTR gene mutation analysis. Therefore, some control individuals, many of whom were siblings or parents of CF patients, could have been carriers and may have skewed the results. This assertion is supported by the study in infants demonstrating that CF carriers had a mean sweat chloride concentration of one standard deviation above control subjects.31
The development of a reliable reference interval requires an adequate sample size. In 1994 Solberg recommended that the sample population should consist of at least 40 participants.18 Three of the 10 studies, had a small number of non-CF subjects to allow definite reference intervals to be stated.27,28,36 The remaining seven studies reported the sweat chloride or sodium concentrations as mean (± standard deviation) without mentioning the distribution of the data. We are now aware of the importance of the distribution, which determines the appropriate statistical analysis to be used. A general rule is that parametric analysis is used for normally distributed data and non-parametric analysis for data that are skewed,37 which is then followed by a series of step-by-step statistical calculations to develop 95% or 99% reference intervals.
Of the ten studies using acceptable methodology, the study of infants by Farrell and Koscik in 1996 is probably the most reliable study as it recruited a large number of subjects (n= 707), performed CFTR gene mutation analysis to determine the carrier status of non-CF subjects, and referred to the distribution of the sweat chloride concentrations.31 The authors of this study recommended that a sweat chloride concentration ≥40 mmol/L be used to distinguish infants with CF, which represented the mean plus three standard deviation values for the group of 128 CF heterozygote carriers identified by gene mutation analysis. Despite acknowledging that the sweat chloride concentration data were skewed in the heterozygote carrier group and in the healthy infants group, they still reported the results as mean (± standard deviation) concentration rather than median.
The question of reference intervals in relation to age was raised in seven of the 17 studies. These studies infer that sweat chloride and sodium concentrations increase with age.15,17–20,22,31 The study by Doery and Huang in 1993 reported increasing sweat chloride and sodium concentrations with age in non-CF subjects, however they had only a small number of subjects in the 10+ year age group.36 This was the only study to take a systematic approach to sweat electrolytes with increasing age, but provides little information to guide clinicians requesting sweat tests in adolescents and adults. This is an increasing problem as the CF phenotype can include patients presenting beyond infancy and childhood. At The Royal Children’s Hospital (Melbourne, Victoria, Australia) 9% of the patients referred for a sweat test were older than 15 years in 2004 and 13% in 2005 (unpublished data). Accurate interpretation of sweat chloride and sodium concentrations in these patients requires knowledge of the age-related changes in sweat chloride and sodium concentrations.
In conclusion, we identified 17 studies over a 50 year period that reported sweat chloride with or without sodium concentrations for the diagnosis of CF. All 17 studies had limitations according to the current guidelines. Most studies did not include a ‘healthy’ control group, were performed prior to the availability of the CFTR gene mutation analysis and did not comply with the currently accepted sweat test method. This systematic review has revealed that there is no definitive study that quantitates the sweat chloride and sodium concentration in a truly normal population. Further work is required to re-establish sweat electrolyte reference intervals, using the current standardised sweat test, CFTR gene mutation analysis to exclude carriers, and correct statistical analyses in healthy participants with ages spanning from infancy to adulthood.
Acknowledgements
We gratefully acknowledge Angela Chiriano for critical reading of the manuscript.
Footnotes
Competing Interests: None declared.
References
- 1.Wilcken B, Wiley V, Sherry G, Bayliss U. Neonatal screening for cystic fibrosis: a comparison of two strategies for case detection in 1.2 million babies. J Pediatr. 1995;127:965–70. doi: 10.1016/s0022-3476(95)70040-4. [DOI] [PubMed] [Google Scholar]
- 2.Welsh MJ, Ramsay BW, Accurso F, Cutting GR. Cystic Fibrosis. In: Scriver ABC, Sly WS, Valle D, editors. The Molecular and Metabolic Basis of Inherited Disease. New York: McGraw-Hill; 2001:5121–5188.
- 3.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–73. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 4.Rommens JM, Iannuzzi MC, Kerem B, Drumm ML, Melmer G, Dean M, et al. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989;245:1059–65. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- 5.Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, et al. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989;245:1073–80. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- 6.The Cystic Fibrosis Genotype-Phenotype Consortium. (Accessed 1 October 2006). http://www.genet.sickkids.on.ca
- 7.Mishra A, Greaves R, Massie J. The relevance of sweat testing for the diagnosis of cystic fibrosis in the genomic era. Clin Biochem Rev. 2005;26:135–153. [PMC free article] [PubMed] [Google Scholar]
- 8.Di Sant’Agnese PA, Darling RC, Perera GA, Shea E. Sweat electrolyte disturbances associated with childhood pancreatic disease. Am J Med. 1953;15:777–83. doi: 10.1016/0002-9343(53)90169-7. [DOI] [PubMed] [Google Scholar]
- 9.Gibson LE, Cooke RE. A test for concentration of electrolytes in sweat in cystic fibrosis of the pancreas utilizing pilocarpine by iontophoresis. Pediatrics. 1959;23:545–9. [PubMed] [Google Scholar]
- 10.Webster HL. Laboratory diagnosis of cystic fibrosis. Critical Reviews in Clinical Laboratory Sciences. 1983;18:313–38. doi: 10.3109/10408368209085074. [DOI] [PubMed] [Google Scholar]
- 11.Carter EP, Barrett AD, Heeley AF, Kuzemko JA. Improved sweat test method for the diagnosis of cystic fibrosis. Archives of Disease in Childhood. 1984;59:919–22. doi: 10.1136/adc.59.10.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wescor Inc. (Accessed 2 August 2006). http://www.wescor.com
- 13.Green A, Kirk J. Guidelines for the performance of the sweat test for the diagnosis of cystic fibrosis. Ann Clin Biochem. 2007;44:25–34. doi: 10.1258/000456307779596011. [DOI] [PubMed] [Google Scholar]
- 14.NCCLS. Sweat testing: Sample Collection and Quantitative Analysis; Approved Guideline - Second edition. Document C34-A2. Wayne: The Committee 2000.
- 15.Coakley J, Scott S, Doery J, Greaves R, Talsma P, Whitham E, et al. Australian guidelines for the performance of the sweat test for the diagnosis of cystic fibrosis. Clin Biochem Rev. 2006;27:S1–S7. [PMC free article] [PubMed] [Google Scholar]
- 16.LeGrys VA. Sweat testing for the diagnosis of cystic fibrosis: practical considerations. Journal of Pediatrics. 1996;129:892–7. doi: 10.1016/s0022-3476(96)70034-3. [DOI] [PubMed] [Google Scholar]
- 17.Green A. Guidelines for the performance of the sweat test for the investigation of cystic fibrosis in the UK. (Accessed 27 January 2006). http://www.ukneqas.org.uk/nov2003guideline.pdf
- 18.Solberg HE. Establishment and use of reference values. In: Burtis CA, Ashwood ER, editors. Tietz textbook of clinical chemistry. second ed. Philadelphia: W.B. Saunders; 1994:454–484.
- 19.Anderson CM, Freeman M. ‘Sweat test’ results in normal persons of different ages compared with families with fibrocystic disease of the pancreas. Arch Dis Child. 1960;35:581–7. doi: 10.1136/adc.35.184.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson CM, Freeman M. A simple method of sweat collection with analysis of electrolytes in patients with fibrocystic disease of the pancreas and their families. Med J Aust. 1958;1:419–422. doi: 10.5694/j.1326-5377.1958.tb86426.x. [DOI] [PubMed] [Google Scholar]
- 21.Webb BW, Flute PT, Smith MJH. The electrolyte content of the sweat in fibrocystic disease of the pancreas. Arch Dis Child. 1957;32:82–84. doi: 10.1136/adc.32.162.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hodson ME, Beldon I, Power R, Duncan FR, Bamber M, Batten JC. Sweat tests to diagnose cystic fibrosis in adults. British Medical Journal Clinical Research Ed. 1983;286:1381–3. doi: 10.1136/bmj.286.6375.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nielsen OH, Flenborg EW. Reference values of sweat sodium obtained by pilocarpine iontophoresis. Current Therapeutic Research. 1987;41:367–373. [Google Scholar]
- 24.Kirk JM, Keston M, McIntosh I, al Essa S. Variation of sweat sodium and chloride with age in cystic fibrosis and normal populations: further investigations in equivocal cases. Annals of Clinical Biochemistry. 1992;29:145–52. doi: 10.1177/000456329202900204. [DOI] [PubMed] [Google Scholar]
- 25.Kirk JM, Westwood A. Interpretation of sweat sodium results--the effect of patient age. Annals of Clinical Biochemistry. 1989;26:38–43. doi: 10.1177/000456328902600105. [DOI] [PubMed] [Google Scholar]
- 26.Shwachman H, Mahmoodian A, Neff RK. The sweat test: sodium and chloride values. Journal of Pediatrics. 1981;98:576–8. doi: 10.1016/s0022-3476(81)80764-0. [DOI] [PubMed] [Google Scholar]
- 27.Hall SK, Stableforth DE, Green A. Sweat sodium and chloride concentrations-essential criteria for the diagnosis of cystic fibrosis in adults. Annals of Clinical Biochemistry. 1990;27:318–20. doi: 10.1177/000456329002700406. [DOI] [PubMed] [Google Scholar]
- 28.Andrews BF, Bruton OC, Knoblock EC. Sweat chloride concentration in children with allergy and with the cystic fibrosis of the pancreas. Pediatrics. 1962;29:204–8. [PubMed] [Google Scholar]
- 29.Davis PB, Del Rio S, Muntz JA, Dieckman L. Sweat chloride concentration in adults with pulmonary diseases. American Review of Respiratory Disease. 1983;128:34–7. doi: 10.1164/arrd.1983.128.1.34. [DOI] [PubMed] [Google Scholar]
- 30.Warwick WJ, Hansen L. Measurement of chloride in sweat with the chloride-selective electrode. Clinical Chemistry. 1978;24:2050–3. [PubMed] [Google Scholar]
- 31.Farrell PM, Koscik RE. Sweat chloride concentrations in infants homozygous or heterozygous for F508 cystic fibrosis. Pediatrics. 1996;97:524–8. [PubMed] [Google Scholar]
- 32.Gharib R, Joos HA, Hilty LB. Sweat chloride concentration. Am J Dis Child. 1965;109:66–68. [PubMed] [Google Scholar]
- 33.Massie J, Gaskin K, Van Asperen P, Wilcken B. Sweat testing following newborn screening for cystic fibrosis. Pediatric Pulmonology. 2000;29:452–6. doi: 10.1002/(sici)1099-0496(200006)29:6<452::aid-ppul7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 34.Green A, Dodds P, Pennock C. A study of sweat sodium and chloride; criteria for the diagnosis of cystic fibrosis. Annals of Clinical Biochemistry. 1985;22:171–4. doi: 10.1177/000456328502200212. [DOI] [PubMed] [Google Scholar]
- 35.Wilschanski M, Zielenski J, Markiewicz D, Tsui LC, Corey M, Levison H, et al. Correlation of sweat chloride concentration with classes of the cystic fibrosis transmembrane conductance regulator gene mutations. Journal of Pediatrics. 1995;127:705–10. doi: 10.1016/s0022-3476(95)70157-5. [DOI] [PubMed] [Google Scholar]
- 36.Doery J, Huang N. Effect of age on the composition of normal sweat. (Abstract). In: XV International Congree of Clinical Chemistry; 1993.
- 37.Royston P. Constructing time-specific reference ranges. Statistics in Medicine. 1991;10:675–90. doi: 10.1002/sim.4780100502. [DOI] [PubMed] [Google Scholar]
- 38.Denning CR, Huang NN, Cuasay LR, Shwachman H, Tocci P, Warwick WJ, et al. Cooperative study comparing three methods of performing sweat tests to diagnose cystic fibrosis. Pediatrics. 1980;66:752–757. [PubMed] [Google Scholar]