Abstract
A positron-emitting paclitaxel (PAC) derivative could allow in-vivo measurement of multidrug resistance in tumors and, therefore, predict a potential chemotherapeutic benefit to patients. [18F]Paclitaxel was produced using a 2-reaction vessel automated synthesizer followed by HPLC purification. Optimized reaction conditions resulted in radiochemical yields of 21.2 ± 9.6% at end of bombardment, radiochemical purity > 99%, and specific activity of 159 ± 43 GBq/μmol. [18F]Paclitaxel activities of 1.33 ± 0.729 GBq (n=7) were obtained in sterile, pyrogen-free solution for IV administration.
Keywords: [18F]Paclitaxel, Multidrug resistance, PET, Automated synthesis system, fluorine-18
1. Introduction
Automated Synthesis of 18F Analogue of Paclitaxel (PAC): [18F]Paclitaxel (FPAC)
Chemotherapeutic failure due to multidrug resistance (MDR) is a common problem in cancer treatment. In general, MDR refers to a phenotype, whereby a tumor develops resistance to a large number of natural chemotherapeutic drugs such as anthracyclines, vinca alkaloids, epipodophyllotoxins, and taxanes (Tan et al., 2000). While several different genes were shown to be associated with a multidrug resistance phenotype, MDR1 (Borst et al., 2000), which codes for a P-glycoprotein (P-gp) pump, represents one of the most characterized barriers to chemotherapy (Ambudkar et al., 1999; Gottesman and Pastan, 1993). P-gp, found in normal human hepatocytes, epithelial cells of the colon and jejunum, and renal proximal tubules (Thiebaut et al., 1987), effects the removal of a wide variety of cytotoxic substances by functioning as an adenosine triphosphate (ATP) dependent efflux transporter (Ambukar et al., 1999).
The choice of a systemic chemotherapeutic is based on a priori analysis of tumor markers to assess the presence or absence of a molecular pathway or target for a given agent. Measurement of MDR is one important marker for planning therapy. Imaging with a radiopharmaceutical that is transported by P-gp may identify non-invasively those tumors in which the transporter is not only expressed, but also functional. Presently, few Single Photon Emission Tomography (SPECT) and Positron Emission Tomography (PET) radiopharmaceuticals exist that can assess transporter mediated resistance (Sharma, 2004).
Paclitaxel (PAC) is a complex diterpene natural product that has application as a chemotherapeutic agent in a number of solid tumors. However, the P-gp efflux pump reduces the effectiveness of paclitaxel in treating some tumors (Ambudkar et al., 2006). Measurement of the in vivo concentration of a positron-emitting PAC derivative in tumor could non-invasively predict, prior to chemotherapy, therapeutic efficacy. In vitro studies have shown that it is possible to completely reverse the MDR phenotype in tumor cell lines by inhibiting the P-gp pump with a variety of chemical modulators (Lan et al., 1996; Moins et al., 2000; Plumb et al., 1994). The derivative [18F]-Paclitaxel ([18F]FPAC) was found sensitive to the presence or absence of P-gp in knockout mice (Kieseweter et al., 2003) and sensitive to administration of XR9576 modulator in monkeys (Kurdziel et al., 2003).
To date, only a manual procedure for the synthesis of [18F]FPAC has been published (Kieseweter et al., 2003). An automated procedure would provide radiation protection to workers, reproducible yields, improved efficiency, and include attributes toward cGMP compliance (FDA, 2005). In this paper we describe [18F]FPAC synthesis using a modified fully automated, two-reaction vessel synthesizer. The system provides a sterile, pyrogen-free product of high-activity and high-purity within 95 minutes from start of synthesis. Radiosynthesis of [18F]FPAC followed method B (Kiesewetter et al., 2003) of the published manual procedure except for minor modifications necessitated by the lower flexibility of the synthesizer.
2. Materials and Methods
2.1 Hardware and Software
The dual reaction vessel synthesizer TRACERlab FXF-N (GE Medical Systems-Functional Imaging GmbH, Münster, Germany) (Figure 1) is based on the single vessel system used for [18F] nucleophilic substitution reactions. The synthesizer contains 10 glass vials for loading reagents prior to [18F] delivery. A 15 mL glassy carbon reaction vessel (RXN#1) and a 2 mL glass reaction vessel (RXN#2) are each thermally controlled by a microprocessor. Exhaust gases are transferred to a monitored delay line and carbon filter trap prior to venting to atmosphere.
Figure 1.
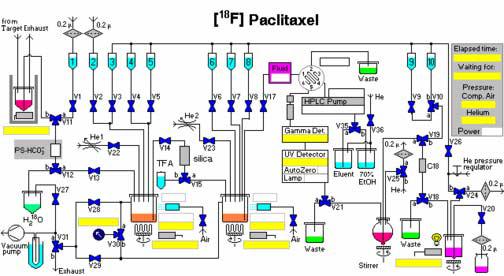
Schematic of the GE TRACERlab FX for synthesis of [18F]Paclitaxel
Due to the corrosive properties of trifluoroacetic acid (Sigma-Aldrich, St. Louis, MO), a separate l mL V-vial (Wheaton Science Products, Millville, NJ) was modified and positioned between the reaction vessels. The time-list synthesis program NINA version 4.9 (GE Medical Systems-Functional Imaging GmbH, Münster, Germany) was modified to allow delivery of the acid to RXN#2 by vacuum transfer.
2.2 Reagents and Apparatus
The reagents obtained were of either American Chemical Society (ACS) or United States Pharmacopoeia (USP) reagent grade where applicable and were used without further purification. H218O water was obtained from Rotem Industries, LTD, Beer Sheva, Israel. Sigma-Aldrich, St. Louis, MO supplied the potassium carbonate, Krytofix® [2.2.2], acetonitrile, diisopropylethylamine, acetic acid, ethanol, trifluoroacetic acid, and anisole. Pentamethylbenzyl trimethyl-ammoniumbenzoate triflate was obtained from ABX, Radeberg, Germany. The paclitaxel primary amine was obtained from Natural Pharmaceuticals, Inc. Beverly, MA. Diethylcyanophosphonate was obtained from Acros Organics, Geel, Belgium. The sterile water was obtained from Hyclone, Logan, UT and the 0.9% Sodium Chloride from Abbott Laboratories, Abbott Park, IL. The 50 mg silica gel cartridge was obtained from Alltech Associates, Deerfield, IL. The C-18 (16 × 250 mm) semi-preparative column, C-18 (30 × 16 mm) guard column, and the PS-HCO3 cartridges were obtained from Macherey-Nagel, Inc, Easton, PA. The 200 mg (40 μm) C-18 BondElut® cartridge was obtained from Varian, Inc, Palo Alto, CA and the Millex-LG 0.2 μm Hydrophilic Fluoropore (PTFE) filter and the Millex-GS 0.2 μm filter were obtained from Millipore, Billerica, MA.
Limulus amebocyte lysate reagent was obtained from Charles River Endosafe (Charleston, SC). Radioactivity was measured using a calibrated ion chamber (CRC-15PET, Capintec, Inc., Ramsey, NJ) and a sodium iodide gamma well counter (model 1282 Compugamma CS Universal Gamma Counter, LKB Wallac, Turku, Finland). [18F]FPAC purification was performed on a system provided with the synthesizer (S1021 pump, Sykam GmBH-Systeme & Komponenten Analytischer Meβtechnik, Eresing, Germany; K-2001 UV detector, Knauer.ASI, Berlin, Germany; and radioactive detector) that included a semi-preparative reverse phase C18 column (16 × 250 mm, Nucleosil 100-7, Macherey-Nagel GmBH & Co.KG, Düren, Germany). The semi-preparative HPLC system was controlled by the GINA Radio Chromato-Graphic System software version 3.0 (Raytest Isotopenmessageräte GmbH, Straubenhard, Germany). Analytical quality control of the final product was performed on a Luna C-18 column (4.6 × 150 mm, 5μm, Phenomenex, Torrance, CA) and a Series III HPLC pump with Model 500 UV detector (Lab Alliance, State College, PA) and Model 105S solid-state radiation detector system (Carroll/Ramsey Associates, Berkeley, CA). The system was controlled by PeakSimple software version 2.83 (SRI Instruments, Torrance, CA).
2.3 Production of [18F]PAC
Prior to [18F]FPAC synthesis all vials and tubing were automatically cleaned with either water, ethanol or acetone and dried under helium flow. The semi-preparative HPLC column, loop and final product tubing were cleaned and disinfected with 70% ethanol (4 mL/min for 30 min) using a dedicated clean program. A membrane filter (Millex-GS, 0.22 μm) was installed on each of four ambient air inlets and the final product delivery tubing (Millex-LG, 0.20 μm). Reagents were loaded into respective vials and separation columns installed.
Kiesewetter et al (2003) described the general chemical method for the synthesis of [18F]FPAC ( method B) and a summary of the operational production is presented in table 1.
Table 1.
Summary of the operational production of [18F]FPAC
|
No carrier-added aqueous [18F] fluoride was produced by the 18O(p,n)18F reaction (PETTrace cyclotron, GE Medical Systems, Waukesha, WI) by the irradiation of an isotopically enriched [18O] water target using a 16 MeV proton beam (15 minute bombardment at 60 μA) (Ruth and Wolf, 1979). At the start of synthesis, the enriched water containing [18F]fluoride was passed by vacuum through a previously conditioned PSHCO3 column. The H218O was recovered and the [18F]fluoride eluted from the resin using aqueous K2CO3 and transferred to RXN#1. Kryptofix® [2.2.2] in acetonitrile was delivered to RXN#1, and the solution evaporated at 95°C for 3 minutes under a helium gas flow.
Pentamethylbenzyl 4-trimethyl-ammoniumbenzoate triflate in acetonitrile was delivered to the residue and heated at 100°C for 7 min. The residue was cooled to room temperature and diluted with acetonitrile. The mixture was transferred to a pre-conditioned silica gel column, washed with acetonitrile, and delivered to RXN#2 containing anisole. The solution was dried under helium flow for 7 minutes at room temperature and the residue treated with trifluoroacetic acid for 5 min at room temperature to give [18F]fluorobenzoic acid. The [18F]fluorobenzoic acid was dried at room temperature with a helium gas flow for 5 minutes and then treated with diisopropylethylamine and the paclitaxel primary amine. The [18F]fluorobenzoic acid was then coupled to the paclitaxel primary amine by treatment with diethylcyanophosphonate at 60°C for 5 min. The crude product was diluted with 0.5% acetic acid at room temperature and loaded onto a C-18 semi-preparative HPLC column in series with a C-18 guard column by a compressed air actuated valve (model C6W, VICI Valco Instruments Co. Inc, Houston, TX). The injectate was eluted with 60% acetonitrile: 40% water containing 0.5% acetic acid at a flow rate of 5 mL/min. The effluent was monitored for UV absorbance (254 nm) and radioactivity. The product, which eluted within 20 min, was diverted to a round bottom flask containing 15 mL of water, mixed, and transferred to a preconditioned C-18 BondElut® column. Adsorbed [18F]FPAC was washed with 2 mL sterile water and isolated by elution of the column with 2 ml ethanol. The ethanolic solution was delivered through a sterilizing filter (Millex-LG, 0.2 μm) to a final product multidose vial containing 9.5 mL isotonic sodium chloride. The process controlling software supported Good Manufacturing Practice.
2.4 Quality control of [18F]FPAC
Samples of the final product were obtained for the quantitation of residual solvents by gas chromatography (SRI 8610C, SRI Instruments, Torrance, CA), half-life for radionuclidic purity, semi-quantitation of residual Kryptofix® [2.2.2] (Mock et al., 1997), sterility, pyrogenicity, and pH. A bubble point procedure was performed on the final filter to test filter integrity.
Identity of the final product was confirmed by co-elution of [18F]FPAC and unlabelled FPAC by reverse phase HPLC, using 55% acetonitrile: 45% water at 1 mL/min. The effluent was monitored for absorbance with a UV detector at 230 nm and radioactivity.
Results and Discussion
The automated synthesis method provides [18F]FPAC in 21.24 ± 9.6% (n = 7) radiochemical yield (EOB) in a synthesis time of 95 min. Radiochemical purity was greater than 99% as determined by a single radiation peak that co-eluted with authentic product at about 7 minutes. Specific activity (mean ± standard deviation) was 159 ± 43 GBq/μmol (4.29 ± 1.16 Ci/μmol) (n=7). These results are similar to those obtained by the manual synthesis (Kiesewetter et al., 2003).
Analysis of final product for residual acetone and acetonitrile were all within acceptable limits of 5,000 ppm and 400 ppm, respectively (FDA, 2003). Radionuclidic half-life was determined using a dose calibrator. The value was calculated from the slope of six measurements obtained at 5-minute intervals. [18F] half-life was found to be within the USP 28 acceptable limit of 108-110 minutes. The Kryptofix® [2.2.2] spot test demonstrated all final products to contain <50 μg per administered dose. The final product was tested for sterility and pyrogenicity by USP methods and found sterile and free from pyrogens. The final filter demonstrated integrity as tested by the bubble point apparatus (Millex/Sterivex Integrity Tester, SLTE ST00 00, Millipore, Billerica, MA).
Conclusion
We developed a fully automated two-reaction vessel synthesis for the routine production of [18F]FPAC. The 95-minute synthesis provided a product of high radiochemical purity (>99%) in a yield of 10-30%. A synthesis beginning with 18.5 GBq (500mCi) of [18F]fluoride at EOB would provide 1.33 GBq (36 mCi) [18F]FPAC for administration to patients. Flexible programming of the GE TRACERlab FX 2-reaction synthesizer allowed complete automation of the process. The automated synthesis provides a low-exposure method that complies with good manufacturing practices.
Acknowledgements
This work was supported by grant IRG-100036 from the American Cancer Society and NIH R21CA098334. The authors would like to thank Dr. J.D. Wilson for his interesting comments and Ms. Celina Thadigiri for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Biochemical, cellular, and pharmacological aspects of multidrug transporter. Annu. Rev. Pharamacol. Toxicol. 1999;39:361–398. doi: 10.1146/annurev.pharmtox.39.1.361. [DOI] [PubMed] [Google Scholar]
- Ambudkar SV, Kim IW, Sauna ZE. The Power of the pump: Mechanisms of action of P-glycoprotein (ABCB1) Eur. J. Pharma. Sci. 2006;27(5):392–400. doi: 10.1016/j.ejps.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Borst P, Evers R, Kool M, Wijnholds J. A Family of Drug Transporters: the Multidrug Resistance-Associated Protiens. J. Natl. Cancer. Inst. 2000;92(16):1295–1302. doi: 10.1093/jnci/92.16.1295. [DOI] [PubMed] [Google Scholar]
- Gottesman MM, Pastan I. Biochemistry of Multidrug Resistance Mediated by the Multidrug Transporter. Annu. Rev. Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- Kiesewetter DO, Jagoda EM, Kao CH, Ma Y, Ravasi YL, Shimoji K, Szajek LP, Eckelman WC. Fluoro-, bromo-, and iodopaclitaxel derivatives: synthesis and biological evaluation. Nucl. Med. Biol. 2003;30(1):11–24. doi: 10.1016/s0969-8051(02)00351-7. [DOI] [PubMed] [Google Scholar]
- Kurdziel KA, Kiesewetter DO, Carson RE, Eckelman WC, Herscovitch P. Biodistribution, Radiation Dose Estimates and In Vivo Pgp Modulation Studies of 18F-Paclitaxel in Nonhuman Primates. J. Nucl. Med. 2003;44(8):1330–1339. [PubMed] [Google Scholar]
- Lan LB, Ayesh S, Lyubimov E, Pashinsky I, Stein WD. Kinetic parameters for reversal of the multidrug pump as measured for drug accumulation and cell killing. Cancer. Chemother. Pharmacol. 1996;38(2):181–190. doi: 10.1007/s002800050468. [DOI] [PubMed] [Google Scholar]
- Mock BH, Winkle W, Vavrek MT. A color spot test for the detection of Kryptofix 2.2.2 in [18F]FDG preparations. Nucl. Med. Biol. 1997;24(2):193–195. doi: 10.1016/s0969-8051(96)00212-0. [DOI] [PubMed] [Google Scholar]
- Moins N, Cayre A, Chevillard S, Maublant J, Verrelle P, Finat-Duclos F. Effects of MDR reversing agent combinations on the H-3-daunomycin accumulation in drug-sensitive and drug-resistant human cancer cells. Anticancer. Res. 2000;20(4):2617–2623. [PubMed] [Google Scholar]
- Plumb JA, Wishart GC, Setanoians A, Morrison JG, Hamilton T, Bicknell SR, Kaye SB. Identification of a multidrugresistance modulator with clinical potential by analysis of synergistic activity in-vitro, toxicity in-vivo and growth delay in a solid human tumor xenograft. Biochem. Pharmacol. 1994;47(2):257–266. doi: 10.1016/0006-2952(94)90015-9. [DOI] [PubMed] [Google Scholar]
- Ruth TJ, Wolf A. Absolute cross sections for the production of 18F via the 18O(p,n)18F reaction. Radiochim. Acta. 1979;26:21. [Google Scholar]
- Sharma V. Radiopharmaceuticals for Assessment of Multidrug Resistance P-Glycoprotein-Mediated Drug Transport Activity. Bioconjugate. Chem. 2004;15(6):1464–1474. doi: 10.1021/bc0498469. [DOI] [PubMed] [Google Scholar]
- Tan B, Piwnica-Worms D, Ratner L. Multidrug resistance transporters and modulation. Curr. Opin. Oncol. 2000;12(5):450–458. doi: 10.1097/00001622-200009000-00011. [DOI] [PubMed] [Google Scholar]
- Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular-localization of the multidrug-resistance gene-product P-glycoprotein in normal human tissues. Proc. Natl. Acad. Sci. 1987;84(21):7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration Draft Guidance PET Drug Products - Current Good Manufacturing Practice (CGMP) 2005 September; [Google Scholar]
- U.S. Food and Drug Administration: International Conference on Harmonization (ICH) Guidance; Guide for Industry: @ -Tables and Literature 11-12-03.


