Abstract
The RNA Recognition Motif (RRM) type RNA binding protein Bruno is required for the differentiation of cystoblasts, the committed daughters of germline stem cells in the Drosophila ovary. To understand how Bruno controls cystoblast differentiation, we used a bioinformatics approach to identify potential mRNA targets of Bruno. One such target is the Sex-lethal (Sxl) transcript MS11, which contains four Bruno Response Elements (BREs) in its 3′ untranslated region. Electrophoresis mobility shift assays demonstrated that Bruno specifically binds to the BREs of Sxl MS11 mRNA. Tagged transgenic Sxl MS11 cDNA constructs were used to show that Bruno represses the translation of the Sxl MS11 mRNA via the BRE-containing region in the 3′UTR. The lack of either Bruno or the BRE-containing region leads to overexpression of SXL, which in turn causes defects in cystoblast differentiation similar to the Bruno mutant phenotype. Therefore, Sxl MS11 represents a novel target of Bruno-mediated translational repression required for cystoblast differentiation.
Keywords: oogenesis, stem cell differentiation, translational regulation, Sex-lethal, Bruno
INTRODUCTION
Stem cells are capable of both self-renewal and differentiation. The differentiation process involves lineage commitment and restriction through which a stem cell daughter gradually loses its potential to adopt alternative cell fates and becomes a more specialized cell. This dynamic process is well demonstrated during Drosophila oogenesis in which a germline stem cell (GSC) continuously undergoes asymmetric self-renewing divisions. Each division produces a committed daughter cell called a cystoblast. The cystoblast then divides four times with incomplete cytokinesis to produce a cyst containing 16 interconnected germ cells called cystocytes. Following 16-cell cyst formation, 15 cystocytes differentiate into nurse cells while one becomes an oocyte. The cyst then becomes enveloped by somatic-derived follicle cells, and together they form an egg chamber. These events occur in the germarium, a structure at the tip of the ovarian functional unit called the ovariole. In the germarium, GSCs are the 2-3 most anteriorly located germ cells. Cystoblasts, dividing cysts, and egg chambers are located at increasingly posterior positions according to their developmental stages. The differentiation of the cystoblast is marked by the transformation of a cytoplasmic organelle called the spectrosome into its derivative structure called fusome (Lin and Spradling, 1995; Lin et al., 1994). The spectrosome is initially a discrete sphere in a GSC or a cystoblast. However, during the four divisions of the cystoblast, it grows into an intercellular structure called the fusome that connects individual cystocytes within a cyst (Lin and Spradling, 1995; Lin et al., 1994).
The Sex lethal (Sxl) gene has been implicated in germ cell proliferation, differentiation and meiotic recombination during early oogenesis in Drosophila (Bopp et al., 1999; SchuLtt et al., 1998; Schupbach, 1985; Vied et al., 2003; Vied and Horabin, 2001). Sxl was initially discovered as a master switch gene of sex determination in Drosophila (Cline, 1978). It encodes a member of a conserved RNA binding protein family that contains RNA-Recognition-Motifs (RRM). The SXL protein regulates the splicing and translation of its target RNAs (Penalva and Sanchez, 2003). It is encoded by two separate sets of at least 10 alternatively spliced mRNAs produced by the early and late promoter, respectively (FlyBase Genome, 2004; Samuels et al., 1991). In female embryos, the early promoter is activated. It produces the early SXL protein, which splices the pre-mRNAs produced by the late promoter into mRNAs that encode the SXL protein, thus defining the female mode of development. One of these female-specifically spliced late RNAs is called MS11. MS11 contains an open reading frame (ORF) encoding SXL and a second ORF with unknown translational capacity (Samuels et al., 1991). During early oogenesis, SXL is present at high levels in the cytoplasm of GSCs, cystoblasts, and 2-cell cysts but is sharply reduced and localized to the nucleus in 4-cell and more advanced cysts (Bopp et al., 1993). The expression of Sxl in the germ cells and its localization to the nucleus appears to be regulated by the Hedgehog signaling pathway and an antizyme encoded by gut feeling (guf). Meanwhile, guf expression also is regulated by Sxl (Vied et al., 2003; Vied and Horabin, 2001). Although the Sxl-guf regulatory loop has been implicated in regulating Cyclin B expression (Vied et al., 2003), its role in cystoblast differentiation has not been definitively characterized.
We have previously demonstrated that cystoblast differentiation requires the Bruno (a.k.a., arrest, aret) gene that also encodes a RRM-containing RNA binding protein (Parisi et al., 2001). The Bruno protein has been best characterized for its role during late oogenesis, where Bruno represses the translation of oskar (osk) mRNA by several conserved cis-elements in its 3′-UTR called Bruno-Responsive-Elements (BREs) (Castagnetti et al., 2000; Kim-Ha et al., 1995; Webster et al., 1997). The repression of oskar translation by Bruno also requires several transacting factors, including Apontic, Bicaudal-C, Me31B, and p50 (Johnstone and Lasko, 2001; Wilkie et al., 2003). Bruno mediated repression of oskar is achieved both by interacting Cup and eIF4E to control the translational initiation, and by oligomerizing mRNA into silencing particles (Chekulaeva et al., 2006; Nakamura et al., 2004).
Although Bruno is required for cystoblast differentiation (Parisi et al., 2001), known target mRNAs of Bruno, oskar and gurken (Filardo and Ephrussi, 2003), do not appear to be involved in this process, because their expression is not detected during cyst differentiation (Kim-Ha et al., 1991; Neuman-Silberberg and Schupbach, 1993). Nor does the ectopic expression of oskar or gurken display either a GSC or cystoblast differentiation phenotype (Kim-Ha et al., 1995; Neuman-Silberberg and Schupbach, 1994). Thus, Bruno must regulate other targets to achieve its function in cystoblast differentiation. In this study, we investigate the function of Bruno in regulating cystoblast differentiation by identifying potential mRNA targets, by examining the Bruno regulation of these targets, and by exploring the biological effects of this regulation. We show that the Sxl MS11 transcript is a novel target of Bruno. Moreover, MS11 is likely to be the main target whose translational repression by Bruno in the germline is essential for cystoblast differentiation.
MATERIALS AND METHODS
Prediction of potential BRE-containing mRNAs
BRE searching program (available upon request) was developed with PERL (http://www.perl.com/). The Drosophila EST database and whole genome cDNA database were obtained from the Berkeley Drosophila Genome Project website (http://www.fruitfly.org/). The list of ovarian expressing genes from Flybase (http://flybase.bio.indiana.edu/) was used to judge whether a gene is expressed in the ovary.
Bruno protein production
Full-length Bruno cDNA was a gift from Dr. P. Lasko (Webster et al., 1997) and was cloned into pQE vectors (Qiagen, Inc. Valencia, CA, USA). Recombinant Bruno protein was expressed and purified according to (Castagnetti et al., 2000).
Electrophoresis Mobility Shift Assay (EMSA)
The oskar cDNA was kindly provided by Dr. R. Lehmann (Ephrussi et al., 1991). DNA templates used to produce RNA were PCR fragments prepared as follows: a PCR fragment containing osk BREs was amplified from oskar cDNA (2557-2739bp) using a sense primer (TTGTGCTAGCTAACGCTTAGTTTTAATATG) and an anti-sense primer (TTGTGCTAGCGTGTGCAGAAAATCAATG). The PCR fragment was subsequently cloned into the pBlueScript SK(+) vector at the NheI site (underlined) and RNA was transcribed from the T3 promoter on the vector. Sxl BREs were amplified from MS11 cDNA (2600-2955bp) using a sense primer, AGTGAATTGTAATACGACTCACTATAGGGAGTTGAGAAGCGTATGA (T7 promoter is underlined) and an anti-sense primer, CGTCGATTTTTCATTCTTTA. RNA was generated directly from the PCR product. The non-specific RNA control was transcribed from the T3 promoter to the KpnI site (658-788bp) of the pBlueScript SK(+) vector.
EMSA was performed according to (Black et al., 1998) with some modifications. In vitro binding was done in the following reaction mix: 10mM HEPES pH 7.5, 50 mM KCl, 0.2% Tween-20, 2mM DTT, 0.05 mg/ml BSA, 200 U/ml RNase-OUT (Invitrogen, Inc. Carlsbad, CA, USA), 2 mM vanadyl ribonucleotide complex (GIBCO BRL, Gaithersburg, MD, USA), 1mg/ml yeast tRNA and 1mg/ml poly (A). Radioactively labeled Sxl RNA containing 4 BREs was used as the substrate of the assay. Purified Bruno protein and cold RNA competitors were added to the reaction mix as needed. The mixture was incubated at room temperature for 30 minutes to allow binding. RNA-protein complexes were resolved on a 4% non-denaturing polyacrylamide gel.
Fly stocks and culture
The aretQB72 allele is a null Bruno mutant kindly provided by Dr. T. Schüpbach (Schupbach and Wieschaus, 1991; Webster et al., 1997). The nos-Gal4 strain was kindly provided by Dr. P. Rørth (Rorth, 1998). All Drosophila stocks were grown at 25°C on yeast-containing corn meal/molasses medium.
Cloning of tagged Sxl MS11 transgene with and without BREs
The Sxl transcript MS11 cDNA was a gift from Dr. P. Schedl (Samuels et al., 1991). To study the translational regulation of Sxl MS11, the first open reading frame (ORF1) of MS11, which encodes SXL, was tagged with two copies of a DNA sequence encoding the MYC (EQKLISEEDL) epitope, one just after the starting codon AUG and the other at the NdeI site (1364). The second ORF (ORF2) was tagged by a DNA sequence encoding the HA (YPYDVPDYA) epitope at the NheI site (2245). The tagged construct (SXL-FL) was subsequently inserted into pUASP vector (a gift of Dr. P.Rørth) (Rorth, 1998) so that the transcription of the tagged cDNA is under the control of the UASP promoter. This construct produces tagged Sxl MS11 mRNA with an intact 3′UTR. The SXLΔBRE construct was generated by removing the the NheI (2245)-NheI (3379) fragment from the tagged SXL-FL construct.
The SXL-FL and SXLΔBRE constructs were injected into w1118 embryos at the Duke Model System Genomics Transgenic Facility. Male transformants bearing these constructs were crossed to nos-Gal4 virgin females. Ovaries from progeny that have one copy of nos-Gal4 and tagged Sxl constructs were examined for the expression of tagged Sxl constructs by immuno-staining. Similarly, the expression of the tagged Sxl constructs was also examined in the aretQB72 mutant background.
Site-directed mutagenesis and in vitro translation
To test whether the HA epitope in ORF2 is detectable by western blot, ORF1 and ORF-2 were fused by mutating the TAA stop codon on SXL-FL at 1524 to TAC, using a QuikChange™ in vitro mutagenesis kit (Stratagene Inc., La Jolla, CA, USA). The “read-through” construct was in vitro translated using the TNT™ wheat germ lysate kit (Promega Inc., Madison WI, USA) according to the product manual. The expression of MYC and HA epitope tags was tested by western blot.
Immunofluorescence microscopy
Drosophila ovaries were dissected and stained according to (Lin et al., 1994). Rabbit anti-VASA antibodies (Hay et al., 1990), a gift from Dr. Yuh-Nung Jan, was used at 1:1000 dilution to label germline cells. Rabbit anti-Bruno antibody, a gift from Dr. Paul Lasko, was used as 1:100. Monoclonal anti-SXL (M18) (Bopp et al., 1991), anti-MYC and anti-1B1 (Zaccai and Lipshitz, 1996) antibodies were obtained from the University of Iowa Hybridoma Bank, and were used at 1:10, 1:5, and 1:2 dilutions for immuno-staining, respectively. Anti-MYC was used as 1:200 for immunoblotting. Rat monoclonal anti-HA high affinity antibody was purchased from Roche Inc. (Basel, Switzerland) and was used at 100ng/ml for immunoblotting. Fluorescently labeled secondary antibodies were obtained from Jackson Immunoresearch Laboratories (West Grove, PA, USA) and were used at 1:200 dilutions from a 1mg/ml stock. The DNA dye DAPI ( Sigma, St. Louis, MO, USA) was used at 0.5 μg/ml.
All samples were examined by epifluorescence microscopy using a Zeiss Axioplan microscope. Confocal microscopy was conducted with a Zeiss LSM510 META system. All images were processed using Adobe Photoshop 7.0™.
RESULTS AND DISCUSSION
The Sxl MS11 transcript contains four Bruno binding sites
To systematically identify putative Bruno target mRNAs, we searched the Drosophila genome databases for cDNAs or ESTs that contain the consensus UU(G/A)U(A/G)U(G/A)U sequence called the Bruno-Response-Element (BRE) (Kim-Ha et al., 1995)(see Materials and Methods). The following three criteria were used for selection. First, a candidate mRNA should contain four or more BREs, since oskar and gurken mRNA contain six and four BREs, respectively ((Kim-Ha et al., 1995) and data not shown). Second, the BREs should be located in the 3′-UTR of the transcript. Third, the candidate mRNA should be expressed during oogenesis. Using these criteria, we found 13 candidates in the Whole Genome Release 3 Dataset (Table 1). Interestingly, this list does not include cyclin A, which has been demonstrated to be a target of Bruno to regulate mitosis (Sugimura and Lilly, 2006), indicating our criteria is too stringent to include all true targets. Some of the targets do not seem to be directly related to Bruno, for example, toucan is known for the development of follicle cells where Bruno is not present. However, some of these target mRNAs do have potential roles in Bruno-regulated oogenic processes, such as failure in fusome formation and increased necrosis (Parisi et al., 2001). For example, trio encodes a protein involved in actin organization that is essential for fusome formation, and Wrinkled (W) encodes a protein that is involved in apoptosis that may contribute to the cell death in aret mutants (Table 1).
Table 1.
Putative ovarian mRNA targets of Bruno that contain at least four BREs*
| Name | Symbol | #BRE | Start, Sequence | Biological Functions† |
|---|---|---|---|---|
| toucan | toc | 4 | 7691, TTATATAT 7787, TTATATAT 7873, TTATATGT 7949, TTATGTGT |
ovarian follicle cell development |
|
polypeptide GalNAc transferase 2 |
pgant2 | 4 | 1541, TTGTATGT 1620, TTATATAT 1787, TTGTATAT 2441, TTGTATAT |
polysaccharide metabolism; protein glycosylation |
| Sex-lethal | Sxl | 4 | 2649, TTATATAT 2664, TTATATAT 2791, TTGTATGT 2912, TTATATAT |
dosage compensation; female somatic sex determination; female germ-line sex determination; meiotic recombination germ-cell development; oogenesis |
|
dilute class unconventional myosin |
didum | 4 | 4570, TTATGTGT 5681, TTGTGTAT 6163, TTATGTAT 6300, TTATATAT |
RNA localization; intracellular protein transport |
| mastermind | mam | 4 | 5784, TTATATGT 5893, TTGTATGT 7452, TTATATAT 7503, TTATATAT |
mesoderm cell fate determination |
| - | Lap1 | 4 | 3170, TTGTATAT 3620, TTATATAT 3704, TTATATGT 3984, TTATATAT |
cell adhesion; cyclic nucleotide metabolism; defense response; synaptic transmission; protein phosphorylation; transmembrane receptor protein serine/threonine kinase signaling |
| - | trio | 4 | 7372, TTGTATGT 7494, TTGTGTGT 8352, TTATATGT 9155, TTGTATGT |
G-protein coupled receptor protein signaling; Rho protein signal transduction; actin cytoskeleton organization and biogenesis; axon guidance; nervous system development |
|
Cyclic-AMP response element binding protein A |
CrebA | 4 | 2588, TTGTATGT 3190, TTGTATAT 4205, TTATATAT 4275, TTGTATGT |
salivary gland development |
| Wrinkled | W | 4 | 2504, TTGTATAT 3194, TTGTATAT 3667, TTGTATAT 3990, TTATATAT |
induction of apoptosis |
| oskar | osk | 4 | 1986, TTATATGT 2018, TTATATGT 2050, TTGTATAT 2060, TTGTGTGT |
posterior development; germ cell formation; long-term memory |
|
suppressor of white-apricot |
su(wa) | 5 | 3327, TTATGTAT 3335, TTATGTAT 3375, TTGTATGT 3471, TTATGTAT 4004, TTGTGTAT |
mRNA splicing via spliceosome |
| corkscrew | csw | 4 | 2931, TTGTGTGT 3973, TTGTGTGT 3991, TTGTATAT 4094, TTGTGTGT |
R7 cell fate commitment; epidermal growth factor receptor signaling; protein dephosphorylation; torso signaling |
| - | RhoGAPp190 | 5 | 4849, TTGTGTAT 5115, TTGTGTGT 5168, TTATATAT 5615, TTGTGTGT 6504, TTGTGTAT |
ectoderm development |
The above list of genes were generated by running the BRE-prediction PERL program against the whole genome Release 3 dataset (available at: ftp://flybase.net/genomes/Drosophila_melanogaster/current/FASTA/whole_genome_transcript_dmel_RELEASE3-1.FASTA.gz) (see Materials and Methods). This release included 18,860 cDNA sequences.
The biological functions of the candidates were obtained from Flybase according to the Gene Ontology.
One of the targets is a transcript of Sex-lethal (Sxl), a master switch gene of sex determination in Drosophila (Cline, 1978). This Sxl transcript, MS11 (GenBank accession no. M59448) (Samuels et al., 1991), contains four predicted BRE sites in the 3′-UTR (Fig. 1A, B). Because Sxl has also been implicated in germ cell proliferation and differentiation (Bopp et al., 1999; SchuLtt et al., 1998; Schupbach, 1985; Vied et al., 2003; Vied and Horabin, 2001), it is likely that Sxl is a target of Bruno in promoting cystoblast differentiation.
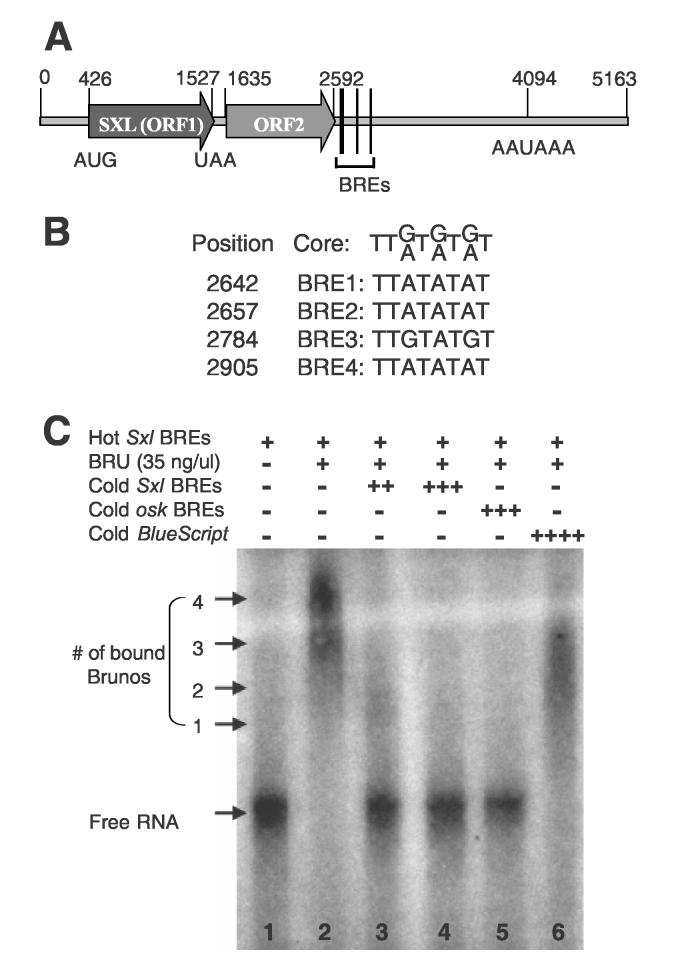
Fig. 1.
Bruno binds to predicted BREs in the 3′-UTR of Sxl MS11 mRNA.
(A) The sequence feature of the Sxl MS11 mRNA. Number denotes nucleotide position. (B) The alignment of the consensus BRE core sequence (Kim-Ha et al., 1995) with the four predicted BREs of MS11. (C) EMSA using purified recombinant Bruno (BRU) protein and a RNA derived from Sxl MS11 cDNA MS11 (2600-2955bp) containing BREs. All lanes contain 5nM radioactive labeled Sxl BREs probe. Lanes 2-6 also contain 140 ng of Bruno protein. Lanes 3, 4, 5, and 6 contain 100 nM unlabeled Sxl BREs RNA probe, 500 nM unlabeled Sxl BREs RNA probe, 700 nM unlabeled osk BREs RNA probe, and about 5μM unlabeled BlueScript RNA probe, respectively. Bracket shows Bruno-RNA complexes. Numbers indicate the number of Bruno molecules bound to a RNA molecule.
To test whether Bruno binds to the predicted BREs of the Sxl MS11 mRNA, we first conducted the Electrophoresis Mobility Shift Assay (EMSA) using a recombinant Bruno protein and a Sxl MS11 RNA fragment that contains the four potential BRE sites (see Materials and Methods). Recombinant Bruno binds to the Sxl MS11 RNA specifically (Fig. 1C). Only an excess of RNAs that contain either Sxl BREs or oskar BREs can effectively compete the binding of Bruno (Fig. 1C, lanes 2-5). In contrast, a non-specific RNA transcribed from pBlueScript, even at 1,000-fold excess, is still an ineffective competitor (Fig. 1C, lane 6). Furthermore, as indicated by the position of the shifted protein/RNA complexes, most labeled Sxl M11 RNA molecules appear to be bound by four Bruno molecules (Fig. 1C, lane 2). A small fraction is bound by three, and only a negligible amount is bound by 1-2 molecules. When approximately 20-fold of cold Sxl MS11 RNA was added as a competitor, most of the labeled RNA was shifted to the unbound position, while a small fraction was still bound by 1-2 Bruno molecules (Fig. 1C, lane 3). Eventually, with the presence of 100-fold of cold Sxl MS11 RNA, the bound protein/RNA complexes are eliminated (Fig. 1C, lane 4). These results indicate that all four BREs of the Sxl MS11 mRNA can be bound by Bruno.
SXL and Bruno display complementary expression patterns during cystoblast differentiation
To investigate whether in vitro binding of the Sxl MS11 RNA sequence by Bruno bears functional significance in cystoblast differentiation, we first examined whether SXL protein is expressed during early oogenesis. As previously reported, the SXL protein has a very dynamic expression pattern during early oogenesis (Bopp et al., 1991). It is present at high levels in the cytoplasm of GSCs, cystoblasts and 2-cell cysts (Fig.2A and green in C), whereas in 4-cell and more advanced cysts, SXL is greatly reduced in abundance and is localized to the nucleus. In contrast, Bruno is detectable in 4-cell cysts and reaches high levels in 16-cell cysts, where cytoplasmic SXL is barely detectable (Fig. 2B and red in C). Given that Sxl RNAs are uniformly distributed in region 1 of the germarium (Bopp et al., 1993), the decreased abundance of SXL in Bruno-expressing cells indicates the possibility that SXL translation is negatively regulated by Bruno during germline cyst differentiation.
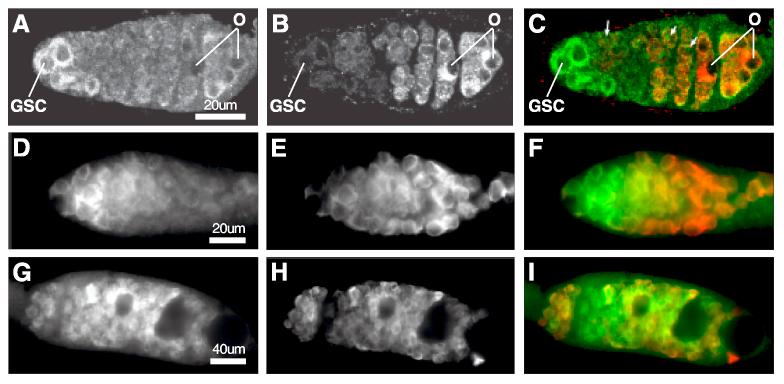
Fig. 2.
The expression of SXL and Bruno during early oogenesis in wildtype and aret mutants ovaries.
(A-C) Confocal Images of a wildtype germarium stained with SXL (A, and green in C), Bruno (B, and red in C). O designates oocyte. Arrows in C point to nuclear SXL. (D-F) Images of an aretQB72 mutant germarium stained with SXL (D, and green in F) and VASA to label germ cells (E, and red in F). (G-I) Images of an aretQB72 mutant pseudo egg chamber stained with SXL (G, and green in I) and VASA (H, and red in I).
Bruno is required for the repression of Sxl MS11
To test whether Bruno indeed negatively regulates Sxl translation in 4-cell and more advanced cysts, we first examined whether SXL is over-expressed in Bruno mutants. In a null mutant of Bruno, aretQB72, SXL is present at high levels in the cytoplasm of GSCs and cystoblasts (Fig. 2D, G, and green in F, I), similar to wildtype (Fig. 2A, C). In four-cell and more advanced cysts, the level of SXL is decreased. However, the down-regulation of SXL is not as sharply as observed in wild type cysts of equivalent stages (Fig. 2E, and green in F). The moderate down-regulation in aretQB72 might be due to proteolysis, an activity that has been reported to exist in the wildtype germline cysts (Vied and Horabin, 2001). Furthermore, SXL is again present at high levels in both the cytoplasm and the nucleus of all the germ cells in aretQB72 mutant “pseudo” egg chambers that contain ill-differentiated germline cysts (Fig. 2H, and green in I). This increased level of cytoplasmic SXL in the aretQB72 mutant is consistent with a role of Bruno in down-regulating SXL in 4-cell and more advanced cysts.
Bruno represses the expression of SXL via BREs
To further test whether Bruno represses the translation of Sxl MS11 RNA through BREs in the 3′UTR, we compared the translation efficiency of Sxl MS11 with and without the BREs. The Sxl MS11 mRNA contains an open reading frame (ORF) encoding SXL and a second ORF with unknown translational capacity (Samuels et al., 1991). We found that such bicistronic mRNAs with non-overlapping ORFs represent 1-2% of the Drosophila transcriptome. To investigate effect of Bruno on the translation of SXL ORF and the translatability of the second ORF, we first designed epitope-tagged MS11 cDNA constructs to follow the translation of these two ORFs individually (Fig. 3A). To distinguish ORFs 1 and 2, MYC and HA epitope tags were inserted into them, respectively (see Materials and Methods, Fig.3A). The expression of these two tags was verified by the following tests: (1) ORFs 1 and 2 were fused into one ORF by mutating the single UAA stop codon to UAC (see Materials and Methods). When this mutated cDNA was translated in vitro, both MYC and HA were detected in a single fusion protein of the expected size (data not shown). (2) When non-mutated MS11 was translated in vitro, only MYC was detected in a protein corresponding to the size of MYC-SXL. We designate the tagged, non-mutated Sxl MS11 cDNA as SXL-FL. Then, we deleted the BRE region in SXL-FL to generate SXLΔBRE (Fig. 3A).
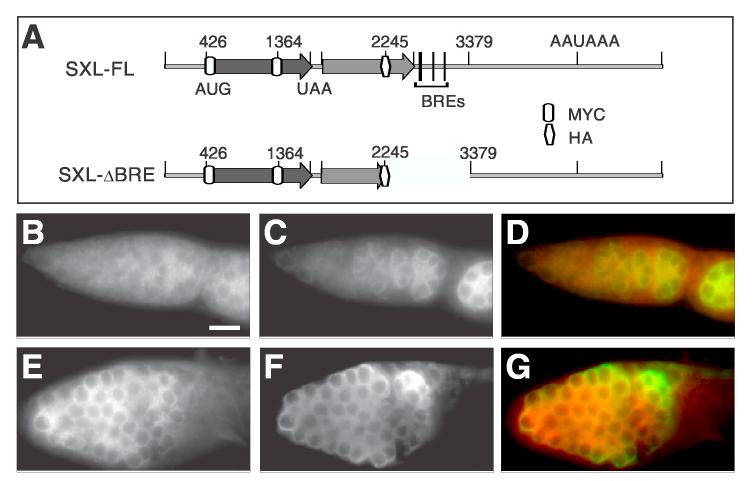
Fig. 3.
The expression of SXL from the SXLΔBRE transgene is elevated in the germarium.
(A) Diagrams show the tagged constructs of MS11 with and without BREs. (B-G) The expression of MYC-SXL (B, E and red in D, G) in SXL-FL (B-D) and SXLΔBRE (E-G) germaria. The anti-VASA antibody staining (C and F, green in D and G) was used to label germ cells. SXL-FL and SXLΔBRE ovaries were from flies of similar ages, and were stained with the same procedure. Images were obtained with the same level of contrast for comparison. Bar in B denotes 20 μm for panels B-G. All germaria are oriented with apical end to the left.
We then introduced tagged SXL-FL and SXLΔBRE cDNAs under the control of the pUASP promoter into the fly genome (Materials and Methods). These cDNAs were transcribed in the germline of the transgenic flies under the control of a nos-Gal4 transgene. Western blotting and immunofluorescence microscopy experiments detected MYC-SXL in the ovaries of either SXL-FL or SXLΔBRE transgenic flies (data not shown). However, the HA tag was not detected, suggesting that ORF2 is not translated in the ovary. Therefore, we focused on the regulation of SXL translation by Bruno.
To assay BRE regulation of MYC-SXL expression, we stained ovaries of the nos-Gal4; SXL-FL and nos-Gal4; SXLΔBRE transgenic flies with anti-MYC antibody. The MYC-SXL level in SXLΔBRE germaria (Fig. 3E) is much higher than in SXL-FL germaria (Fig. 3B). Such a difference was consistently observed across five independent transgenic nos-Gal4; SXLΔBRE lines and four independent transgenic nos-Gal4; SXL-FL lines, ruling out the influence of position effect variegation. Furthermore, the transcription of SXLΔBRE and SXL-FL in all transgenic lines is activated by the same nos-Gal4 transgenic line, and thus should be transcribed at the same level. These results together indicate that the significantly elevated level of MYCSXL in SXLΔBRE germaria is likely due to the failure in translational repression by Bruno owing to the absence of BREs in Sxl M11 RNA. However, because the BRE region deletion also removes six of 12 potential SXL binding sites, the observed results could be influenced by the failure in SXL's negative auto-regulation (Deshpande et al., 1999).
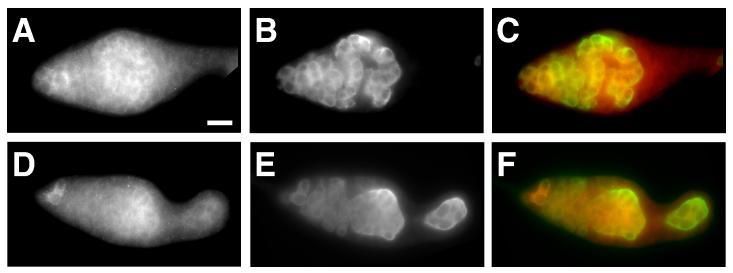
To further test whether the elevated level of MYC-SXL in SXLΔBRE germaria is due to the failure in translational repression by Bruno, we examined the expression of SXLΔBRE and SXL-FL transgenes in the aretQB72 mutant ovaries. As expected, the level of MYC-SXL from the SXL-FL transgene is significantly elevated in aretQB72 mutant germaria; the difference between the expression of MYC-SXL from the SXL-FL and the SXLΔBRE transgene is diminished (Fig. 4). In aretQB72 mutant flies carrying either SXL-FL or SXLΔBRE transgenes, MYC-SXL is highly expressed in GSCs, cystoblasts and early cysts, and down-regulated in cysts in the middle region of the germarium. Finally, MYC-SXL regains high levels of expression in the poorly differentiated germ cells that accumulate in germaria and pseudo egg chambers. This pattern of MYC-SXL expression is very similar to that of endogenous SXL in aretQB72 mutant germaria (Fig. 2), and further confirms that Bruno represses the translation of Sxl MS11 mRNA via the BRE elements.
Fig. 4.
The repression of SXL expression requires Bruno.
The expression of MYC-SXL (A, D, and red in C, F) in aretQB72 mutant ovaries carrying a SXL-FL (A-C) or a SXLΔBRE (D-F) transgene. The Anti-VASA antibody staining (B, E, and green in C, F) was used to label germ cells. Bar in A denotes 20 μm for all panels. All germaria are oriented with apical end to the left.
Overexpression of SXL severely affects the differentiation of germline cysts
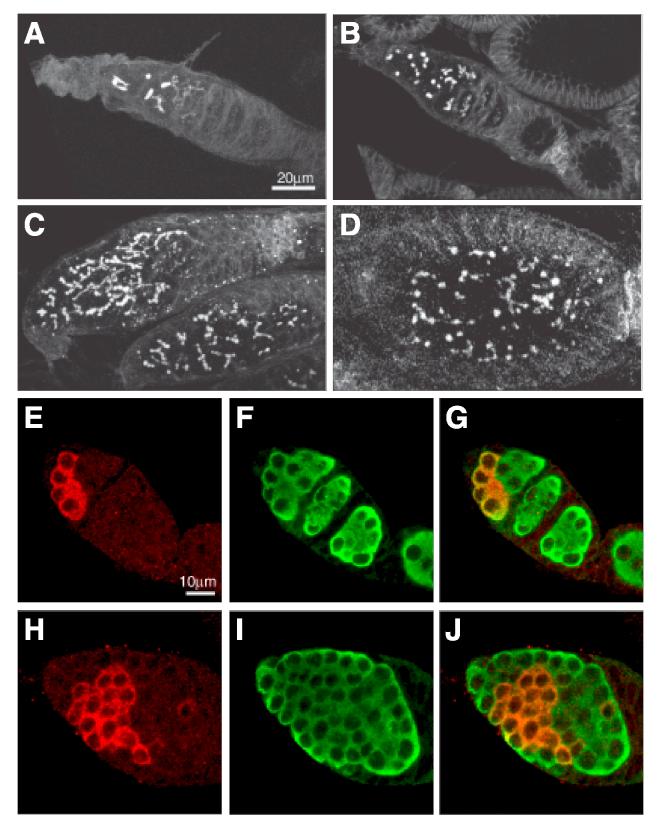
The overexpression of SXL caused by the lack of Bruno-mediated repression leads to defects in germline cyst differentiation. In SXL-FL transgenic flies in which MYC-SXL is properly repressed, 75% of ovarioles (n=898) contain a normal number of differentiating germline cysts (Fig.5A, E-G). Only 25% of ovarioles contain slightly over-proliferated germline cysts, as judged by the presence of small fusomes (Fig. 5B) and a normal number of germline cysts expressing Bam-C, a cyst-specific marker (McKearin and Ohlstein, 1995)(data not shown). In contrast, in all SXLΔBRE fly ovarioles (100%, n=516), cysts severely over-proliferate, as indicated by the presence of large fusomes with elaborate branching patterns (Fig. 5C, D) and an increase in Bam-C-expressing germ cysts (Fig. 5H-J). No normally developing egg chamber is found. Instead, “pseudo egg chambers” that are filled with ill-differentiated cysts, as marked by the presence of fusomes, are frequently observed (Fig. 5D). This mildly tumorous phenotype resembles the previously reported aretQB72 mutant phenotype (Parisi et al., 2001). In addition, it suggests that the MYC-tagging does not abolish the activity of SXL — a conclusion consistent with the fact that the MYC insertion sites do not disrupt the RNA binding domain of SXL. Meanwhile, these data also implicate that Sxl is likely the main target of Bruno-mediated translational repression that is responsible for the proper differentiation of germline cysts.
Fig. 5.
The ectopic expression of SXLΔBRE causes cyst overproliferation and underdifferentiation.
(A-D) Confocal images showing fusome morphology in ovaries from MS11 transgenic flies. In SXL-FL transgenic flies (A), cyst proliferation is largely normal in most germaria. Only in a very small number of germaria (B), GSCs or cystoblasts appear to be slightly overproliferated, as indicated by the number of fusomes. In contrast, in SXLΔBRE transgenic flies (C), all germaria are filled with overproliferating cysts that contain many branched fusomes. In addition, fusomes are present in pseudo egg chambers (D), a defect seldom seen in SXL-FL transgenic flies. (E-J) Confocal images of ovaries from MS11 transgenic flies stained for Bam-C (red) to label dividing cysts and VASA (green) stains all germ cells. Notice that a SXL-FL germarium (E-G) contains a normal number of Bam-C positive cysts, while a SXLΔBRE germarium (H-J) has more Bam-C positive cysts. All germaria are oriented with apical end to the upper left corner.
CONCLUSIONS
Our bioinformatics analyses suggest the existence of multiple Bruno target mRNAs during oogenesis. Furthermore, our experiments demonstrate that Sxl appears to be a main functional target of Bruno in germline cyst differentiation, defining a likely novel function for Sxl in germline cyst development in addition to its known role in sex determination, germ cell proliferation, differentiation, and meiotic recombination. Finally, our results indicate that the ORF2 of Sxl MS11 does not appear to be expressed in the ovary, and does not interfere with the translational repression of Bruno towards ORF1 via the BRE elements in the 3′UTR. This may be illustrative of the translational regulation of other bicistronic mRNAs that represent approximately 1-2% of the total transcripts in Drosophila.
ACKNOWLEDGEMENTS
We thank Brigid Hogan for valuable comments, and Seth Findley, Andrea Kirby, Heather Megosh, and Krystle Nomie for critical reading of the manuscript. We also thank Paul Schedl for MS11 cDNA, Paul Lasko for Bruno cDNA, Ruth Lehmann for osk cDNA, Pernille Røth for nos-Gal4 flies and the pUSAp vector, and Trudi Schüpbach for aret mutants. This work was supported by NIH (HD33760).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Black DL, Chan R, Min H, Wang J, Bell L. The elecrophoretic mobility shift assay for RNA binding proteins. In: Smith CWJ, editor. RNA:Protein Interactions. A practical approach. Oxford University Press; New York: 1998. pp. 109–136. [Google Scholar]
- Bopp D, Bell LR, Cline TW, Schedl P. Developmental distribution of female-specific Sex-lethal proteins in Drosophila melanogaster. Genes Dev. 1991;5:403–15. doi: 10.1101/gad.5.3.403. [DOI] [PubMed] [Google Scholar]
- Bopp D, Horabin JI, Lersch RA, Cline TW, Schedl P. Expression of the Sex-lethal gene is controlled at multiple levels during Drosophila oogenesis. Development. 1993;118:797–812. doi: 10.1242/dev.118.3.797. [DOI] [PubMed] [Google Scholar]
- Bopp D, Schutt C, Puro J, Huang H, Nothiger R. Recombination and disjunction in female germ cells of Drosophila depend on the germline activity of the gene sex-lethal. Development. 1999;126:5785–94. doi: 10.1242/dev.126.24.5785. [DOI] [PubMed] [Google Scholar]
- Castagnetti S, Hentze MW, Ephrussi A, Gebauer F. Control of oskar mRNA translation by Bruno in a novel cell-free system from Drosophila ovaries. Development. 2000;127:1063–8. doi: 10.1242/dev.127.5.1063. [DOI] [PubMed] [Google Scholar]
- Chekulaeva M, Hentze MW, Ephrussi A. Bruno acts as a dual repressor of oskar translation, promoting mRNA oligomerization and formation of silencing particles. Cell. 2006;124:521–33. doi: 10.1016/j.cell.2006.01.031. [DOI] [PubMed] [Google Scholar]
- Cline TW. Two closely linked mutations in Drosophila melanogaster that are lethal to opposite sexes and interact with daughterless. Genetics. 1978;90:683–98. doi: 10.1093/genetics/90.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande G, Calhoun G, Schedl PD. The N-terminal domain of Sxl protein disrupts Sxl autoregulation in females and promotes female-specific splicing of tra in males. Development. 1999;126:2841–53. doi: 10.1242/dev.126.13.2841. [DOI] [PubMed] [Google Scholar]
- Ephrussi A, Dickinson LK, Lehmann R. Oskar organizes the germ plasm and directs localization of the posterior determinant nanos. Cell. 1991;66:37–50. doi: 10.1016/0092-8674(91)90137-n. [DOI] [PubMed] [Google Scholar]
- Filardo P, Ephrussi A. Bruno regulates gurken during Drosophila oogenesis. Mech Dev. 2003;120:289–97. doi: 10.1016/s0925-4773(02)00454-9. [DOI] [PubMed] [Google Scholar]
- FlyBase Genome A. Release 3.2 of the annotated Drosophila melanogaster genome. 2004 [Google Scholar]
- Hay B, Jan LY, Jan YN. Localization of vasa, a component of Drosophila polar granules, in maternal-effect mutants that alter embryonic anteroposterior polarity. Development. 1990;109:425–33. doi: 10.1242/dev.109.2.425. [DOI] [PubMed] [Google Scholar]
- Johnstone O, Lasko P. Translational regulation and RNA localization in Drosophila oocytes and embryos. Annu Rev Genet. 2001;35:365–406. doi: 10.1146/annurev.genet.35.102401.090756. [DOI] [PubMed] [Google Scholar]
- Kim-Ha J, Kerr K, Macdonald PM. Translational regulation of oskar mRNA by bruno, an ovarian RNA-binding protein, is essential. Cell. 1995;81:403–12. doi: 10.1016/0092-8674(95)90393-3. [DOI] [PubMed] [Google Scholar]
- Kim-Ha J, Smith JL, Macdonald PM. oskar mRNA is localized to the posterior pole of the Drosophila oocyte. Cell. 1991;66:23–35. doi: 10.1016/0092-8674(91)90136-m. [DOI] [PubMed] [Google Scholar]
- Lin H, Spradling AC. Fusome asymmetry and oocyte determination in Drosophila. Dev Genet. 1995;16:6–12. doi: 10.1002/dvg.1020160104. [DOI] [PubMed] [Google Scholar]
- Lin H, Yue L, Spradling AC. The Drosophila fusome, a germline-specific organelle, contains membrane skeletal proteins and functions in cyst formation. Development. 1994;120:947–56. doi: 10.1242/dev.120.4.947. [DOI] [PubMed] [Google Scholar]
- McKearin D, Ohlstein B. A role for the Drosophila bag-of-marbles protein in the differentiation of cystoblasts from germline stem cells. Development. 1995;121:2937–47. doi: 10.1242/dev.121.9.2937. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Sato K, Hanyu-Nakamura K. Drosophila cup is an eIF4E binding protein that associates with Bruno and regulates oskar mRNA translation in oogenesis. Dev Cell. 2004;6:69–78. doi: 10.1016/s1534-5807(03)00400-3. [DOI] [PubMed] [Google Scholar]
- Neuman-Silberberg FS, Schupbach T. The Drosophila dorsoventral patterning gene gurken produces a dorsally localized RNA and encodes a TGF alpha-like protein. Cell. 1993;75:165–74. [PubMed] [Google Scholar]
- Neuman-Silberberg FS, Schupbach T. Dorsoventral axis formation in Drosophila depends on the correct dosage of the gene gurken. Development. 1994;120:2457–63. doi: 10.1242/dev.120.9.2457. [DOI] [PubMed] [Google Scholar]
- Parisi MJ, Deng W, Wang Z, Lin H. The arrest gene is required for germline cyst formation during Drosophila oogenesis. Genesis. 2001;29:196–209. doi: 10.1002/gene.1024. [DOI] [PubMed] [Google Scholar]
- Penalva LO, Sanchez L. RNA binding protein sex-lethal (Sxl) and control of Drosophila sex determination and dosage compensation. Microbiol Mol Biol Rev. 2003;67:343–59. doi: 10.1128/MMBR.67.3.343-359.2003. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorth P. Gal4 in the Drosophila female germline. Mech Dev. 1998;78:113–8. doi: 10.1016/s0925-4773(98)00157-9. [DOI] [PubMed] [Google Scholar]
- Samuels ME, Schedl P, Cline TW. The complex set of late transcripts from the Drosophila sex determination gene sex-lethal encodes multiple related polypeptides. Mol Cell Biol. 1991;11:3584–602. doi: 10.1128/mcb.11.7.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SchuLtt C, Hilfiker A, Nothiger R. virilizer regulates Sex-lethal in the germline of Drosophila melanogaster. Development. 1998;125:1501–7. doi: 10.1242/dev.125.8.1501. [DOI] [PubMed] [Google Scholar]
- Schupbach T. Normal female germ cell differentiation requires the female X chromosome to autosome ratio and expression of sex-lethal in Drosophila melanogaster. Genetics. 1985;109:529–48. doi: 10.1093/genetics/109.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupbach T, Wieschaus E. Female sterile mutations on the second chromosome of Drosophila melanogaster. II. Mutations blocking oogenesis or altering egg morphology. Genetics. 1991;129:1119–36. doi: 10.1093/genetics/129.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimura I, Lilly MA. Bruno inhibits the expression of mitotic cyclins during the prophase I meiotic arrest of Drosophila oocytes. Dev Cell. 2006;10:127–35. doi: 10.1016/j.devcel.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Vied C, Halachmi N, Salzberg A, Horabin JI. Antizyme is a target of sex-lethal in the Drosophila germline and appears to act downstream of hedgehog to regulate sex-lethal and cyclin B. Dev Biol. 2003;253:214–29. doi: 10.1016/s0012-1606(02)00012-x. [DOI] [PubMed] [Google Scholar]
- Vied C, Horabin JI. The sex determination master switch, Sex-lethal, responds to Hedgehog signaling in the Drosophila germline. Development. 2001;128:2649–60. doi: 10.1242/dev.128.14.2649. [DOI] [PubMed] [Google Scholar]
- Webster PJ, Liang L, Berg CA, Lasko P, Macdonald PM. Translational repressor bruno plays multiple roles in development and is widely conserved. Genes Dev. 1997;11:2510–21. doi: 10.1101/gad.11.19.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie GS, Dickson KS, Gray NK. Regulation of mRNA translation by 5'-and 3'-UTR-binding factors. Trends Biochem Sci. 2003;28:182–8. doi: 10.1016/S0968-0004(03)00051-3. [DOI] [PubMed] [Google Scholar]
- Zaccai M, Lipshitz HD. Differential distributions of two adducin-like protein isoforms in the Drosophila ovary and early embryo. Zygote. 1996;4:159–66. doi: 10.1017/s096719940000304x. [DOI] [PubMed] [Google Scholar]