Abstract
We examined the reinforcer-specificity of Pavlovian conditioning in the control of appetitive and consummatory behaviors in Pavlovian-to-instrumental transfer, cue-potentiated eating, and devaluation procedures. Rats received pairings of one conditioned stimulus with sucrose and another conditioned stimulus with maltodextrin. In Experiment 1, rats were also trained to earn sucrose for one instrumental response and maltodextrin for another. In a transfer test, the Pavlovian cues enhanced the rate of instrumental responding more when the food reinforcer predicted by the instrumental response and the Pavlovian cue were consistent than when they were inconsistent, but both cues enhanced both responses. In Experiment 2, sated rats' consumption of each food was potentiated in the presence of a cue for that food, but not in the presence of a cue for the other food. In Experiment 3, one food was devalued by pairing it with lithium chloride, prior to testing food consumption and food-cup directed behaviors. The food cues selectively controlled food-cup related behaviors, regardless of the presence of the devalued or nondevalued foods in the food cup. Together, these results are consistent with the view that conditioned cues modulate appetitive and consummatory behaviors with increasing levels of specificity. The closer an action comes to ingestion, the more it is controlled by sensory properties conveyed by learned cues. These data are discussed in the context of allostatic regulation of food foraging and intake.
Keywords: appetite, conditioning, cue-potentiated feeding, devaluation, food consumption, Pavlovian-to-instrumental transfer
1. Introduction
Overeating has become a problem of epidemic proportions. Thus, understanding the events that trigger appetitive and consummatory behaviors is of utmost importance. Considerable scientific attention has been focused on roles of food availability, meal size, energy homeostasis and pharmacological variables in determining feeding. The importance of learning and memory is often overlooked. The experiments reported here examined the effects of learned signals for foods on various aspects of appetitive and consummatory behavior. Progress in understanding the impact of learning on food-procuring and consuming behaviors should contribute to understanding the impact of environmental cues and food availability on eating habits.
Consideration of roles for learning in feeding behavior is consistent with the notion of allostasis [1], which has increasingly supplemented traditional homeostatic views of feeding behavior. From this perspective, relative stability of body weight is the product of mechanisms that predict future demands rather than merely adjust to physiologically determined setpoints. Ingestive behavior may be described as allostatic not only because animals forage and eat before their energy stores are depleted, but also because those behaviors are determined by information about the future availability or scarcity of food as well as by the animal's current deprivation state. When a future shortage of food is predicted, an animal acts upon that prediction, although its actual state may not have gone below a determined homeostatic setpoint or threshold. Thus, allostatic mechanisms are readily adaptable to constantly changing environmental challenges and demands.
Allostatic systems may deal with current or future scarcities of resources. For example, induction of a sodium deficit enhances long-term salt palatability beyond the moment of restoration of normal sodium levels [2,3]. From an allostatic standpoint, this form of sensory and/or hedonic stimulation of appetite is highly adaptive. Although the exquisite relation of sodium deficits and salt palatability may be unique, studies of acquired flavor preferences and aversions show that animals can alter their evaluations of foods that are enriched or deficient in particular nutrients. These learned changes in evaluation or preference may come under the control of a variety of cues, such as other sensory properties of those foods, or environmental cues associated with those foods during periods of nutrient scarcity. Furthermore, these cues might later guide procurement and eating of those foods and hence prevent depletion of that nutrient in the face of future scarcity. Thus, an important question addressed in the present experiments is the extent to which the influence of learned signals for foods on appetitive and consummatory behaviors is specific to the particular foods being sought or consumed.
The development of specific hungers and preferences might be especially advantageous if they were accompanied by the acquisition of corresponding food-specific foraging behaviors. For example, if a cue correlated with carbohydrate in a period of food scarcity especially enhances consumption of carbohydrate-laden foods, it would be advantageous if it also enhanced foraging behaviors that secure possession of those particular foods. Experiments 1 and 2 were designed to examine the influence of learned cues for specific foods on both the consumption of those foods and on operant “foraging” for them, in the same rats. In Experiment 1 we addressed the question of the specificity of learned cues' effects on foraging behaviors in the context of Pavlovian-to-Instrumental Transfer (PIT), in which cues previously paired with the delivery of specific foods were presented while rats were performing instrumental lever-press responses that earned the same or different foods.
In Experiment 2 we examined whether cues paired with specific reinforcers while rats were food-deprived selectively enhanced consumption when the rats were food-sated. A number of studies have shown that learned visual or auditory cues that originally signaled food while animals were food-deprived can potentiate eating later, when those animals are food-sated [4-6]. However, in those experiments, animals were trained and tested with only one type of food. Thus, it is of considerable interest to determine whether cues associated with particular foods in periods of food scarcity may act selectively to enhance consumption of those same foods later in times of dietary repletion.
Finally, it is useful for animals to modify their behavior in response to food-related cues if subsequent experience alters the desirability of the foods signaled by those cues. For example, the induction of illness after consuming a particular food not only reduces consumption of that food but also reduces the likelihood that cues for that food will elicit approach to the source of that food [7]. In Experiment 3, we examined both food-source approach and food-consumption behaviors in the presence of food cues, after establishing an aversion to the taste of one of a pair of foods.
2. Experiment 1
Experiment 1 was designed to measure the sensory-specific effects of superimposing Pavlovian conditioned stimuli (CSs) on instrumental behavior. This Pavlovian-to-instrumental transfer (PIT) procedure is commonly used to evaluate the motivational effects of Pavlovian stimuli on ongoing goal-directed behavior. Specifically, we examined the effects of a CS that predicted one food (e.g., sucrose) on instrumental responding that was trained with that same food as the reinforcer, and on instrumental responding that was trained with a different food (e.g., maltodextrin).
2.1. Method
2.1.1. Subjects
The subjects were 16 male naive Long-Evans strain rats (Charles River Laboratories, Raleigh, NC, USA) which weighed between 300–350 g when they arrived in the laboratory vivarium. They had free access to lab chow (2018 Rodent Diet, Harlan Teklad Laboratory, Madison, WI, USA) for a week before their food was restricted to maintain them at 85% of their ad-libitum weights. The rats were caged individually in a colony room illuminated from 6:00am to 6:00pm. The research was approved by the Johns Hopkins University Animal Care and Use committee.
2.1.2. Apparatus
We used eight training chambers (22.9 × 20.3 × 20.3 cm) with aluminum front and back walls and clear acrylic side walls and top. An infrared activity monitor (Coulbourn Instruments, Allentown, PA, USA) and a panel of infrared lights used to illuminate the chamber for video recording were placed on the top of each chamber. An illuminated clear acrylic food cup, with a capacity of about 1.7 ml, was placed behind a square hole in the center the front wall. A photocell beam in the food cup was used to detect head entries and time spent in the cup. An aluminum lever (2.0 × 2.0 cm) was mounted on each side of the food cup, centered between the cup and the side walls; throughout the Pavlovian training sessions, they were covered with aluminum boxes (3.0 × 2.0 × 3.0 cm). A speaker which was used to present auditory cues was placed on the back wall of a double-walled sound-resistant shell which enclosed each experimental chamber. A television camera was placed 18 cm above the speaker to record the rat's behavior, and a second camera was placed under the transparent food cup, to record consummatory responses. Images from each camera were digitized, and were recorded and displayed in real time on video monitors, each of which showed 4 chambers or food cups. Video data are not presented in this article.
2.1.3. Pavlovian conditioning
Rats first received four 64-minute sessions designed to train them to approach the food cup and consume each of the 2 food unconditioned stimuli (USs). Each of these sessions included 16 0.1-ml deliveries of either 4% sucrose (2 sessions) or 4% maltodextrin solution (2 sessions), which served as the 2 USs. Each food was administered in a 4% (w/v) solution because pilot data from our laboratory indicated that at this concentration Long-Evans rats consume equal amounts of these foods. Solutions were delivered by infusion pumps located outside the double-walled sound-attenuating shells. Next, rats were given 17 32-min training sessions intended to establish two Pavlovian associations, CS1-US1 and CS2-US2. The CSs were a 78-dB, 1500-hz tone and an 80-dB white noise and were each 2 min in duration. Intertrial intervals were variable, and averaged 90 s. Four USs were delivered at random times within each CS presentation. The identities of the CSs, USs and their combinations were completely counterbalanced. Each of the first four training sessions included 8 presentations of only one of the CS-US combinations (two sessions with each). Each of the remaining sessions included 5 CS1-US1 trials and 5 CS2-US2 trials, randomly intermixed. Table 1 provides an outline of the procedures of Experiment 1.
Table 1.
Summary of experimental designs
| Phase 1 | Phase 2 | Phase 3 | Test |
|---|---|---|---|
| Experiment 1 | |||
| Pavlovian training | Instrumental training | Transfer test | |
| CS1 → US1 | R1 → US1 | R1: CS1(cons), CS2(incons), no CS | |
| CS2 → US2 | R2 → US2 | R2: CS1(incons), CS2(cons), no CS | |
| Experiment 2 | |||
| Pavlovian training | Ad-lib feeding | Consumption tests | |
| CS1 → US1 | 1 wk chow | US1: CS1(cons), CS2(incons), or no CS | |
| CS2 → US2 | US2: CS1(incons), CS2(cons), or no CS | ||
| Experiment 3 | |||
| Pavlovian training | Devaluation | Ad-lib feeding | Consumption tests |
| CS1 → US1 | US1 → LiCL | 1 wk chow | US1: CS1(cons), CS2(incons), or no CS |
| CS2 → US2 | US2 → nothing | US2: CS1(incons), CS2(cons), or no CS | |
Note: CS1, CS2 refer to tone and noise conditioned stimuli (counterbalanced); US1, US2 refer to 4% sucrose and maltodextrin reinforcement (counterbalanced); R1, R2 refer to left and right instrumental lever manipulanda (counterbalanced); LiCl = Lithium chloride injections; cons = CS whose outcome was consistent with the outcome of the available response (Experiment 1) or US (Experiments 2 and 3); incons = CS whose outcome was not consistent with the outcome of the available response (Experiment 1) or US (Experiments 2 and 3). In Experiment 1, one transfer test was conduced with R1 available and the other with R2 available; in that experiment “no CS” refers to baseline response periods in the intertrial intervals equivalent to the CS intervals. In Experiment 2, each rat received 6 consumption tests, 3 with each US. In Experiment 3, each rat received 3 consumption tests; half of the rats were tested with US1 and half with US2. In Experiments 2 and 3 “no CS” refers to test sessions with no CS present in the test period.
2.1.4. Instrumental training
After the 10th Pavlovian session, the rats received instrumental training in which pressing one lever was reinforced with 0.1 ml sucrose and pressing the other lever was reinforced with 0.1 ml maltodextrin (counterbalanced across left and right levers). In the shaping phase, one lever was uncovered in the experimental boxes and rats were reinforced for each lever press until they pressed the lever 50 times. These levers were baited with lab chow paste in order to favor initial lever presses. The next day, the other lever was introduced (and the first one covered) in a session identical to the previous one, except that these levers were not baited, and the other reinforcer was contingent on responding. Subsequently, rats received 30-min sessions during which lever pressing was reinforced on a random interval (RI) 30-s schedule. Rats were trained with each of the two levers in separate sessions, in a mostly alternating order (LRLRLRLRLRRLRLRLLR). After 8 sessions, the schedule was changed to RI 60-s for the remaining 10 sessions. Instrumental and Pavlovian sessions were intercalated on a 2:1 ratio.
2.1.5. Pavlovian to Instrumental transfer tests
Pavlovian-to-instrumental transfer tests (PIT) were conducted immediately after training. In these tests, lever pressing was not reinforced. In the first 30-min session only the left lever was uncovered. On the next day, during the second 30-min test session, only the right lever was available. In each session, 2-min CS1 and CS2 trials were each presented three times in random order with 2-min intertrial intervals.
2.2 Results
2.2.1. Pavlovian and instrumental training
Pavlovian conditioning occurred normally. We evaluated learned responding by examining responding during CSs prior to the delivery of the first US on any trial. Overall, the rats entered the food cup more frequently during CS presentations (22.7 ± 1.6 entries/min) than during intertrial intervals (11.8 ± 1.1 entries/min) [F(1,12)= 313.17, p<0.001). Similarly, they spent more time in the food cup during the CS periods (31.1 ± 2.5%) than during ITIs (15.5 ± 1.4%) [F(1,12)=106.91, p<0.001]. Finally, on either of these measures, the rats did not show differences in responding between tone and noise [F(1,12)=0.04, p=0.853); F(1,12)=0.05, p=0.834), respectively], or between signals for sucrose and maltodextrin [F(1,12)=0.07, p=0.795); F(1,12)=0.02, p=0.893].
By the end of the instrumental training sessions, the mean ± sem lever press/min rate was 9.6 ± 0.7. There was no evident preference for either the left or right lever [F(1,14)=2.35, p=0.148], or for pressing for sucrose or maltodextrin [F(1,14)=0.50, p=0.492].
2.2.2. PIT tests
Both specific and general PIT effects were observed (Figure 1a). An analysis of variance (ANOVA) that included test period (baseline, consistent CS and inconsistent CS), the 2 counterbalancing variables (CS and US identity), and test session (first or second) as variables showed a significant effect of test period [F(2,24)= 18.39; p<.001]. Planned comparisons showed that presentation of the consistent CS (the CS that had been paired with the same US that had been used as the reinforcer for responses on the available lever) enhanced lever presses significantly more than presentation of the inconsistent CS [F(1,12)=12.76, p=0.004]; thus we observed specific PIT. Nevertheless, we also observed a more general PIT: the inconsistent CS also increased responding relative to equivalent no-CS empty intervals [F(1,12)=7.97, p=0.015]. Test period did not interact with the counterbalancing variables [Fs < 1, ps > 0.403]. Because instrumental responding was not reinforced, its frequency decreased both within and between sessions. Rats responded significantly less in the second test [F(1,12)=8.45, p=0.013].
Figure 1.
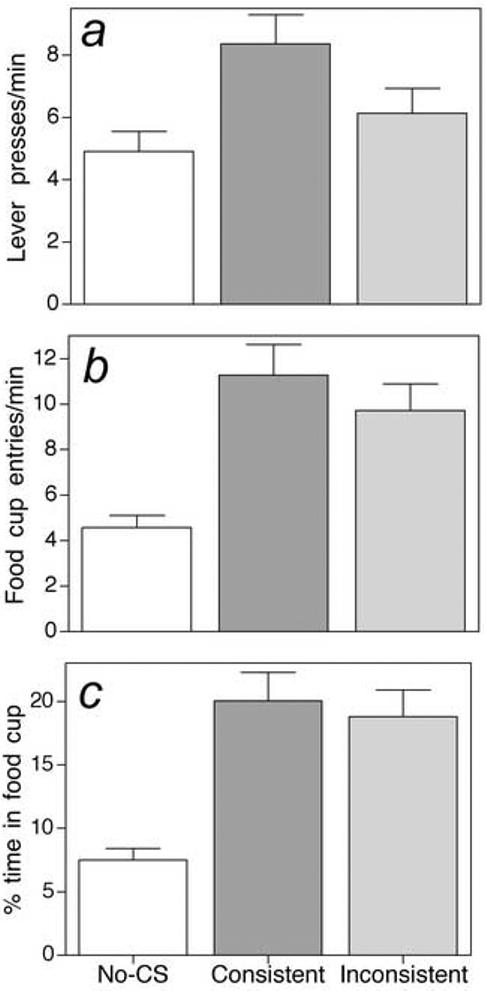
Mean rates of instrumental (lever) responding (a), entries into the food cup (b) and the percent time spent in the food cup (c) during the test for Pavlovian-to-instrumental transfer in Experiment 1. Testing was conducted in 2 consecutive sessions, during which reinforcement was not available and only one lever was present. The bars labeled “consistent” refer to responding during presentations of the CS that had been paired with the same US (sucrose or maltodextrin) that had been used as the reinforcer for the available lever and the bars labeled “inconsistent” refer to responding during presentations of the CS that had been paired with the other US. The bars labeled “no CS” refer to responding during equivalent periods in which no CS was present (i.e., the intertrial interval). Note that both general and selective PIT effects were observed: Both CSs elevated lever press responding above the no-CS level, but presentations of the consistent CS produced greater increases than the inconsistent CS. Presentation of either CS increased the rate of food cup entries (b) and the time spent in the food cup (c). Error bars indicate ± SEM.
Presentation of either CS also increased the rate of food cup entries (Figure 1b) [F(1,12)=58.18, p < 0.001 for the consistent CS and F(1,12)=50.39, p < 0.001 for the inconsistent CS] and the time spent in the food cup (Figure 1c) [F(1,24)=54.65 and 64.80, respectively, ps < 0.001], compared to responding in the No-CS intervals. Not surprisingly, there was no difference in food cup entry rates [F(1,12)=2.78, p=0.121] or time spent in the food cup [F(1,12)=0.59, p=0.458] during the consistent versus inconsistent CSs. The insensitivity of these measures to the nature of the CS (unlike lever pressing, which was greater during consistent CSs) suggests that these responses were controlled by Pavlovian mechanisms and were at least partially dissociated from the instrumental responding. As with lever pressing, rats entered the food cup significantly more often [F(1,12)=42.72, p<0.001] and spent more time there [F(1,12)=47.59, p<0.001] during the first than during the second test session.
2.3. Discussion
The results of Experiment 1 show that CSs can invigorate ongoing instrumental behavior, both when the predicted outcome of that action coincides with the US prediction evoked by the CS (the consistent CS), and when it does not (the inconsistent CS). A simple account for these results is that Pavlovian CSs control both outcome-specific and more general processes [8, 9]. Many psychologists agree that a primary consequence of Pavlovian conditioning is the conditioning of emotional or motivational responses [10, 11]. Within this view, CSs acquire general motivational properties of the USs with which they are paired. Thus, a signal for food may acquire general arousal properties that would allow it to modulate instrumental foraging behaviors regardless of the sensory properties of the predicted instrumental outcome. At the same time, Pavlovian CSs often convey outcome-specific information about the upcoming US [12-14], and there is considerable evidence that instrumental lever press conditioning is mediated by the formation of specific response-outcome associations [15,16]. Thus, Pavlovian CSs may enhance instrumental responding by arousing the same expectancy that mediates the instrumental response. From this perspective, all PIT is selective, and observations of general PIT simply reflect generalization between the cues, responses, or reinforcers. Strictly speaking, within this view only consistent CSs, which signal the same outcome as earned by the instrumental response, should support PIT. Indeed, many authors (9, 13) have reported that CSs that predict one instrumental reinforcer depress instrumental responding supported by another reinforcer, as if the evocation of a different US representation produced generalization decrement or interference.
Although Experiment 1 does not address the issue of whether general and specific PIT are the result of distinct psychological processes, brain lesion studies support the idea that outcome-specific and more general PIT effects are separable. Earlier studies [17, 18] found that rats with lesions of the central nucleus of the amygdala (CeA) failed to show PIT, whereas rats with lesions of the basolateral amygdala (BLA) showed normal PIT. Notably, in those studies, rats were trained with a single reinforcer and a single instrumental response, which may have discouraged the formation of outcome-specific expectancies. By contrast, a later study [19] showed that BLA lesions prevented outcome-specific PIT, when rats were trained with two reinforcers and two instrumental responses, as in Experiment 1. Most recently, Corbit and Balleine [9] examined the effects of CeA and BLA lesions on PIT in a more complex experimental procedure, in which a third CS and Pavlovian US was added to a two response, two reinforcer procedure like that used in Experiment 1. In sham-lesioned rats, Corbit and Balleine [9] found both selective and general PIT. First, each CS enhanced instrumental responding that was reinforced by the US associated with that CS, but not instrumental responding that was reinforced by the other US (selective PIT). Second, the third CS, which predicted a third Pavlovian US that had not been used as a reinforcer for either instrumental response, enhanced the rates of both of those responses (general PIT). Rats with CeA lesions showed normal selective PIT but no evidence for general PIT, whereas rats with BLA lesions showed intact general PIT but no evidence for selective PIT.
3. Experiment 2
In Experiment 2, we examined the sensory specificity of cue-potentiated feeding. While food-deprived, rats were trained with two USs, sucrose and maltodextrin, each signaled by a different auditory CS, as in Experiment 1. After satiation on standard lab chow in their home cages, consumption of sucrose and maltodextrin was examined in the experimental chambers in the presence of the previously-trained sucrose signal, the maltodextrin signal, and no signal, in separate test sessions.
3.1. Method
3.1.1. Subjects and apparatus
The rats from Experiment 1 were used in this experiment, which was conducted after the completion of Experiment 1. Except as noted, they were maintained as in Experiment 1. The apparatus was also the same apparatus as used earlier. The levers were covered with aluminum boxes throughout Experiment 2.
3.1.2. Pavlovian retraining and food-satiation
Table 1 provides an outline of the procedures of Experiment 2. Rats were retrained to respond to the two 2-min CSs used in Experiment 1. In each of four 32-minute sessions the rats were given 5 CS1-US1 trials and 5 CS2-US2 trials. The original CS-US associations, (i.e. CS1→US1; CS2→US2) were maintained. Immediately after the 4th session, the rats were given free access to lab chow in their home cages for a week.
3.1.3. CS-Potentiated Feeding Tests
On consecutive days, rats received 6 10-min potentiated feeding tests, in which consumption of each food was examined in the presence of its corresponding CS signal (Consistent tests, CS1+US1 or CS2+US2), the other CS (Inconsistent tests, CS1+US2 or CS2+US1), or no CS (US1 or US2). The no-CS sessions provided an index of spontaneous feeding in the absence of explicit Pavlovian cues. In the first five minutes of each session rats had unlimited access to the test US solution in the absence of any CS (pretest). The purpose of this pretest was to reduce overall consumption of the test US to permit a more sensitive assessment of the effects of the various CS test conditions later. Previous studies [4, 20] showed that chow-sated rats consume substantial amounts of generally more palatable USs when first returned to the experimental context. In the second half of each session (test), the rats had access to the US while two 2-minute CSs were presented, separated by 40 s. Each session ended with a 20-s empty (no CS) interval. The second and fifth test sessions were both no-CS tests. Consistent and Inconsistent tests bracketed each of those tests, and were presented in counterbalanced order. Finally, in each test, half of the rats were tested with sucrose and half with maltodextrin.
The food cups were filled with 1.7 ml of solution before the rats were placed in the experimental chambers. Consumption of the rats was monitored on the video monitors by two experimenters. When the liquid in a cup was nearly depleted, another 0.2 ml was delivered by the experimenter, using a computer program that activated the appropriate infusion pump. The time and number of these deliveries was recorded by the computer, providing a record of the pattern and amount of liquid consumed by each rat.
3.2. Results
3.2.1. Pavlovian retraining
The rats showed more food-cup entries and more time in the food cup during the CSs (20.5 ± 2.3 entries and 42.1 ± 5.7%) than during ITIs (11.1 ± 1.7 and 22.1 ± 3.7%) [Fs(1, 12) = 111.97, 62.27, ps < 0.001]. The effects of the counterbalancing assignment variables, as well as their interactions, were not significant [Fs < 2.95, ps > 0.111].
3.2.2. Feeding test: consummatory responding
The primary dependent variable in this test was food consumption, indexed as the rate of 0.2-ml fluid deliveries needed to maintain solution in the food cup (Figure 2). The most notable finding was that, relative to baseline pretest consumption, consumption was greater in the presence of the consistent CS (the CS that had previously been paired with the fluid US present in that test) than in the presence of the inconsistent CS (Figure 2a). Fluid delivery rates were analyzed with an ANOVA that included the experimental variables of test period (pretest or test) and test type (consistent CS, inconsistent CS, or no CS), and counterbalancing assignment variables of US (sucrose or maltodextrin) and CS (noise or tone) identity in test. Overall, mean liquid consumption during the test (68.1 ± 2.8 deliveries) was lower than during the pretest (85.0 ± 2.9 deliveries) [F(1,12) = 15.53, p = 0.002]. However, the magnitude of this reduction depended on the CS type in test; test period interacted with the effects of CS type [F(2, 24)=6.63, p = 0.005]. Thus, the effects of the test conditions were evaluated against a falling baseline of consumption. The levels of consumption dropped significantly more in the inconsistent CS and no CS tests than during the consistent CS test. Orthogonal contrasts used to partition the test period X test type interaction showed that the pretest-test difference (Figure 2a) was smaller in the consistent than in the inconsistent CS test condition, [F(1, 12) = 13.31, p = 0.003] or the no-CS condition [F(1, 12) = 7.81, p = 0.016], which did not differ from each other [F (1, 12) = 1.44, 0 = 0.253]. Finally, neither of the counterbalancing variables (CS and US assignment) interacted significantly with any other variable, Fs(1, 12) < 3.07, ps > 0.105. However, overall, mean sucrose consumption (174.7 ± 6.1 deliveries) was significantly greater than maltodextrin consumption (131.5 ± 5.2 deliveries) [F(1,12) = 52.11, p<0.001].
Figure 2.
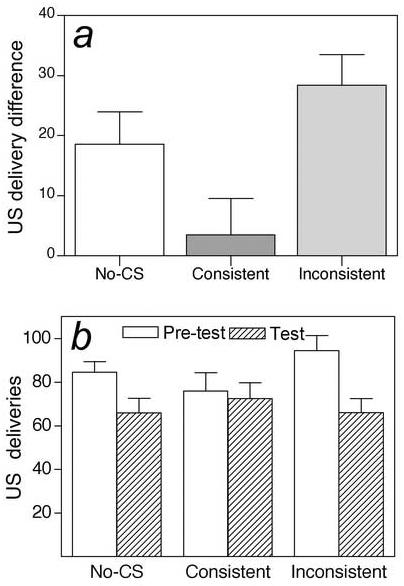
Mean liquid-food consumption during the tests for potentiated feeding in Experiment 2. On consecutive sessions, rats received six 10-min feeding tests during which consumption of each food (sucrose or maltodextrin) was assessed in the presence of its corresponding conditioned stimulus (CS) signal (Consistent tests, CS1+US1 or CS2+US2), the other CS (Inconsistent tests, CS1+US2 or CS2+US1), or no CS (US1 or US2). The no-CS sessions provided an index of spontaneous feeding in the absence of Pavlovian food cues. To reduce baseline levels of consumption, during the first five minutes of each session, rats had unlimited access to the test unconditioned stimulus (US) solution in the absence of any CS (pretest). Data are expressed as the difference in US deliveries (1 US delivery unit = 0.2 ml) needed to maintain constant liquid in the food cup, relative to baseline pretest US deliveries (a), or as the total number of US deliveries during the pretest and test sessions (b). Note that, relative to pretest consumption levels, consumption was greater in the presence of the consistent CS (the CS that had previously been paired with the fluid US present in that test) than in the presence of the inconsistent CS (a). Although inspection of the pretest data (b) raises the possibility that this effect is due to differences in pretest consumption levels, post-hoc analyses failed to support this argument (see text). Error bars indicate ± SEM.
It might be argued from the data in Figure 2b that the specificity effect is derived mostly from the higher pretest consumption before inconsistent tests and lower pretest consumption before consistent tests. Indeed, in the first set of tests, that was the case. Consumption during the test period was 63.6 ± 5.6 deliveries for the consistent CS and 60.3 ± 6.6 deliveries in the inconsistent test, whereas pretest consumption was 53.5 ± 5.1 and 88.9 ± 6.7 deliveries, respectively. Although consumption during the CSs themselves did not differ [F < 1], as with the overall test data, the reduction in consumption from pretest to test was significantly less for the consistent test [F(1, 12) = 18.77, p < 0.001]. One concern is that chance differences in pretest consumption led to differential satiation at the beginning of the test periods when the CSs were presented. We evaluated this possibility several ways.
First, from this argument, test consumption should be negatively correlated with pretest consumption within each session. However, neither simple correlation nor multiple regression analyses revealed any evidence for such negative correlations. Indeed, regression analyses showed significant positive relations between test consumption on inconsistent-CS and no-CS tests, and both body weight and pretest consumption [R2 = 0.35, p = 0.002; R2 = 0.30, p = 0.005, respectively]. By contrast, consumption during the consistent test was not reliably predicted by these factors [R2 = 0.11, p = 0.183; R2 = 0.20, p=0.311, respectively]. Thus, the consistent CS apparently enhanced consumption regardless of pretest consumption, overriding the normal relation between body weight and consumption, and between initial (baseline) and subsequent (test) consumption.
Second, ANCOVA of test consumption using pretest consumption as a covariate revealed the same pattern of effects and significances as described previously. Finally, it is notable that the difference in pretest consumption occurred only before the first consistent and inconsistent test sessions (described previously). In the second set of test sessions, pretest consumption was 98.3 ± 7.4 deliveries before the consistent test and 100.0 ± 6.7 deliveries before the inconsistent test. Even with these nearly identical baselines, consumption in the second consistent test (81.3 ± 8.3 deliveries) was significantly greater than consumption in the second inconsistent test (71.8 ± 6.6 deliveries) [F(1, 12) = 19.27, p < 0.001]. Thus, we think Figure 2a appropriately reflects greater enhancement of consumption by the consistent CS than the inconsistent CS.
3.2.3. Test data: appetitive response measures
As with food consumption itself, the rats spent significantly less time in the food cup during the test periods (61.9 ± 2.1%) than during the pretest periods (75.5 ± 1.7%) [F(1,12) = 27.56, p<0.001]. The test period × test type condition interaction was not significant for time spent in the cup [F(2,24) = 1.70, p = 0.204]. Thus, the time spent in the food cup was not an accurate index of the amount of consumption. Nevertheless, as with food consumption, the amount of time spent in the food cup was significantly greater while sucrose was presented than when maltodextrin was delivered (76.7 ± 11.1% and 60.7 ± 8.8%, respectively) [F(1,12) = 67.04, p<0.001]. The effects of the counterbalancing variables and their interactions were not significant [ps > 0.104].
By contrast, the rate at which the animals entered the food cup was greater in the test periods (5.4 ± 0.2 entries/min) than in the pretest periods (3.4 ± 0.2 entries/min), [F(1,12)=53.93, p<0.001], as might be anticipated if the rats tended to drink uninterruptedly during the pretest period. Furthermore, the interaction of test type condition with this test-pretest difference was significant [F(2,24) = 4.11, p=.029]: the entry rate difference was larger in the inconsistent CS test (2.63 ±0.45) than in either the consistent CS test (1.55 ± 0.59) or the no CS test (1.95 ± 0.57) [F(1, 12) = 14.40, p = 0.003], which did not differ from each other [F < 1, p > 0.400]. This observation is consistent with the greater consumption in the inconsistent pretests, discussed earlier. Food cup entry rate was unaffected by whether sucrose or maltodextrin was present in the test, and the effects of the counterbalancing assignment variables, as well as their interactions, were not significant [Fs < 1, ps > 0.355].
3.3. Discussion
In Experiment 2, CSs modulated consummatory behavior only when the CS-elicited representation of a specific food and the food present in the food cup were the same. This specificity, which occurred despite the comparable familiarity and preference for the two foods, is informative about the nature of the cue-potentiated feeding effect. First, because in this experiment both foods were delivered to the same food cup, enhanced eating with consistent CS presentations relative to inconsistent CS presentations can not be simply the result of learned food-cup approach behaviors acquired during initial training. This observation complements Holland et al.'s [4] finding of cue-potentiated feeding effects even when the test food was presented at a site remote from the food cup. Second, it is unlikely that these learned cues acted by inducing a general state of hunger [21] or by overriding general satiety signals [22]. Instead, any account that invokes learned motivational processes must assume some specificity, for example the induction of specific appetites or the suppression of satiety in a sensory-specific fashion [23], or the enhancement of the palatability or incentive value of the predicted food [12, 24, 25].
Why might the effects of CSs on consumption that we observed in this experiment be more specific than their effects on instrumental behavior in Experiment 1? In normal circumstances, the chain of food-related behaviors culminates with ingestion. This last link is of utmost importance, because it determines whether an animal will enjoy the nutritional benefits of a certain food, or experience unpleasant consequences. On the contrary, pre-consummatory behaviors do not necessary lead to irreversible consequences. In this way, appetitive behaviors can be guided by both general food properties and by specific ones (as in Experiment 1). Consummatory actions, instead, should be very specific when considering the sensory properties of what is about to be eaten. Any variation in some quality of known food (e.g., its smell) could potentially mean that it is not safe to eat.
4. Experiment 3
The purpose of Experiment 3 was to investigate the specificity of appetitive and consummatory behavior of food-sated rats in the presence of CSs in the context of a devaluation procedure. After selective pairings of two CSs and USs for each rat, e.g., tone→sucrose and noise→maltodextrin, the value of one of the two USs (e.g., sucrose) was reduced by pairing it with LiCl-induced illness, in the absence of the CSs. Responding to both CSs was then assessed after 10 days of free access to chow in the home cages. Unlike in most devaluation experiments [e.g., 7-8, 12-13, 15-16, 26-29, but see 30], in which responding to CSs is assessed in extinction, in Experiment 3, the rats were tested with unlimited access to an experimental food US, as in Experiment 2. For half of the rats, the devalued reinforcer was present in the food cup, and for the other half, the other, nondevalued reinforcer was present. In this way, the relative influence of information conveyed by the CS, the US itself, and the CS-US relation (consistent or inconsistent) on both appetitive (approach and entry to the food cup) and consummatory (consumption of the food) behavior was examined.
4.1. Method
4.1.1. Subjects and apparatus
The subjects and apparatus were the same as those used in Experiment 2. At the conclusion of that experiment, the rats were returned to their 85% weights in preparation for Experiment 3.
4.1.2. Training Procedures
The rats first received 4 32-minute Pavlovian retraining sessions, with 10 CS1→US1 pairings in sessions 1 and 3 and 10 CS2→US2 pairings in sessions 2 and 4. Then, the foods were presented in the absence of conditioned cues during 8 32-min sessions, with the same frequency as in the previous retraining sessions but in the absence of CSs. Each food (4% sucrose or 4% maltodextrin) was made available in alternate daily sessions. After the first, third, fifth and seventh sessions rats received an intraperitoneal injection of 0.3 M LiCl solution (5 ml/kg). Thus, each rat received an injection immediately after one food, whereas the other food was never followed by LiCl-induced illness.
After taste aversion training, the rats were given 10 days access to lab chow in their home cages, and then 3 post-devaluation tests, similar to the potentiated feeding tests of Experiment 2. However, in this study each rat was tested with only one type of food. Each of the three tests (conducted on consecutive days) consisted of a single 10-min session. As in Experiment 2, in the first five min of each session rats had unlimited access to a food US in the absence of any CS (pretest). In the second half of the session rats had access to the US while two 2-min CSs were presented with a 40-s separation between them (test). Each session ended with a 20-s empty interval (see table1). These tests were designed to examine the effects of conditioned cues on appetitive and consummatory behavior when the CS presented was either consistent (CS1+US1; CS2+US2) or inconsistent (CS2+US1; CS1+US2) with the food found in the food cup. Additionally, all rats were tested in a control test in which no cues were presented during either pretest or test periods. Consumption was indexed by the rate of US deliveries needed to maintain a constant level of fluid in the wells throughout the test session, and the appetitive measures included the percentage of time spent in the food cup, the latency to make the first head entry, and the rate of head entries during the first 20 s of CS (or empty interval) presentations. We chose the first 20-s period of CS presentation for our measures of appetitive behaviors to enhance the likelihood of observing behavior controlled by the CS rather than the liquid present in the food cup.
4.2. Results
4.2.1. Taste aversion training
Over sessions that included the devalued US, the time spent in the food cup decreased (Figure 3a) and the latency to enter the food cup after the first US delivery increased (Figure 3b). By the final pair of aversion training sessions, rats spent less time in the food cup [F(1,15)=81.42, p<0.001] and showed longer latencies [F(1,15)=30.51, p<0.001] when the devalued food was presented than when the other food was presented. Interestingly, the devaluation treatment did not differentially affect the number of times the animals entered the food cup (not shown [F(1,15)=.47, p<0.505]); we will comment on this observation in the discussion.
Figure 3.
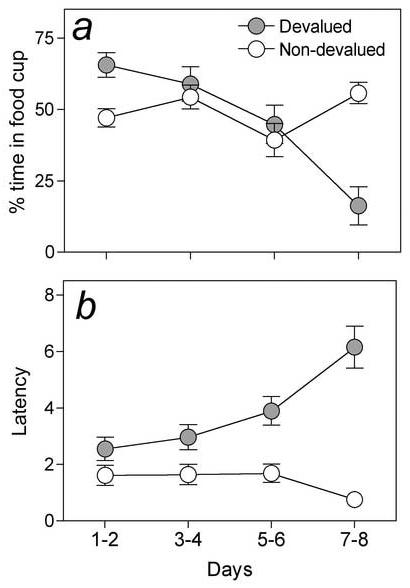
Mean percent time in the session spent in the food cup (a) and the mean latency to respond after the 1st food delivery (b) during the selective taste aversion devaluation phase of Experiment 3. The two food unconditioned stimuli (USs) were available in the absence of conditioned cues on alternate days for a total of 8 32-min sessions. Selective devaluation of one liquid US (4% sucrose or maltodextrin) was induced by administering an intraperitoneal injection of 0.3 M LiCl solution immediately after each session with the to-be-devalued US, but not after sessions that included the other US. Note that the percent time spent in the liquid-food cup (a) and the latency to begin drinking (b) were significantly decreased for the LiCl-devalued US by the end of the devaluation phase. Error bars indicate ± SEM.
4.2.2. Testing
The primary data of Experiment 3 are the appetitive and consummatory behaviors produced in the presence of the CSs and USs after devaluation of one of the USs. These data are summarized in Table 2. Figure 4 shows behavior during the first 20 s of CS presentation. Consider first the performance of the rats that were tested with the devalued US present in the food cup (right side of each panel of Figure 4). Not surprisingly, these rats failed to consume the devalued US (Figure 4a), regardless of the CS present [F < 1]. Nevertheless, they displayed considerable appetitive behavior in the presence of the inconsistent CS (which had previously been paired with the nondevalued food US). These rats showed higher rates of food cup entry (Figure 4b) and spent more time in the food cup (Figure 4c) in the presence of the inconsistent (nondevalued) CS than during either the consistent (devalued) CS [F(1,4) = 14.52, p = 0.019, and F(1,4) = 12.30, p = 0.025, respectively] or the empty, no-CS test intervals [F(1,4) = 10.23, p = 0.033, and F(1,4) = 10.32, p = 0.033). Performance during the devalued CS and empty test intervals was indistinguishable. Thus, with these measures the devaluation effect on appetitive behaviors controlled by the CSs was complete.
Table 2.
Experiment 3 - Results
| Non-devalued food present | Devalued food present | |
|---|---|---|
| Consumption | ||
| Consistent cue | ↔ | ↔ |
| Inconsistent cue | ↔ | ↔ |
| Rate of cup entries | ||
| Consistent cue | ↑ | ↔ |
| Inconsistent cue | ↑ | ↑ |
| Time spent in cup | ||
| Consistent cue | ↔ | ↓* |
| Inconsistent cue | ↓ | ↑ |
Note: Summary of the effects of consistent and inconsistent conditioned stimuli on consummatory and appetitive behaviors, compared to a no-cue condition, in Experiment 3. Arrows indicate changes in behavior compared to the no-CS tests; ↔: no change. ↑↓: positive or negative differences (p<.05); except for ↓* which indicates a non-significant trend (F(1,4)=5.01, p=0.089).
Figure 4.
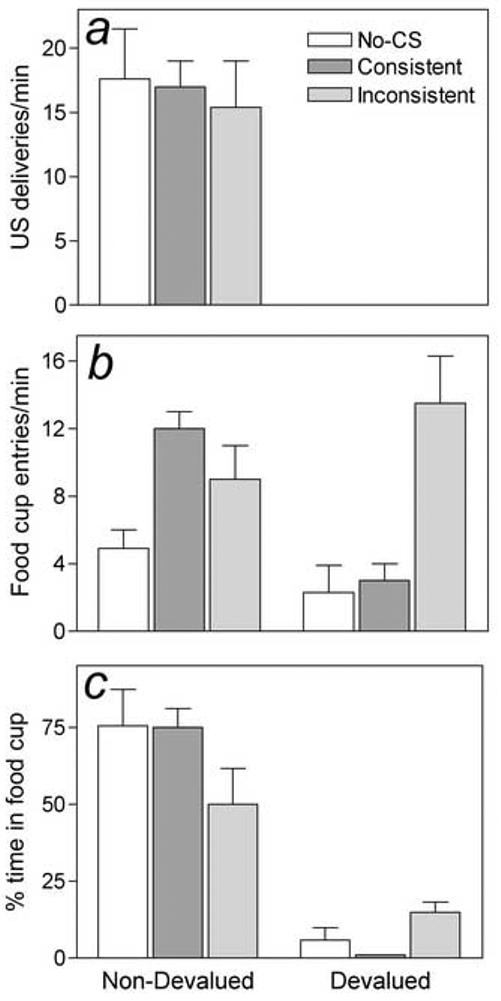
Mean number of unconditioned stimulus (US) deliveries needed to maintain a constant level of liquid in the food cup (a), mean entries into the food cup (b) and mean percent time spent in the liquid-food cup (c) during the first 20 s of conditioned stimulus (CS) presentation in the tests for potentiated feeding following selective devaluation in Experiment 3. Each rat was tested with 1 US, either the devalued (right 3 bars in each panel) or the non-devalued (left 3 bars) US. In separate sessions, the rats received presentations of the CS that signaled the food present in the food cup (Consistent CS, CS1+US1 or CS2+US2), the CS that signaled the other US (Inconsistent CS, CS1+US2 or CS2+US1), or no CS. To reduce baseline levels of consumption, during the first five minutes of each test session, rats had unlimited access to the test US solution in the absence of any CS (pretest; data not shown). Note that, in the presence of the devalued US (a; right 3 bars) rats failed to consume the (devalued) food altogether, regardless of the CS present, while at the same time showing higher rates of food cup entry (b) and spending more time in the food cup (c) in the presence of the inconsistent CS, which signaled the nondevalued US. In the presence of the non-devalued US (a; left 3 bars), the inconsistent CS (which predicted the devalued US) suppressed the time spent in the food cup (c), and both CSs elevated the rate of food cup entry (b). Error bars indicate ± SEM.
Next, consider behavior of the rats tested with the nondevalued food US present in the food cup (left side of each panel of Figure 4). These rats consumed substantial amounts of the nondevalued US in all tests (Figure 4a), regardless of the CS present [F < 1]. Nevertheless, despite the presence of an attractive US in the food cup in all tests, appetitive behaviors were modulated by the CSs. First, the devalued (inconsistent) CS suppressed the time spent in the food cup (Figure 4c), relative to the nondevalued (consistent) or no-CS intervals [F(1,4) = 13.88, p = 0.020 and F(1,4) = 9.32, p=0.038, respectively]. Second, both the nondevalued and devalued CSs elevated the rate of food cup entry (Figure 4b) relative to responding during the no-CS test [F(1,4) = 12.45, p=.024 and F(1,4) = 11.00, p = 0.029]; entry rate during the two CS types did not differ [F(1,4) = 1.032, p = 0.367]. This observation of maintained cup entry rates to the devalued CS contrasts both with the suppression of time in the food cup observed in these rats and with the suppression of food cup entry rates in the rats that received the devalued food in the test sessions. However, it is consistent with our observation of maintained cup entry rates (but suppressed time spent in the food cup) during the taste aversion training phase itself, when food consumption was not yet completely suppressed. Thus, initial entry to the food cup may be less sensitive to devaluation than a maintained presence in that cup, regardless of the substance present in it.
The analyses just described involved a limited sample of behavior near the beginning of the CS presentation (or the equivalent no CS period) in the test sessions. This sample was intended to maximize the observation of conditioned appetitive behaviors controlled by the CS rather than by the liquid present in the food cup. Previously (4), we noted that effects of CSs on food consumption were not visible immediately after CS presentations, but instead occurred considerably later. Thus, it is of interest to examine food consumption over a more protracted period, as in Experiment 2. Over that test period, for the rats that were given the nondevalued US, consumption was 89.4 ± 17.2, 76.9 ± 10.6, and 62.4 ± 13.7 deliveries in the no-CS, consistent (nondevalued) CS, and inconsistent (devalued) CS tests, respectively. Unlike in Experiment 2, there were no differences in pretest consumption (91.6 ± 4.7, 95.6 ± 8.4, and 91.6 ± 4.7 deliveries, respectively). ANCOVA of test consumption with pretest consumption as a covariate showed a significant effect of test [F(2, 12) = 4.39, p = 0.037). Post-hoc comparisons using the Tukey honestly significant difference procedure showed that consumption of the nondevalued US was reliably suppressed by the inconsistent CS [p = 0.047] relative to the no-CS test. Thus the CS that signaled the devalued US suppressed consumption of the nondevalued US. A similar, but insignificant suppression by the consistent (nondevalued) CS (rather than potentiation as in Experiment 2) may reflect generalization between the CSs. Finally, even with the longer sampling period, the rats presented with the devalued US did not consume it.
4.3. Discussion
Experiment 3 extended the generality of the devaluation paradigm, in which responding to the CSs is typically evaluated in extinction, to testing in the presence of the devalued and nondevalued USs. As in most other devaluation studies, the CSs controlled appetitive behavior in a stimulus-specific manner [8, 12, 15, 19, 26-29]. Appetitive responding was lower in the presence of the CS that had previously signaled the devalued US than in the presence of the CS that signaled the nondevalued US. This result was observed both when rats were tested with the devalued US present and when they were tested with the nondevalued US present. Similarly, when the nondevalued US was present, the CS that signaled the devalued US suppressed consumption. It is not clear whether this suppression was a direct effect of that CS on consumption, for example, reducing the palatability of the nondevalued US, or was derived from a reduction in the amount of time spent in the food cup. Nevertheless, it is notable that in Experiment 2, test differences in food consumption were not reflected in differences in time spent in the food cup. Finally, neither of the CSs influenced consumption of the devalued US, despite the substantial number of entries into the food cup elicited by the CS that signaled the nondevalued US. Thus, this experiment provided no evidence that a CS could override taste aversion learning to enhance consumption of an averted flavor, despite acting to bring the rat into close proximity to the food repeatedly.
In a related study, which also examined devaluation performance in food-sated rats, but which did not examine food-specificity of devaluation, Weingarten and Martin [30] failed to observe devaluation. In their study, conducted entirely in the rats' home cages while they had free access to lab chow, a 4.5-min CS was first paired with a 30-s presentation of a liquid food, which overlapped the final 30 s of the CS, and which over the course of the 6 daily CS-food pairings provided 70% of the rats' daily consumption of that food (which was freely available). Later, for half of the rats, food-LiCl pairings were intermingled with CS-food training sessions. Although these rats rapidly reduced their food consumption during the 30-s CS+food periods, they did not show reductions of food cup entries during the 4-min CS-alone periods, compared to rats that received food and LiCl unpaired. Given the many previous demonstrations of devaluation effects [7-9, 12-13, 15, 16, 19, 26-29] and the substantial differences between our procedures and those of Weingarten and Martin [30] , for example, home-cage versus separate chamber training, long versus short CSs, large versus small USs, training while chow-sated versus training while deprived, the use of single versus multiple reinforcers, testing CS responding in the absence of the US versus only in the presence of a US, we are not inclined to speculate on the basis of the difference between their results and ours.
5. General discussion
The experiments described in this article showed that conditioned stimuli convey sensory-specific reinforcement information, which can modulate multiple aspects of feeding behavior. First, in Experiment 1, the rate of instrumental responding, perhaps akin to foraging behaviors, was enhanced more by a signal for the same reinforcer as was normally earned by that response, than by a signal for a different reinforcer. Nevertheless, we observed a general transfer effect as well; even the signal for a different reinforcer enhanced instrumental responding above baseline. Second, in some circumstances, Pavlovian behaviors directed to the food cup were also reinforcer-specific. Although in Experiments 1 and 2, food cup behaviors showed no such specificity (which is not surprising because both liquids were delivered to the same cup), in Experiment 3, the time spent in the food cup food during initial portions of the CS reflected the current value of the US with which that CS had previously been paired, regardless of the food that was physically present in the food cup. Similarly, when the devalued food was present, food cup entry rates also reflected the current value of the US with which a CS had been paired. Third, in Experiment 2, food consumption itself was controlled by learned cues in a sensory-selective manner: only a CS that previously predicted the same CS as was present in the food cup enhanced consumption relative to baseline, no-CS conditions.
Historically, learning theorists have often distinguished between the conditioning of relatively general motivational responses [11, 31, 32] and of more specific outcome expectancies [14, 16], to Pavlovian CSs. However, this traditional distinction confounds two variables, that of the content of learning (specific vs. general) and its function (“motivational” vs. “cognitive”) in the control of behavior. A priori there is no reason to deny potential roles for generic knowledge in stimulus control functions (“lever presses are followed by food”) or for specific outcome information in hunger, appetite, or craving (“I want maltodextrin”). The present experiments were not designed to distinguish between these functions. For example, In Experiment 1, a Pavlovian CS for maltodextrin might specifically enhance responding on the right lever by providing additional cues for pressing the right lever or by eliciting a craving or appetite for maltodextrin. Although it may be more intuitive to account for the specificity of cue-induced eating in Experiment 2 to the arousal of a specific appetite by the CS, the failure of the inconsistent CS to potentiate feeding in that experiment may reflect a novelty-induced suppression of feeding produced by the presence of one food in the presence of an expectancy for another [33, 34].
It may be instructive to speculate on the ultimate origins of behaviors like those described here. It is possible that the differential modulation of appetitive and consummatory behaviors under various experimental conditions reflects the outcome of allostatic processes that have evolved to anticipate rather than react to events. As in most laboratory studies of learning, in our studies, learning about cue-food relations occurred under conditions in which the delivery of small amounts of food were presented while the rats were significantly deprived of food. Thus, CS-activation of a representation of food may be accompanied by an induction of appetite that further enhances both food-procuring and consummatory behaviors, allowing an animal to forage and eat beyond its present needs to be prepared for future famine. Well-learned cues therefore may potentiate feeding even under conditions of satiation. Similarly, although the integration of new information about the desirability of certain foodstuffs into established foraging patterns may direct animals' foraging to some extent, the control of consumption itself is likely to be governed more by proximal characteristics of the food such as taste, which more accurately predict the food's post-ingestive consequences. Hence, in Experiment 3, although rats' food cup entry in the presence of food cues depended on the current evaluation of the food signaled by that cue, once the cup was entered, consumption of the food found there was mostly determined by the properties of that food itself.
The processes by which cue-potentiated feeding, PIT, and devaluation occur seem to have evolved to deal with famine and potentially unsafe foods. Understanding how these processes operate in conditions of food abundance and pervasive food-related environmental cues, such as print and video advertisements and salient but uniform design cues in roadside chain restaurants, is important in understanding the origins of maladaptive overeating of humans [35-37]. For example, Fedoroff et al. [38] examined the effects of brief presentation of the smell of chocolate chip cookies or pizza on ratings of appetite for, and subsequent consumption of, those foods. In that study, both restrained eaters (dieters) and unrestrained eaters reported stronger specific appetite or craving for pizza after the pizza cue than after the cookie cue or no cue, but only restrained eaters actually ate more pizza after the pizza cue. Interestingly, the enhanced consumption by restrained eaters was highly specific; those participants also showed greater consumption of cookies after the cookie cue than after the pizza or no cue. Given that cue-potentiated feeding has been observed in human infants [39] and young children [40], a better grasp of the determinants of this phenomenon may prove useful in understanding the development of both normal and pathological eating.
The lines of research described in the present report may provide elements to untangle the complex relationships between external learned stimuli and both regulatory and non-regulatory, allostatic processes [41]. The experimental methods used in these studies provide promising animal models to explore the brain mechanisms underlying the effects of environment on human bingeing and overeating [35, 42, 43].
Acknowledgement
This research was supported by NIMH grants MH53667 and MH65879.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sterling P. Principles of Allostasis: Optimal Design, Predictive Regulation, Pathophysiology, and Rational Therapeutics. In: Schulkin J, editor. Allostasis, Homeostasis, and the Costs of Adaptation. MIT Press; Cambridge: 2004. pp. 1–35. [Google Scholar]
- 2.Leshem M. The ontogeny of salt hunger in the rat. Neurosci. Biobehav. Rev. 1999;23:649–659. doi: 10.1016/s0149-7634(98)00059-1. [DOI] [PubMed] [Google Scholar]
- 3.Sakai RR, Fine WB, Epstein AN, Frankmann SP. Salt appetite is enhanced by one prior episode of sodium depletion in the rat. Behav. Neurosci. 1987;101:724–731. doi: 10.1037//0735-7044.101.5.724. [DOI] [PubMed] [Google Scholar]
- 4.Holland PC, Petrovich GD, Gallagher M. The effects of amygdala lesions on conditioned stimulus-potentiated eating in rats. Physiol. Behav. 2002;76:117–129. doi: 10.1016/s0031-9384(02)00688-1. [DOI] [PubMed] [Google Scholar]
- 5.Petrovich GD, Setlow B, Holland PC, Gallagher M. Amygdalo-hypothalamic circuit allows learned cues to override satiety and promote eating. J. Neurosci. 2002;22:8748–8753. doi: 10.1523/JNEUROSCI.22-19-08748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weingarten HP. Conditioned cues elicit feeding in sated rats: a role for learning in meal initiation. Science. 1983;220:431–433. doi: 10.1126/science.6836286. [DOI] [PubMed] [Google Scholar]
- 7.Holland PC, Rescorla RA. Effect of 2 ways of devaluing unconditioned stimulus after 1st order and 2nd-order appetitive conditioning. J. exp. psychol., Anim. behav. processes. 1975;104:355–363. doi: 10.1037//0097-7403.1.4.355. [DOI] [PubMed] [Google Scholar]
- 8.Holland PC. Relations between Pavlovian-instrumental transfer and reinforcer devaluation. J. exp. psychol., Anim. behav. processes. 2004;30:104–117. doi: 10.1037/0097-7403.30.2.104. [DOI] [PubMed] [Google Scholar]
- 9.Corbit LH, Balleine BW. Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of pavlovian-instrumental transfer. J. Neurosci. 2005;25:962–970. doi: 10.1523/JNEUROSCI.4507-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mowrer OH. On the dual nature of learning: A re-interpretation of ‘conditioning’ and ‘problem-solving’. Harvard Educational Review. 1947;17:102–148. [Google Scholar]
- 11.Rescorla RA, Solomon RL. Two-process learning theory: Relationships between Pavlovian conditioning and instrumental learning. Psychol. Rev. 1967;74:151–82. doi: 10.1037/h0024475. [DOI] [PubMed] [Google Scholar]
- 12.Holland PC. Event Representation in Pavlovian conditioning - Image and action. Cognition. 1990;37:105–131. doi: 10.1016/0010-0277(90)90020-k. [DOI] [PubMed] [Google Scholar]
- 13.Colwill RM, Rescorla RA. Associations between the Discriminative Stimulus and the Reinforcer in Instrumental Learning. J. exp. psychol., Anim. behav. processes. 1988;14:155–164. [Google Scholar]
- 14.Trapold MA, Overmier JB. The second learning process in instrumental training. In: Black A, Prokasy WF, editors. Classical conditioning II. Appleton-Century-Crofts; New York: 1972. pp. 427–452. [Google Scholar]
- 15.Balleine BW, Dickinson A. The role of incentive learning in instrumental outcome revaluation by sensory-specific satiety. Anim. learn. behav. 1998;26:46–59. [Google Scholar]
- 16.Colwill RM, Rescorla RA. Associative structures in instrumental learning. In: Bower GH, editor. The Psychology of Learning and Motivation. Vol. 20. Academic Press; San Diego: 1986. pp. 55–104. [Google Scholar]
- 17.Hall J, Parkinson JA, Connor TM, Dickinson A, Everitt BJ. Involvement of the central nucleus of the amygdala and nucleus accumbens core in mediating Pavlovian influences on instrumental behaviour. Eur. J. Neurosci. 2001;13:1984–1992. doi: 10.1046/j.0953-816x.2001.01577.x. [DOI] [PubMed] [Google Scholar]
- 18.Holland PC, Gallagher M. Double dissociation of the effects of lesions of basolateral and central amygdala on conditioned stimulus-potentiated feeding and Pavlovian-instrumental transfer. Eur. J. Neurosci. 2003;17:1680–1694. doi: 10.1046/j.1460-9568.2003.02585.x. [DOI] [PubMed] [Google Scholar]
- 19.Blundell P, Hall G, Killcross S. Lesions of the basolateral amygdala disrupt selective aspects of reinforcer representation in rats. J. Neurosci. 2001;21:9018–9026. doi: 10.1523/JNEUROSCI.21-22-09018.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holland PC, Hatfield T, Gallagher M. Rats with basolateral amygdala lesions show normal increases in conditioned stimulus processing but reduced conditioned potentiation of eating. Behav. Neurosci. 2001;115:945–950. [PubMed] [Google Scholar]
- 21.Hull CL. Principles of Behavior. Appleton-century-crofts; New York: 1943. [Google Scholar]
- 22.Petrovich GD, Holland PC, Gallagher M. Amygdalar and prefrontal pathways to the lateral hypothalamus are activated by a learned cue that stimulates eating. J. Neurosci. 2005;25:8295–8302. doi: 10.1523/JNEUROSCI.2480-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Booth DA. Food-Conditioned Eating Preferences and Aversions with Interoceptive Elements - Conditioned Appetites and Satieties. Ann. N.Y. Acad. Sci. 1985;443:22–41. doi: 10.1111/j.1749-6632.1985.tb27061.x. [DOI] [PubMed] [Google Scholar]
- 24.Delamater AR, LoLordo VM, Berridge KC. Control of fluid palatability by exteroceptive Pavlovian signals. J. exp. psychol., Anim. behav. processes. 1986;12:143–152. [PubMed] [Google Scholar]
- 25.Weingarten HP. Stimulus-Control of Eating - Implications for a 2-Factor Theory of Hunger. Appetite. 1985;6:387–401. doi: 10.1016/s0195-6663(85)80006-4. [DOI] [PubMed] [Google Scholar]
- 26.Colwill RM, Motzkin DK. Encoding of the Unconditioned Stimulus in Pavlovian Conditioning. Animal Learning & Behavior. 1994;22:384–394. [Google Scholar]
- 27.Holland PC. Excitation and inhibition in unblocking. Journal of Experimental Psychology: Animal Behavior Processes. 1988;14:261–279. [PubMed] [Google Scholar]
- 28.Holland PC. Amount of training affects associatively-activated event representation. Neuropharmacology. 1998;37:461–469. doi: 10.1016/s0028-3908(98)00038-0. [DOI] [PubMed] [Google Scholar]
- 29.Balleine BW. Neural bases of food-seeking: Affect, arousal and reward in corticostriatolimbic circuits. Physiology & Behavior. 2005;86:717–730. doi: 10.1016/j.physbeh.2005.08.061. [DOI] [PubMed] [Google Scholar]
- 30.Weingarten HP, Martin GM. Mechanisms of conditioned meal initiation. Physiology & Behavior. 1989;45:735–40. doi: 10.1016/0031-9384(89)90287-4. [DOI] [PubMed] [Google Scholar]
- 31.Bindra D. A motivational view of learning, performance, and behavior modification. Psychol. Rev. 1974;81:199–213. doi: 10.1037/h0036330. [DOI] [PubMed] [Google Scholar]
- 32.Mowrer OH. Learning theory and behavior. Wiley; New York: 1960. [Google Scholar]
- 33.Elliott MH. The effect of change of reward on the maze performance of rats. University of California Publications in Psychology. 1928;4:19–30. [Google Scholar]
- 34.Tinklepaugh OL. An experimental study of representative factors in monkeys. J. Comp. Psychol. 1928;8:197–236. [Google Scholar]
- 35.Holland PC, Petrovich GD. A neural systems analysis of the potentiation of feeding by conditioned stimuli. Physiol. Behav. 2005;86:747–61. doi: 10.1016/j.physbeh.2005.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levitsky DA. The non-regulation of food intake in humans: Hope for reversing the epidemic of obesity. Physiol. Behav. 2005;86:623–632. doi: 10.1016/j.physbeh.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 37.Popkin B, Duffey K, Gordon-Larsen P. Environmental influences on food choice, physical activity and energy balance. Physiol. Behav. 2005;86:603–13. doi: 10.1016/j.physbeh.2005.08.051. [DOI] [PubMed] [Google Scholar]
- 38.Fedoroff I, Polivy J, Herman CP. The specificity of restrained versus unrestrained eaters' responses to food cues: general desire to eat, or craving for the cued food? Appetite. 2003;41:7–13. doi: 10.1016/s0195-6663(03)00026-6. [DOI] [PubMed] [Google Scholar]
- 39.Coyle S, Arnold HM, Goldberg-Arnold JS, Rubin DC, Hall WG. Olfactory conditioning facilitates diet transition in human infants. Dev. Psychobiol. 2000;37:144–52. doi: 10.1002/1098-2302(200011)37:3<144::aid-dev3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 40.Birch LL, Mcphee L, Sullivan S, Johnson S. Conditioned Meal Initiation in Young-Children. Appetite. 1989;13:105–113. doi: 10.1016/0195-6663(89)90108-6. [DOI] [PubMed] [Google Scholar]
- 41.Woods SC. Signals that influence food intake and body weight. Physiol. Behav. 2005;86:709–16. doi: 10.1016/j.physbeh.2005.08.060. [DOI] [PubMed] [Google Scholar]
- 42.Jansen A. A learning model of binge eating: cue reactivity and cue exposure. Behav Res Ther. 1998;36:257–72. doi: 10.1016/s0005-7967(98)00055-2. [DOI] [PubMed] [Google Scholar]
- 43.Sobik L, Hutchison K, Craighead L. Cue-elicited craving for food: a fresh approach to the study of binge eating. Appetite. 2005;44:253–61. doi: 10.1016/j.appet.2004.12.001. [DOI] [PubMed] [Google Scholar]


