Abstract
Activation of the dorsal periaqueductal gray (PAG) evokes defense-like behavior including a marked increase in sympathetic drive and resetting of baroreflex function. The goal of this study was to investigate the role of the lateral parabrachial nucleus (LPBN) in mediating dorsal PAG modulation of the arterial baroreflex. Reflex responses were elicited by electrical stimulation of the aortic depressor nerve (ADN) at 5 Hz or 15 Hz in urethane anesthetized rats (n=18). Electrical stimulation of the dorsal PAG at 10 Hz did not alter baseline mean arterial pressure (MAP) but did significantly attenuate baroreflex control of heart rate (HR) evoked by low frequency ADN stimulation. Alternatively, 40 Hz dorsal PAG stimulation increased baseline MAP (43±3 mmHg) and HR (33±3 bpm) and attenuated baroreflex control of HR at both ADN stimulation frequencies. Reflex control of MAP was generally unchanged by dorsal PAG stimulation. Bilateral inhibition of neurons in LPBN area (n=6) with muscimol (0.45 nmol per side) reduced dorsal PAG evoked increases in MAP and HR by 50±4% and 95±4%, respectively, and significantly reduced, but did not completely eliminate dorsal PAG attenuation of the cardiac baroreflex. Bilateral blockade of glutamate receptors in the LPBN area (n=6) with kynurenic acid (1.8 nmol) had a similar effect on dorsal PAG evoked increases in MAP, HR and cardiac baroreflex function. Reflex control of MAP was unchanged with either treatment. These findings suggest that the LPBN area is one of several brainstem regions involved in descending modulation of the cardiac baroreflex function during defensive behavior.
Keywords: dorsal periaqueductal gray, defense response, lateral parabrachial nucleus and sympathoexcitation
1. INTRODUCTION
In both cat and rat activation of the dorsal cell column of the periaqueductal gray (PAG) elicits a behavioral response similar to that evoked by threatening or stressful stimuli, including flight or fight responses (1–3, 5, 10, 20, 22). Coupled with these behavioral changes are coordinated cardiorespiratory responses, including sympathoexcitation, tachycardia, hyperventilation, and modulation of baroreflex function (7, 13, 32, 37, 40). Direct neuroanatomical projections from the dorsal PAG to select sympathoexcitatory regions of the medulla, including the paragigantocellular nucleus (PGi) and rostral ventrolateral medulla (RVLM) have been identified (4, 6, 8, 17, 34, 39), suggesting that a portion of the cardiovascular response evoked by dorsal PAG activation is mediated through a direct pathway to presympathetic bulbospinal neurons (18, 27–29, 40).
Although direct projections to the RVLM have been identified, current evidence suggests that dorsal PAG modulation of sympatho-excitation also involves an important relay through the rostral pons. In 1993 Nosaka and colleagues first demonstrated that large electrolytic or chemical lesions of the dorsal pons, localized to the region of the parabrachial nucleus (PBN), markedly reduced dorsal PAG evoked increases in mean arterial pressure (MAP) (32). In a more recent study from our lab, utilizing smaller microinjection volumes, we confirmed that activation of lateral PBN (LPBN) neurons was essential for dorsal PAG evoked increases in MAP and heart rate (HR), as well as changes in breath timing (16). This observation corroborated neuroanatomical data showing that dorsal PAG neurons primarily project to the LPBN and not the medial PBN (23). Unexpectedly however, we demonstrated that bilateral blockade of LPBN area neurons with the GABA-A receptor agonist muscimol or the glutamate receptor antagonist kynurenic acid was approximately 20% more effective at attenuating dorsal PAG evoked increases in HR than changes in MAP. This raised the possibility that dorsal PAG modulation of HR is primarily mediated through input to the LPBN, while descending control of sympathetic drive to the vasculature involves an alternative pathway to the RVLM.
In addition to inducing increases in sympathetic drive, activation of the dorsal PAG also modulates baroreflex function. Previous studies have shown that PAG stimulation raises both the threshold for baroreflex-mediated inhibition of lumbar and splanchnic sympathetic nerve activity and increases the cut-off pressure needed to silence sympathetic drive (40). Additionally, activation of dorsal PAG neurons has been reported to attenuate baroreflex control of HR (7, 32).
Accordingly, in their original study, Nosaka and colleagues demonstrated that large chemical lesions of the PBN also markedly attenuated dorsal PAG modulation of baroreflex control of MAP and HR (32). This provided further evidence for a critical role for PBN area neurons in mediating a portion of dorsal PAG modulation of autonomic function. However, because the lesions utilized in that study were relatively large, it remains to be determined whether dorsal PAG modulation of baroreflex function is primarily mediated through neurons in the LPBN or in surrounding regions. Current evidence suggests that dorsolateral pontine-evoked modulation of the arterial baroreflex primarily originates from the ventrolateral regions of the LPBN and involves descending projections to both the NTS (9, 24) and the RVLM (24–26, 31). Yet, neuroanatomical data suggest that there are few direct projections from the dorsal PAG to the ventrolateral PBN (23). Instead, descending projections from the dorsal PAG primarily terminate in the central and superior lateral regions of the LPBN. Thus it remains to be determined whether blockade of LPBN neurons using smaller, more centrally focused injections are involved in mediating dorsal PAG modulation of baroreflex function.
Based on the above information, the present study was undertaken to test the hypothesis that the integrity of LPBN area neurons is essential for dorsal PAG modulation of baroreflex function. Additionally, we hypothesized that dorsal PAG modulation of baroreflex control is dependent upon glutamatergic input to the LPBN.
2. RESULTS
The mean weight of the adult male Sprague Dawley rats used in this study was 375±9 gm (n=18). The average resting MAP and HR of all rats prior to central microinjection was 94±3 mmHg and 384±7 bpm, respectively. Between treatment-groups there was no significant difference in weight (data not shown), baseline MAP, or HR (see Table 1, p>0.5).
Table 1.
Baseline mean arterial pressure (MAP) and heart rate (HR) before and following bilateral microinjection into the LPBN for each treatment group of rats.
| MAP (mmHg) Pre vs. Post drug | HR (bpm) Pre vs. Post drug | |
|---|---|---|
| aCSF (n=6) | 96±3 vs. 96±5 | 388±15 vs. 401v17 |
| Muscimol (n=6) | 97±7 vs. 107±8 | 383±5 vs. 396±13 |
| Kynurenic acid (n=6) | 90±6 vs. 97±6 | 380±15 vs. 385±17 |
2.1. Effect of dorsal PAG stimulation on baroreflex function
Figure 1 illustrates the effect of dorsal PAG stimulation on baroreflex function from one animal. The averaged responses from all animals (n=18) are shown in Figure 2. Alone, electrical stimulation of the ADN evoked bradycardia and hypotension (Fig. 1A). Reflex decreases in MAP and HR evoked by 5Hz ADN stimulation (Fig 2A, open bars) were significantly (p<0.005) smaller than those evoked by 15 Hz (Fig. 2B, open bars). Activation of the dorsal PAG at 10 Hz did not did not significantly increase resting MAP (+2±0.5 mmHg) or HR (+1±0.5 mmHg). In contrast, PAG stimulation at 40 Hz induced a ramp increase in baseline MAP and HR that reached a peak approximately 10 s following the onset of PAG stimulation (Fig. 1C) and was significantly different from resting levels (MAP +43±3 mmHg; HR +33±3 mmHg). Co-activation of the dorsal PAG modified baroreflex control of HR to a greater extent than reflex control of MAP and 40 Hz PAG stimulation had a greater effect on reflex control than 10 Hz. Reflex control of MAP was only significantly attenuated when 40 Hz PAG stimulation was applied during low frequency-5 Hz ADN stimulation.
Fig. 1. The effects of dorsal periaqueductal gray (PAG) stimulation on baroreflex function from one animal.
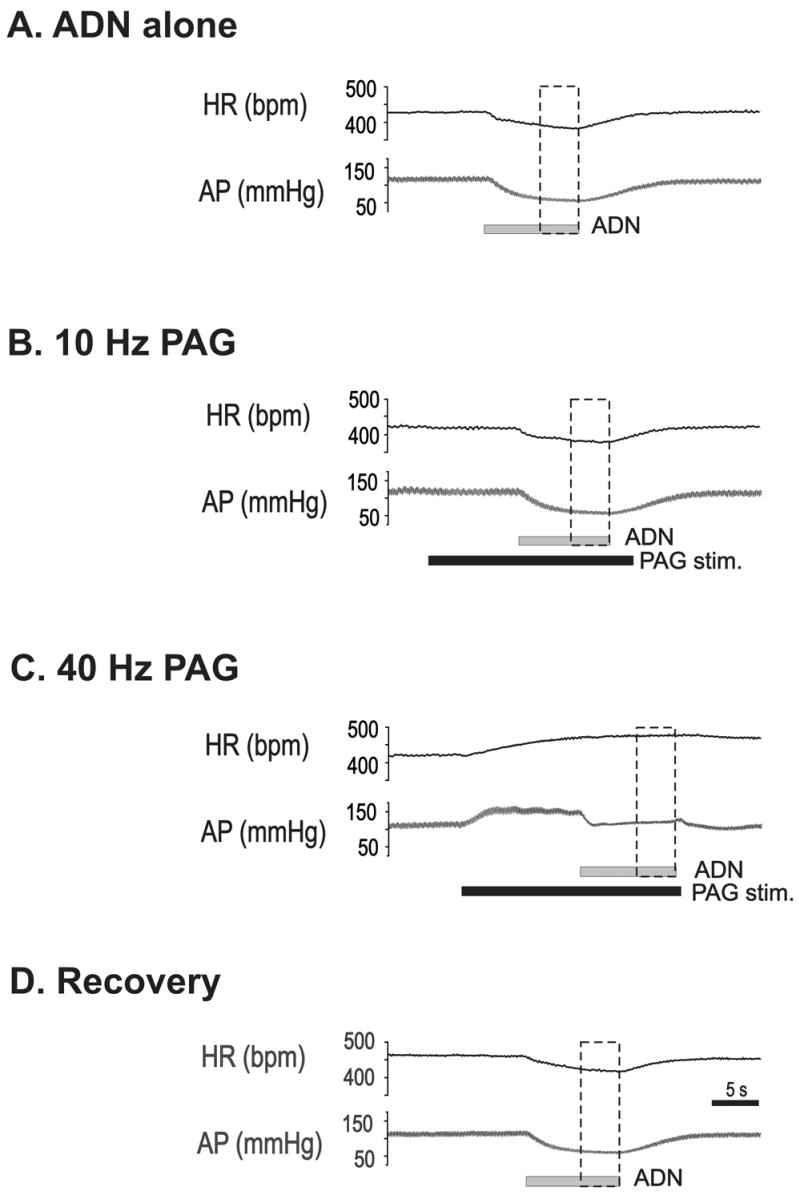
Changes in heart rate (HR), and arterial pressure (AP) during ten seconds of 5 Hz stimulation (horizontal gray bar) of the aortic depressor nerve (ADN; 10V, 2 ms pulse) are shown (A–D). Dashed vertical boxes indicate the two second time windows used for measurement of the effects of ADN stimulation. A. ADN stimulation alone. B. ADN stimulation combined with 10 Hz PAG stimulation (horizontal gray bar). C. ADN stimulation combined with 40 Hz PAG stimulation (horizontal black bar). D. ADN stimulation alone one minute following the offset of 40 Hz PAG stimulation.
Fig. 2. Averaged effects of 10 versus 40 Hz dorsal PAG stimulation on baroreflex-evoked changes in mean arterial pressure (MAP) and heart rate (HR).
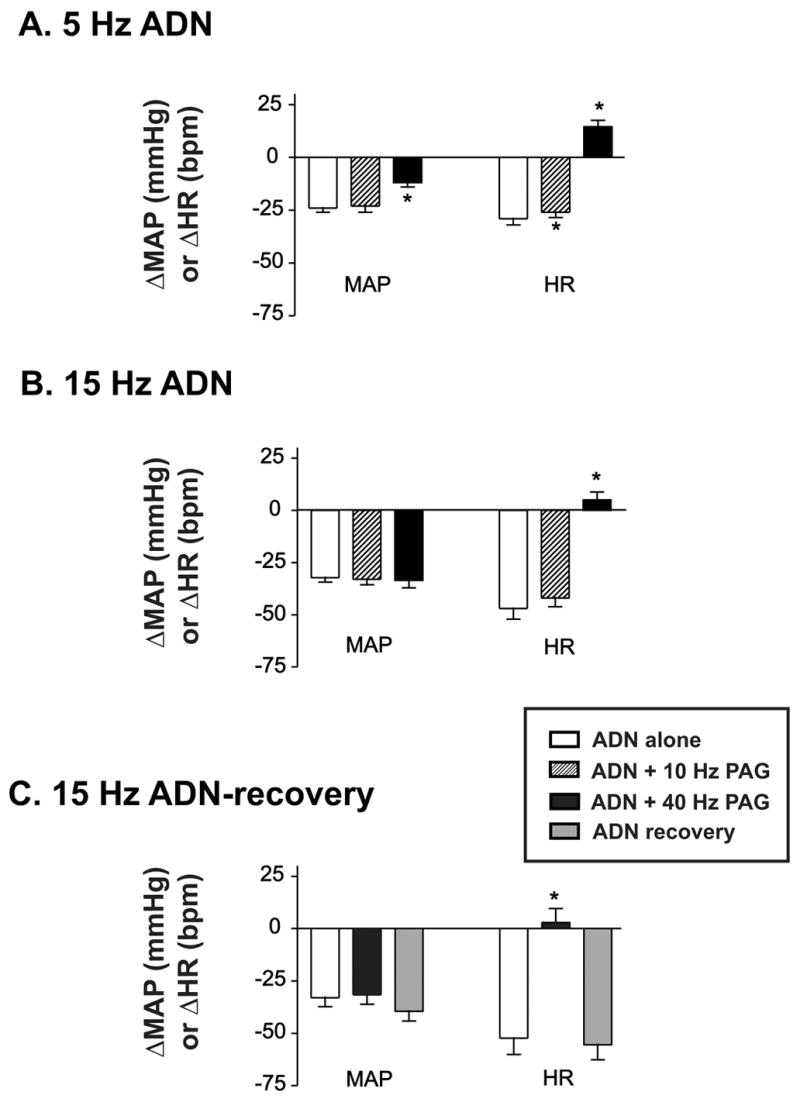
Open bars indicate baroreflex responses evoked by ADN stimulation alone. Hatched bars indicate baroreflex responses evoked by ADN stimulation during 10 Hz PAG stimulation. Black bars indicate responses evoked during 40 Hz PAG stimulation. A. MAP and HR responses during 5 Hz ADN stimulation with or without dorsal PAG stimulation (n=18). B. MAP and HR responses evoked by 15 Hz ADN stimulation with or without dorsal PAG stimulation (n=18). C. MAP and HR responses evoked by 15 Hz ADN stimulation alone (open bar), during 40 Hz PAG (black bar) and one minute following the offset of 40 Hz PAG stimulation (ADN alone, gray bars). Asterisks indicate significantly different (P<0.05) from ADN-alone evoked response.
One minute following the offset of dorsal PAG stimulation at 40 Hz, resting MAP quickly returned to pre-stimulation levels, but baseline HR remained elevated for several minutes (Fig. 1D). In nine animals baroreflex function (15 Hz ADN stimulation) was retested one minute following the offset of 40 Hz dorsal PAG stimulation. In these animals dorsal PAG stimulation (40 Hz) alone induced an increase MAP and HR of 49±4 mmHg and 35±4 bpm above baseline, respectively. One minute following the offset of dorsal PAG stimulation, baseline MAP and HR remained elevated (12±3 mmHg and 31±3 bpm above pre-PAG stimulation levels), but baroreflex control of MAP and HR had returned to control levels (Fig 1D & 2C, open vs. lightly shaded bars).
Figure 3 illustrates the reconstructed stimulation sites from all experiments. In all instances the placement of the stimulation electrode was confined to the dorsal quadrant of the PAG. Because the effects of 10 Hz PAG stimulation on MAP, HR and baroreflex function were minimal, the effect of subthreshold PAG stimulation on baroreflex function following LPBN microinjection will not be discussed further.
Fig. 3. Reconstructed dorsal PAG stimulation sites.
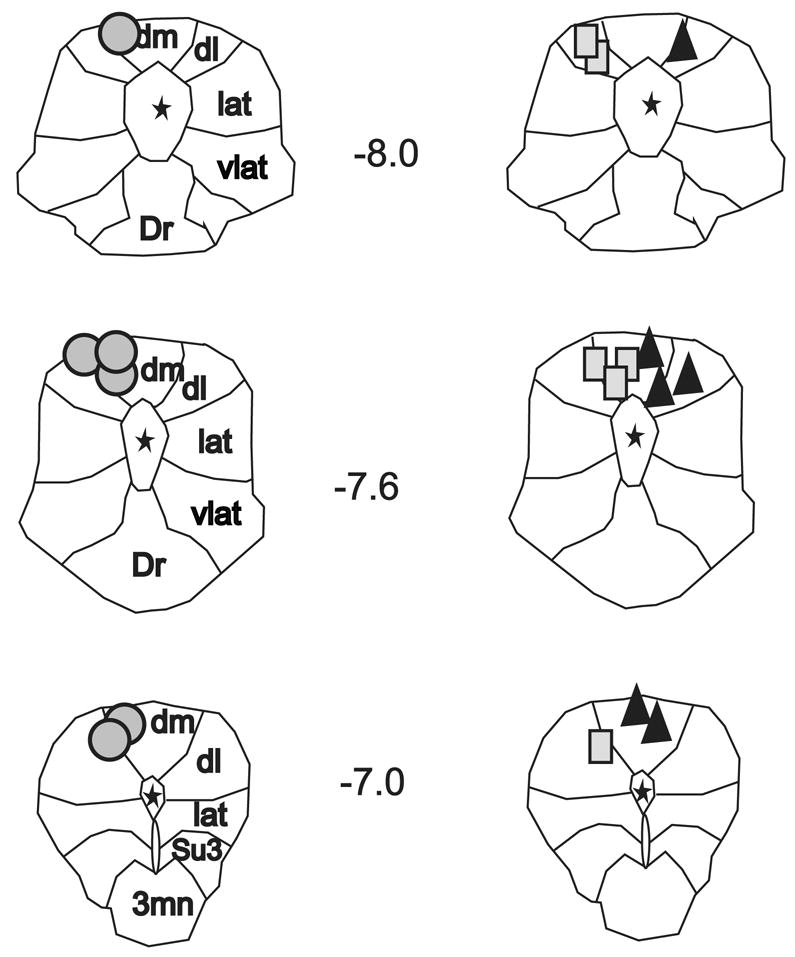
Outline of the rostral, middle, and caudal columns of the PAG from Paxinos & Watson’s Rat Brain Stereotaxic Atlas (35). Reconstructed stimulation sites from all animals are organized by experimental group; circles indicate PAG stimulation sites recovered from muscimol microinjection studies; triangles indicate sites from kynurenic acid studies; and rectangles indicate sites from aCSF studies. Note, for illustration purposes all stimulation sites from kynurenic acid experiments are shown on the right side. Numbers refer to midbrain location relative to bregma. Asterisks indicated location of central aqueduct. Dm, dorsomedial column; dl, dorsolateral column; lat, lateral column; vlat, ventrolateral column; Dr, dorsal raphe; Su3, supraoculomotor nucleus; 3mn, oculomotor nucleus.
2.2. The role of the LPBN in dorsal PAG modulation of baroreflex function
Fig. 4 illustrates the effect of bilateral inhibition of the LPBN from one animal. Prior to LPBN blockade, ADN stimulation alone induced a significant decrease in both MAP and HR (Fig. 4A left panel). Stimulation of the dorsal PAG at 40 Hz evoked a ramp increase in MAP and HR, eliminated baroreflex control of HR, and attenuated reflex control of MAP (Fig. 4A right panel). Following bilateral microinjection of 0.45 nmoles/per side of muscimol into the LPBN, baseline MAP, HR and the baroreflex response were not altered (Fig. 4B, left panel). In contrast, the dorsal PAG stimulation evoked increase in baseline MAP was attenuated and the increase in HR eliminated (Fig. 4B right panel). Furthermore, PAG modulation of baroreflex function was markedly reduced.
Fig. 4. Effects of bilateral inhibition of the lateral parabrachial nucleus (LPBN) on dorsal periaqueductal gray (PAG) modulation of baroreflex function in one animal.
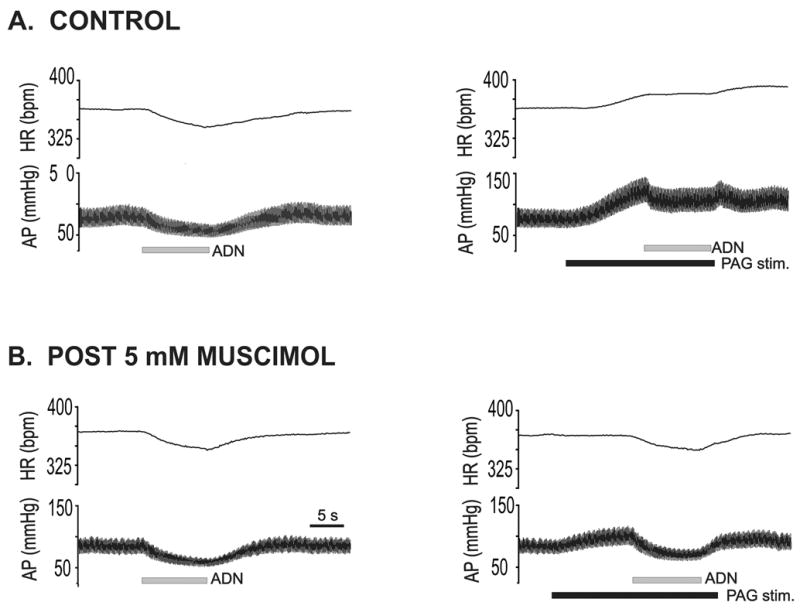
Changes in heart rate (HR) and arterial pressure (AP) recorded during 5 Hz stimulation of the aortic depressor nerve (ADN) (horizontal gray bar) from one rat. A. ADN stimulation alone, pre-treatment (left) and ADN stimulation combined with 40 Hz dorsal PAG stimulation (right; horizontal black bar). B. ADN stimulation alone (left) and during 40 Hz dorsal PAG stimulation (right) approximately 5 minutes following bilateral blockade of the LPBN (0.45 nmols muscimol per side).
In six animals the effect of bilateral inhibition of the LPBN with muscimol on baroreflex function was tested. In a separate group of animals the effects of bilateral microinjection of kynurenic acid in the LPBN was tested. Following LPBN blockade with either muscimol or kynurenic acid there was no significant change in either baseline MAP or HR (Table 1) or baroreflex function (see Table 2). However, dorsal PAG (40 Hz) evoked increases in baseline MAP and HR were significantly attenuated (Table 3). LPBN blockade had a disproportionately greater effect on dorsal PAG-evoked increases in baseline HR (~90–95% reduction) compared to increases in baseline MAP (~50% reduction from control).
Table 2.
Baroreflex control of MAP and HR before and following bilateral microinjection of aCSF, muscimol or kynurenic acid into the LPBN.
| TREATMENT | Delta MAP (mmHg) Pre vs. Post-Drug | Delta HR (bpm) Pre vs. Post-Drug |
|---|---|---|
| 5Hz ADN stim. | ||
|
| ||
| Muscimol (n=6) | −18±2 vs. −28±4 | −20±3 vs. −24±5 |
| Kynurenic Acid (n=6) | −24±5 vs. −30±6 | −34±4 vs. −46±4 |
| aCSF(n=6) | −25±7 vs. −20±6 | −23±7 vs. −32±9 |
|
| ||
| 15 Hz ADN stim. | ||
|
| ||
| Muscimol (n=6) | −39±6 vs. −32±6 | −43±8 vs. −28±8 |
| Kynurenic Acid (n=6) | −40±6 vs. −37±2 | −65±8 vs. −54±13 |
| aCSF(n=6) | −30±3 vs. −32±5 | −53±5 vs. −56±7 |
Table 3.
Dorsal PAG (40 Hz) evoked changes in baseline MAP and HR before and following bilateral microinjection into the LPBN.
| TREATMENT | Delta MAP Pre vs. Post-Drug | Delta HR Pre vs. Post-Drug |
|---|---|---|
| Muscimol (n=6) | 45±5 vs. 20±3* | 28±5 vs. 3±4* |
| Kynurenic Acid (n=6) | 44±10 vs. 27±5* | 37±5 vs. 5±10* |
| aCSF(n=6) | 46±3 vs. 41±8 | 39±6 vs. 36±5 |
Asterisk indicates significantly different from pre-treatment response
Because LPBN microinjection did not significantly alter baroreflex responses evoked by ADN stimulation alone, reflex responses elicited before and after LPBN microinjection were averaged for further comparisons. Figure 5 illustrates the averaged effect of LPBN blockade on dorsal PAG modulation of baroreflex function. The effect of dorsal PAG stimulation on baroreflex control of MAP was not significant changed following LPBN blockade. In contrast, bilateral inhibition of the LPBN with muscimol reversed the effect of dorsal PAG stimulation on baroreflex control of HR. However, cardiac baroreflex responses evoked during dorsal PAG stimulation following LPBN blockade remained significantly different from baroreflex responses by ADN stimulation alone, suggesting LPBN inhibition only partially reversed the effect of dorsal PAG stimulation on baroreflex function. Bilateral blockade of the LPBN with kynurenic acid had a similar effect (Fig. 5 middle column) and significantly attenuated the effect of dorsal PAG stimulation on baroreflex control of HR only.
Fig. 5. Averaged effect of dorsal PAG modulation of baroreflex responses before and following lateral parabrachial nucleus (LPBN) microinjection.
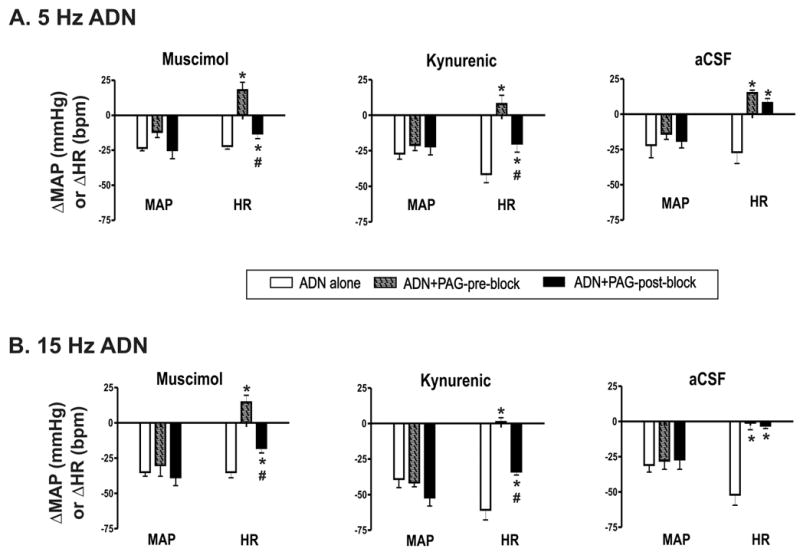
Open bars indicate baroreflex responses evoked by ADN stimulation alone. Hatched bars indicate baroreflex responses elicited during 40 Hz PAG stimulation before bilateral LPBN blockade. Black bars indicate ADN responses elicited during 40 Hz PAG stimulation after bilateral LPBN blockade. A. MAP and HR responses during 5 Hz ADN stimulation before and after LPBN microinjection (n=6/group). B. Baroreflex responses during 15 Hz ADN stimulation before and after LPBN microinjection (n=6/group). Baroreflex responses evoked alone reflect the average response elicited before and following LPBN microinjection. Left panels, muscimol at 0.45 nmol/side; middle panels, kynurenic acid 1.8 nmol/side; aCSF, 90 nl per side. * indicates significantly different (P<0.05) from ADN-alone evoked response. # indicates significantly different from response evoked by ADN + dorsal PAG stimulation before LPBN microinjection (P<0.05).
2.3 Role of LPBN volume injection on dorsal PAG modulation of baroreflex function
To control for the effect of microinjection in the LPBN on reflex and dorsal PAG evoked responses, a third group of animals (n=6) was tested before and following bilateral microinjection of aCSF into the LPBN. Following bilateral microinjection of aCSF into the LPBN, dorsal PAG modulation of baroreflex control of MAP and HR was unchanged (i.e., responses post LPBN microinjection were not significantly different from pre-injection). Additionally, dorsal PAG evoked increases in baseline MAP, HR and baroreflex responses to ADN stimulation alone were unchanged (Tables 2& 3).
2.4 Reconstructed LPBN microinjection sites
Figure 6A shows an example of an image from one microinjection site in the rostral LPBN. A comparison of brightfield versus fluorescent images identified that placement of the microinjectate in this example was in the external lateral quadrant of the rostral LPBN. Reconstructed microinjection sites from all experiments are shown in Fig. 6B–D. There was no apparent difference in the effect of microinjection site (rostral vs. caudal) in the LPBN or VLPAG and effectiveness of blockade of PAG evoked responses. However, in one animal that received bilateral kynurenic acid (Fig. 6C, asterisks), the microinjection sites were later identified to be localized quite ventral to the outside of the boundaries of the LPBN. Microinjection into this site did not significantly alter PAG evoked responses. For example, in this animal PAG stimulation at 40 Hz stimulation evoked a 49 mmHg increase in MAP and a 37 bpm increase in HR before pontine microinjection. Following pontine microinjection, the increase in MAP and HR was 47 mmHg and 34 bpm. This was in contrast to the average 90% reduction in PAG evoked changes in HR following microinjection of kynurenic acid in sites more closely localized to the LPBN. Data from this animal were excluded from the study.
Figure 6. Reconstructed lateral parabrachial (LPBN) bilateral microinjection sites.
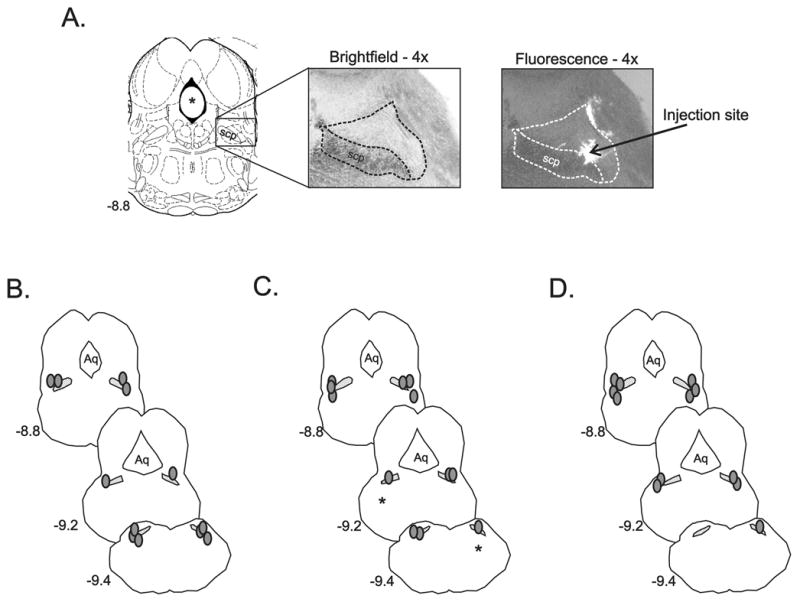
A. Outline of the rostral pons/caudal midbrain from Paxinos & Watson’s (35) and corresponding histological section from one animal illustrating the rostral LPBN viewed with brightfield and fluorescent microscopy. The superior cerebellar peduncle (scp) and boundaries of the LPBN are shown (dashed lines). Arrow indicates the reconstructed center of LPBN microinjection site viewed under fluorescence. B–D. Reconstructed bilateral LPBN microinjection sites (shaded circles) from all muscimol (B, n=6), kynurenic acid (C, n=6), and aCSF (D, n=6), experiments. Asterisks in D illustrate the location of bilateral microinjection sites from one animal made in the ventrolateral pons that did not significantly alter cardiovascular responses evoked from the dorsal PAG and as a result were not included in data analysis. Cross-hatched regions represent location of scp. Numbers to the left of the LPBN images indicate location of the brain section relative to bregma. Aq. indicates location of central aqueduct.
3. DISCUSSION
Previous investigators identified that large lesions, encompassing the dorsal lateral quadrant of the rostral pons, effectively eliminated dorsal PAG-evoked attenuation of the cardiac baroreflex (32). The present study targeted a more focused region and identified that chemical inhibition of neurons in the central region of the LPBN markedly attenuated dorsal PAG modulation of the cardiac baroreflex. Additionally, blockade of excitatory input to the central LPBN was just as effective as neuronal inhibition in attenuating dorsal PAG modulation of the cardiac baroreflex. Furthermore, the effect of LPBN blockade occurred in the absence of any noticeable change in baseline baroreflex function or resting MAP and HR. Our results support previous findings demonstrating a critical role for LPBN area neurons in mediating cardiovascular responses originating from the dorsal PAG (16, 32). However, because the effects of dorsal PAG stimulation on cardiac baroreflex function were only partially reversed by LPBN blockade, our results also support evidence from other investigators demonstrating that other brainstem regions outside the LPBN may be recruited during dorsal PAG stimulation for the purpose of baroreflex modulation (7, 38).
3.1 PAG modulation of the arterial baroreflex control
In the present study stimulation of the dorsal PAG significantly attenuated baroreflex control of HR but had little influence over reflex-evoked hypotension. The threshold for PAG-evoked modulation of reflex control of HR was lower than that needed for modulation of MAP; baroreflex control of HR was significantly attenuated by low frequency dorsal PAG stimulation, but no significant changes in reflex control of MAP were observed until higher frequency PAG stimulation was used. Thus, dorsal PAG modulation of baroreflex function appeared to be dependent upon the level of baroreceptor input; dorsal PAG stimulation had a greater effect on reflex function evoked by lower frequency vs higher frequency ADN stimulation. These observations are in agreement with previous work demonstrating that activation of the defense regions of the brain by either electrical or chemical stimulation can shift or “reset” reflex control to higher arterial pressures (11, 30). Furthermore, current evidence suggests that mechanisms underlying descending modulation of reflex control of HR may be different from those used in modulation of baroreflex control of MAP. For instance, the gain of reflex control of HR may be unchanged at the higher pressures, while the gain of reflex control of sympathetic drive to the vasculature may actually be increased at higher pressures during activation of brain defense regions (30, 32, 38). Similarly, in the present study a small attenuation of reflex control of MAP was observed when high frequency dorsal PAG stimulation was activated during 5 Hz ADN stimulation, but reflex control of MAP evoked by 15 Hz ADN stimulation was apparently unchanged by PAG stimulation.
To date there is good evidence to suggest that descending control of the cardiac baroreflex by the dorsal PAG, as well as other defense regions of the brain, involves activation of both GABA-A and 5-HT3 receptors in the nucleus of the solitary tract (NTS) (7, 21, 38). Interestingly blockade of these receptor subtypes in the NTS does not evoke a parallel change in baseline baroreflex function or dorsal PAG evoked increases in baseline MAP or HR (38). In contrast, the results of the present study and a previous work (16, 32) demonstrate that LPBN neurons play an important role in mediating dorsal PAG evoked increases in baseline MAP and HR, also independent of any changes in baseline baroreflex function. This suggests that changes in autonomic regulation evoked by activation of the dorsal PAG are mediated through excitation (or inhibition) of multiple descending pathways, allowing for independent control of both tonic and reflex regulation of sympathetic and parasympathetic drive.
In present study, it was noted that HR remained elevated for several minutes following the offset of dorsal PAG stimulation. This raised the possibility that some of the effect of midbrain stimulation on baroreflex control may simply reflect summation of sympathetic, parasympathetic, and possibly hormonal inputs peripherally at the level of sinoatrial node. Yet, when baroreflex function was retested one minute following the offset of PAG stimulation, reflex control of HR had returned to control levels. This supports previous evidence that dorsal PAG modulation of the cardiac baroreflex is mediated through active central mechanisms (32, 33, 38). A similar centrally process has been proposed to mediate LPBN modulation of the cardiac baroreflex (24, 25). The results of the present study provide evidence that descending excitation originating from the dorsal PAG reflects one important mechanism through which baroreflex modulating pathways in the LPBN can be activated. The impact of LPBN blockade on cardiovascular responses to threatening stimuli in the conscious animal however remains to be tested.
3.2. LPBN modulation of baroreflex function
In 1982, Mraovitch and colleagues first demonstrated that activation of the PBN attenuated baroreflex function in the anesthetized cat (31). Since that time, PBN modulation of the cardiac baroreflex, similar to that evoked by the dorsal PAG, has been shown to involve activation of GABAergic inputs to the NTS (9, 24, 25). Additionally, there is evidence of a second excitatory projection from the LPBN to the RVLM which is also involved in attenuating or resetting baroreflex function (25, 26). Both GABAergic and glutamatergic neurons in the LPBN with projections to the NTS and RVLM, have been proposed to mediate LPBN-evoked modulation of baroreflex function (25, 26). LPBN area neurons with descending projections to the RVLM and NTS are primarily located in the ventrolateral regions of the LPBN. However it remains to be determined whether activation of these neurons versus other neurons in the LPBN can selectively shift baroreflex control. Indeed, neuroanatomical studies, suggest that dorsal PAG neurons do not send a large number of axons to the ventrolateral LPBN (23), thus it remains to be determined whether this same population of GABAergic/glutamatergic LPBN neurons mediate dorsal PAG modulation of cardiac baroreflex function. Future studies investigating the influence of regional excitation of LPBN neurons on baroreflex function using considerably smaller microinjection volumes (10–20 nl) than utilized in the present study remain to be performed.
3.3 The role of the LPBN in modulating dorsal PAG evoked responses
The role of the dorsolateral pons in mediating dorsal PAG evoked responses was first proposed in 1993 by Nosaka and colleagues (32). In that study, either electrical or chemical activation of the dorsal PAG was shown to increase baseline MAP and simultaneously attenuate the cardiac baroreflex by approximately 80% in beta-blocked animals. Following ipsilateral pontine lesions, which included all PBN subnuclei, and a contralateral pontine transection, dorsal PAG evoked increases in MAP were reduced by 75% and dorsal PAG modulation of baroreflex function was essentially eliminated. In the present study chemical inhibition with muscimol of a smaller region of the PBN, localized to the LPBN, also produced a significant reduction in dorsal PAG evoked increases in MAP (50%). Dorsal PAG evoked increases in HR were attenuated by 90%. Similar results were observed following blockade of glutamate receptors in the LPBN. In both instances however, the effect of dorsal PAG stimulation on reflex control of HR was only partially reversed during higher frequency ADN stimulation. The difference between our results and those of Nosaka et al., may reflect the impact of a more localized injection vs. a larger unilateral lesion combined with the effects of contralateral midbrain transection (32). Additionally, there is now evidence that dorsal PAG modulation of cardiac baroreflex function also involves activation of serotonergic cells groups in the ventromedial medulla which in turn project to the NTS (17). The results of our study suggest that descending activation of these serotonergic neurons may occur through activation of pathways independent of the LPBN.
Based the pattern of descending projections from the dorsal PAG to the LPBN and results from a previous c-Fos study(14), in the present study we primarily targeted the rostral LPBN for our microinjections. In the majority of experiments we were successful in placing our injectate into the intended site. However, in a few instances the microinjection site was positioned in more caudal regions of the LPBN. Interestingly, there appeared to be no difference between the effects of rostral versus caudal injection sites on blocking dorsal PAG evoked responses. This might be explained by the diffusion of the injectate between caudal and rostral sites due to the volume of injectate utilized. Alternatively, there maybe intra-parabrachial connections, which involve both rostral and caudal sites, and blockade of either region can prevent the relay or integration of input from the dorsal PAG to critical sites in the medulla.
3.4 Methodological considerations
Finally, several methodological factors should be considered when interpreting the results of the present study. First, in the present study we chose to only utilize electrical stimulation to activate the dorsal PAG. Electrical stimulation allowed us to tightly regulate the onset and offset of dorsal PAG activation with ADN activation. Electrical stimulation however activates both neurons and fibers of passage. However, previous studies have demonstrated that either electrical or chemical stimulation of the dorsal PAG induces similar cardiovascular responses (13), including significant inhibition of cardiac baroreflex function independent of much change in baroreflex control of MAP (7, 32). This suggests that the primary effect observed in the present study was due to activation of dorsal PAG neurons, however future studies investigating the interconnection between the dorsal PAG and LPBN utilizing chemical activation of the dorsal PAG need to be performed to confirm our results.
Another methodological consideration for the present study is that our experiments were performed in spontaneously breathing animals. In a previous study we demonstrated that chemical blockade of the LPBN markedly altered baseline respiratory timing, including increasing the time of the times of inspiration and expiration and reducing baseline respiratory frequency (16). This change in respiratory control may have independently altered baroreflex function. However, in the present study baseline baroreflex control of MAP and HR were not significantly altered following LPBN blockade with either muscimol or kynurenic acid. Additionally, the study done by Nosaka and colleagues (32) was performed in paralyzed and ventilated rats and their results are consistent with ours, suggesting that descending excitation of LPBN neurons is critical in mediating dorsal PAG evoked modulation of the cardiac baroreflex, independent of changes in respiration.
Interestingly, several previous studies have been reported that the LPBN plays a tonic role in baroreflex function (15, 19, 36). However we did not observe any significant change in reflex function following chemical blockade of the LPBN. Differences between our results and those of other studies may be due to the type of chemical (lidocaine or kainic acid) or the dose of chemical used to block the LPBN. The doses used in the present study were identified to be sufficient to block dorsal PAG evoked responses and it is possible that higher doses are required to completely block other groups of neurons involved in tonic descending control of reflex function. Alternatively, differences in how the arterial baroreflex was evoked between our studies (unilateral ADN stimulation) versus other studies (peripheral vasoconstriction with phenylephrine) or the region of the LPBN we targeted versus other investigators may have contributed to the lack of effect of LPBN blockade on baseline baroreflex function.
3.5 Conclusion
In conclusion, the results of the present study demonstrate that neurons localized in the region of the LPBN are involved in mediating a significant portion of dorsal PAG evoked increases in MAP and HR and descending control of the cardiac baroreflex. Additionally, we have demonstrated for the first time that descending excitation of LPBN neurons is essential for mediating a portion of dorsal PAG-evoked modulation of the cardiac baroreflex. These findings support the hypothesis that the LPBN as an important component of those brainstem pathways known to be involved in mediating autonomic changes associated with the defense response (7).
4. EXPERIMENTAL PROCEDURES
All experiments were performed on adult male Sprague Dawley rats (Harlan, 320–420 g) housed in the University of Florida animal care facility. The rats were exposed to a normal 12 hr light (6AM to 6 PM)/12 hr dark cycle (6 PM to 6AM). All animal handling and experimental protocols were reviewed and approved by the Univ. of Florida Institutional Animal Care and Use Committee and were in compliance with the Animal Welfare Act and the Public Health Service Policy on the Humane Care and Use of Laboratory Animals.
4.1. General Preparation
Animals were initially anesthetized with an intraperitoneal injection of urethane (1.2–1.4 g/kg). No surgical procedures were started until a surgical plane of anesthesia was achieved as evidenced by the absence of a respiratory or motor response to a noxious hindlimb pinch. Femoral arterial and venous catheters were inserted for recording of arterial pressure and administration of supplemental anesthesia and intravenous (i.v.) fluids, respectively. While in the supine position, a mid-line incision was made on the ventral surface of the neck. A tracheotomy was performed and the animal was intubated. All animals were spontaneously breathing and inspired a mixture of room air and 100% oxygen. Next, from a ventral approach the left aortic depressor nerve (ADN) was identified by its insertion into the superior laryngeal nerve and parallel arrangement relative to the vagus nerve. The ADN was separated and isolated from surrounding nerves for stimulation. The animal was then placed in the prone position in a stereotaxic head holder (Kopf Instruments, Tugunga, CA) and the brain regions overlying the PAG and LPBN were exposed by a craniotomy and removal of the dura. Finally, the isolated left ADN was exposed laterally by an incision extending from the base of the ear to the shoulder. The nerve was placed on a bipolar silver wire stimulating electrode, positioned away from the vagus nerve, and covered with a mixture of mineral oil and petroleum jelly (30:70) to prevent drying.
The need for supplemental anesthesia was evaluated every 30–40 min. throughout the experiment by monitoring the response to a noxious pinch of the hindpaw. If fluctuations in blood pressure or heart rate were observed, all experimental procedures were terminated immediately and supplemental anesthesia was administered (0.1 g/kg, i.v. per dose) until a surgical plane of anesthesia was reestablished.
The arterial catheter was attached to a calibrated pressure transducer connected to an amplifier (Stoelting Inc., Wood Dale, IL). The analog output from the blood pressure amplifier was connected to a computer data sampling system (Cambridge Electronics Design [CED] 1401 computer interface, Cambridge, UK). Body temperature was monitored continuously with a rectal temperature probe and maintained within 38±1 °C with a heating blanket (Harvard Bioscience, Inc, Holliston, MA).
An insulated, stainless steel monopolar stimulating electrode (1 M , Fredrick Haer) was secured to a micropositioner (MP-660, Kopf Instruments, Tujunga, CA) and stereotaxically positioned into dorsal PAG at a 45 degree angle. The final position of the electrode tip was between 7.0 and 8.0 mm caudal from bregma, 0.2–0.3 mm lateral from midline and 3.8 and 4.1 mm ventral to the surface of the brain, according to stereotaxic coordinates for the dorsal PAG described by Paxinos and Watson’s Rat Brain in Stereotaxic Coordinates (35). The stimulating electrode was connected to an isolated current stimulator (DS1A, Digitimer, Hertfordshire, UK), in-series with a programmable stimulator (Master8, AMPI, Jerusalem, Israel). Electrical stimulation parameters of the DPAG were set at 70 μA, 0.4 ms pulse width and 10 or 40 Hz. The stimulating electrode for the left ADN was connected to a second isolated voltage stimulator (DS2A, Digitimer) connected in-series with the programmable stimulator. ADN stimulation parameters were set at 10 V, 2 ms and 5 or 15 Hz for 10 seconds.
4.2. Protocol
To test the effect of PAG stimulation on baroreflex control, the cardiovascular response to ADN stimulation alone or ADN stimulation combined with PAG stimulation was recorded. For each trial, following the recording of baseline MAP and HR for 30 seconds, the response to ADN stimulation at a single frequency alone or combined with PAG stimulation was tested. In trials combining dorsal PAG stimulation with ADN stimulation, the onset of PAG stimulation preceded ADN stimulation by a minimum of 10 s. This 10 s interval was based on previous work identifying that this was the minimum amount of time needed for cardiovascular response to dorsal PAG stimulation to stabilize (16). The inter-trial interval was two minutes for the majority of the experiments and the order of ADN and PAG stimulation frequency was randomly presented between trials. Following characterization of the effect of PAG stimulation on ADN evoked responses, a single barrel glass microinjection pipette secured to a micromanipulator (Kopf) was positioned into the left, and then the right, LPBN. Stereotaxic coordinates for the LPBN were 8.9–9.4 caudal from bregma, 2.2 mm lateral and 5.5–5.6 mm ventral to the surface of the brain. The microinjection pipette was connected to a pressure injection system (BH2, Medical systems). One of three solutions was bilaterally microinjected (90 nl per side) into the region of the LPBN. Each animal received only one drug. For chemical inhibition of the LPBN, 0.45 nmoles of the GABA-A receptor agonist muscimol (5 mM solution) was microinjected bilaterally into the LPBN. For selective blockade of glutamatergic input to the LPBN, 1.8 nmoles of the kynurenic acid (20 mM solution) was microinjected bilaterally into the LPBN. The concentrations of both muscimol and kynurenic acid used for blockade were based on previous studies in this lab (12). Both muscimol and kynurenic acid were diluted in artificial cerebrospinal fluid (aCSF) containing: 122 mM NaCl, 3 mM KCl, 25.7 mM NaHCO3-, and 1 mM CaCl2, with pH adjusted to 7.4. Finally, to control for the effects of microinjection alone, one group of animals underwent bilateral microinjection (90 nl per side) of aCSF into the LPBN. Small amounts of fluorescent latex microspheres (Lumafluor Corp, Naples, FL) were mixed into all injectates to facilitate later identification of the microinjection sites. The volume of microinjectate was determined by monitoring the movement of the meniscus in the microinjection pipette with a monocular microscope equipped with a calibrated eye-piece (Titan Tools, Buffalo, NY). Three minutes following microinjection into second side of the LPBN, the effect of dorsal PAG stimulation on baroreflex function was retested.
At the end of the experiment all animals were euthanized and an electrolytic lesion was made in the PAG at the stimulation site (4 mA, 10 s duration). For all animals, the brain was removed and placed in 4% paraformaldehyde solution for 24–72 h. The brains were then frozen to –16 degrees C and the brainstem was sliced into 40-micrometer transverse sections with a cryostat (HM101, Carl Zeiss, Inc, Thornwood, NY). The tissue was mounted on slides and sealed with a coverslip (Antifade, Molecular Probes, Eugene, OR). Microinjection and stimulation sites were imaged with a microscope equipped with both brightfield and epifluorescence.
4.3. Data Analysis
All data were analyzed off-line using Spike2 software (CED). MAP was calculated from the difference between the systolic and diastolic pressures divided by 3, plus the diastolic pressure. HR was calculated from the interval between systolic pressure peaks. Baseline values were averaged over a 10 s period collected just prior to the onset of ADN or dorsal PAG stimulation at the beginning of each trial. The baseline MAP and HR for each animal was calculated from the average of these values before and following LPBN microinjection. Baroreflex responses were calculated as the difference between the MAP and HR measured over a 2 second window just prior to the onset of ADN stimulation and the average of MAP and HR over the last 2 seconds of ADN stimulation (see Fig. 1 for an example of ADN measurement times). The effect of dorsal PAG stimulation on baseline MAP and HR was calculated from the difference between a 2 second average taken just prior to the onset of PAG stimulation versus the 2 second average taken just prior to the onset of ADN stimulation during PAG stimulation.
Data collected before LPBN microinjection were analyzed using a two-way analysis of variance (ANOVA; treatment group vs. ADN stimulation frequency) to identify significant differences between groups (muscimol vs. kynurenic acid or ACSF microinjection). No significant difference between groups was identified, so all pre-treatment data were combined and a one-way ANOVA was used to identify differences between the effects of PAG or ADN stimulation frequency on MAP and HR. A paired t-test with Bonferonni adjustments for multiple comparisons was used to test the influence of PAG stimulation (10 vs. 40 Hz) on ADN evoked responses. Following LPBN microinjection, an ANOVA with repeated measures was used to identify significant effects of drug treatment on baseline measurements and baroreflex responses activated alone or in the presence of PAG stimulation (40 Hz stimulation only) within each ADN stimulation frequency. When significant differences were indicated, interactions were examined with a paired t-test with adjustments for multiple comparisons. Finally, to evaluate changes in baroreflex function, ADN-evoked changes in MAP and HR were plotted from all experiments before and following LPBN microinjection. Changes were considered significant when p<0.05. All data are reported as mean±SEM.
Acknowledgments
The author would like to thank Anisha Gulati for her help in the data analysis and histology for this study.
Footnotes
GRANTS:
This project was supported by funds from the American Heart Association-Florida Puerto Rico Affiliate (0255036B) and NIH (HL 063232 & HL 76518).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: Modules for emotional expression? Trends Neurosci. 1994;17:379–389. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 2.Bandler R, Carrive P. Integrated defence reaction elicited by excitatory amino acid microinjection in the midbrain periaqueductal grey region of the unrestrained cat. Brain Res. 1988;439:95–106. doi: 10.1016/0006-8993(88)91465-5. [DOI] [PubMed] [Google Scholar]
- 3.Bandler R, Prineas S, McCulloch B. Further localization of midbrain neurones mediating the defense reaction in the cat by microinjections of excitatory amino acids. Neurosci Letters. 1985;56:311–316. doi: 10.1016/0304-3940(85)90261-7. [DOI] [PubMed] [Google Scholar]
- 4.Cameron AA, Khan IA, Westlund KN, Willis WD. The efferent projections of the periaqueductal gray in the rat: a phaseolus vulgaris-leucoagglutinin study. II Descending Projections. J Comp Neurol. 1995;351:585–601. doi: 10.1002/cne.903510408. [DOI] [PubMed] [Google Scholar]
- 5.Carrive PSP, Karli P. Flight induced by microinjection of D-tubocurarine or alpha-bungarotoxin into medial hypothalamus or periaqueductal gray matter: cholinergic or GABAergic mediation? Behav Brain Res. 1986;22 doi: 10.1016/0166-4328(86)90068-9. [DOI] [PubMed] [Google Scholar]
- 6.Chen S, Aston-Jones G. Anatomical evidence for inputs to ventrolateral medullary catecholaminergic neurons from the midbrain periaqueductal gray of the rat. Neurosci Lett. 1995;195:140–144. doi: 10.1016/0304-3940(94)11788-k. [DOI] [PubMed] [Google Scholar]
- 7.Comet MA, Sevoz-Couche C, Hanoun N, Hamon M, Laguzzi R. 5-HT-mediated inhibition of cardiovagal baroreceptor reflex response during defense reaction in the rat. Am J Physiol Heart Circ Physiol. 2004;287:H1641–1649. doi: 10.1152/ajpheart.01204.2003. [DOI] [PubMed] [Google Scholar]
- 8.Farkas E, Jansen ASP, Loewy AD. Periaqueductal gray matter input to cardiac-related sympathetic premotor neurons. Brain Res. 1998;792:179–192. doi: 10.1016/s0006-8993(98)00029-8. [DOI] [PubMed] [Google Scholar]
- 9.Felder RB, Mifflin SW. Modulation of carotid sinus afferent input to nucleus tractus solitarius by parabrachial nucleus stimulation. Circ Res. 1988;63:35–49. doi: 10.1161/01.res.63.1.35. [DOI] [PubMed] [Google Scholar]
- 10.Graeff F. Neuroanatomy and neurotransmitter regulation of defensive behaviors and related emotions in mammals. Braz J Med Biol Res. 1994;27:811–829. [PubMed] [Google Scholar]
- 11.Hatton D, Brooks V, Qi Y, McCarron DA. Cardiovascular response to stress: baroreflex resetting and hemodynamics. Am J Physiol. 1997;272:R1588–R1594. doi: 10.1152/ajpregu.1997.272.5.R1588. [DOI] [PubMed] [Google Scholar]
- 12.Hayward L. Evidence for alpha-2 adrenoreceptor modualtion of arterial chemoreflex in the caudal solitary nucleus of the rat. Am J Physiol. 2001;281:R1464–R1473. doi: 10.1152/ajpregu.2001.281.5.R1464. [DOI] [PubMed] [Google Scholar]
- 13.Hayward L, Swartz CL, Davenport PW. Respiratory response to activation or disinhibition of the dorsal periaqueductal gray in rats. J Appl Physiol. 2003;94:913–922. doi: 10.1152/japplphysiol.00740.2002. [DOI] [PubMed] [Google Scholar]
- 14.Hayward LF, Castellanos M. Increased Fos expression in select lateral parabrachial subnuclei following chemical versus electrical stimulation of the dorsal periaqueductal gray in rats. Brain Res. 2003;974:153–166. doi: 10.1016/s0006-8993(03)02573-3. [DOI] [PubMed] [Google Scholar]
- 15.Hayward LF, Felder RB. Lateral parabrachial nucleus modulates baroreflex regulation of sympathetic activity. Am J Physiol. 1998;274:R1274–R1282. doi: 10.1152/ajpregu.1998.274.5.R1274. [DOI] [PubMed] [Google Scholar]
- 16.Hayward LF, Castellanos M, Davenport PW. Parabrachial neurons mediate dorsal periaqueductal gray evoked respiratory responses in the rat. J Appl Physiol. 2004:1146–1154. doi: 10.1152/japplphysiol.00903.2003. [DOI] [PubMed] [Google Scholar]
- 17.Hermann D, Luppi PH, Peyron C, Hinckel P, Jouvet M. Afferent projections to the rat nuclei raphe magnus, raphe pallidus and reticularis gigantocellularis pars alpha demonstrated by iontophoretic application of choleratoxin (subunit b) J Chem Neuroanat. 1997;13:1–21. doi: 10.1016/s0891-0618(97)00019-7. [DOI] [PubMed] [Google Scholar]
- 18.Hilton S, Smith PR. Ventral medullary neurones excited from the hypothalamic and mid-brain defence areas. J Auton Nerv Syst. 1984;11:35–42. doi: 10.1016/0165-1838(84)90006-7. [DOI] [PubMed] [Google Scholar]
- 19.Hubbard JW, Buchholz RA, Keeton TK, Nathan MA. Parabrachial lesions increase plasma norepinephrine concentration, plasma renin activity and enhance baroreflex sensitivity in the conscious rat. Brain Res. 1987;421:226–243. doi: 10.1016/0006-8993(87)91292-3. [DOI] [PubMed] [Google Scholar]
- 20.Hunsperger R. Affective behavior patterns elicited by electrical stimulation of the brain stem and forebrain. J Physiol (Paris) 1963;55:45–98. [PubMed] [Google Scholar]
- 21.Jordan D, Mifflin SW, Spyer KM. Hypothalamic inhibition of neurones in the nucleus tractus solitarius of the cat is gaba mediated. J Physiol. 1988;399:389–404. doi: 10.1113/jphysiol.1988.sp017087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krieger J, Graeff FG. Defensive behavior and hypertension induced by glutamate in the midbrain central gray of the rat. Braz J Med Biol Res. 1985;18(1):61–67. [PubMed] [Google Scholar]
- 23.Krout K, Jansen ASP, Loewy AD. Periaqueductal gray matter projection to the parabrachial nucleus in rat. J Comp Neurol. 1998;401:437–454. [PubMed] [Google Scholar]
- 24.Len W, Chan JY. GABAergic neurotransmission at the nucleus tractus solitarii in the suppression of reflex bradycardia by parabrachial nucleus. Synapse. 2001;42:27–39. doi: 10.1002/syn.1096. [DOI] [PubMed] [Google Scholar]
- 25.Len W, Chan SH, Chan JY. Parabrachial nucleus induces suppression of baroreflex bradycardia by the release of glutamate in the rostral ventrolateral medulla of the rat. J Biomed Sci. 2000;7:401–411. doi: 10.1007/BF02255815. [DOI] [PubMed] [Google Scholar]
- 26.Len WB, Chan JY. Glutamatergic projection to RVLM mediates suppression of reflex bradycardia by parabrachial nucleus. Am J Physiol. 1999;276:H1482–H1492. doi: 10.1152/ajpheart.1999.276.5.H1482. [DOI] [PubMed] [Google Scholar]
- 27.Li P, Lovick TA. Excitatory projections from hypothalamic and midbrain defense regions to nucleus paragiagantocellularis lateralis in the rat. Exp Neurol. 1985;89:543–553. doi: 10.1016/0014-4886(85)90005-6. [DOI] [PubMed] [Google Scholar]
- 28.Lovick T. Midbrain influences on ventrolateral medullo-spinal neurones in the rat. Exp Brain Res. 1992;90:147–152. doi: 10.1007/BF00229266. [DOI] [PubMed] [Google Scholar]
- 29.Lovick T. Ventrolateral medullary lesions block the antinociceptive and vascular responses elicited by stimulating the dorsal periaqueductal gray matter in rats. Pain. 1985;21:241–252. doi: 10.1016/0304-3959(85)90088-0. [DOI] [PubMed] [Google Scholar]
- 30.McDowall L, Horiuchi J, Killinger S, Dampney RA. Modulation of the baroreceptor reflex by the dorsomedial hypothalamic nucleus and perifornical area. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1020–R1026. doi: 10.1152/ajpregu.00541.2005. [DOI] [PubMed] [Google Scholar]
- 31.Mraovitch S, Kumada M, Reis DJ. Role of the nucleus parabrachialis in cardiovascular regulation in cat. Brain Res. 1982;232:57–75. doi: 10.1016/0006-8993(82)90610-2. [DOI] [PubMed] [Google Scholar]
- 32.Nosaka S, Murata K, Inui K, Murase S. Arterial baroreflex inhibition by midbrain periaqueductal grey in anesthetized rats. Pflugers Arch. 1993;424:266–275. doi: 10.1007/BF00384352. [DOI] [PubMed] [Google Scholar]
- 33.Nosaka SIK, Murase S, Murata K. A prejunctional mechanism in midbrain periaqueductal gray inhibition of vagal bradycardia in rats. Am J Physiol. 1996;270:R373–R382. doi: 10.1152/ajpregu.1996.270.2.R373. [DOI] [PubMed] [Google Scholar]
- 34.Odeh F, Antal M. The projections of the midbrain periaqueductal grey to the pons and medulla oblongata in rats. Eur J Neurosci. 2001;14:1275–1286. doi: 10.1046/j.0953-816x.2001.01760.x. [DOI] [PubMed] [Google Scholar]
- 35.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press, Inc.; 1998. [Google Scholar]
- 36.Saleh TM, Connell BJ. Modulation of the cardiac baroreflex following reverse blockade of the parabrachial nucleus in the rat. Brain Res. 1997;767:201–207. doi: 10.1016/s0006-8993(97)00560-x. [DOI] [PubMed] [Google Scholar]
- 37.Schenberg LC, Vasquez EC, da Costa MB. Cardiac baroreflex dynamics during the defence reaction in freely moving rats. Brain Res. 1993;621:50–58. doi: 10.1016/0006-8993(93)90296-y. [DOI] [PubMed] [Google Scholar]
- 38.Sevoz-Couche CCM, Hamon M, Laguzzi R. Role of nucleus tractus solitarius 5-HT3 receptors in the defense reaction-induced inhibition of the aortic baroreflex in rats. J Neurophysiol. 2003;90:2521–2530. doi: 10.1152/jn.00275.2003. [DOI] [PubMed] [Google Scholar]
- 39.Van Bockstaele A-JG, Pieribone VA, Ennis M, Shipley MT. Subregions of the periaqueductal gray topographically innervate the rostral ventral medulla in the rat. J Comp Neurol. 1989;309:305–327. doi: 10.1002/cne.903090303. [DOI] [PubMed] [Google Scholar]
- 40.Verberne AJ, Guyenet PG. Midbrain central gray: influence on medullary sympathoexcitatory neurons and the baroreflex in rats. Am J Physiol. 1992;263:R24–R33. doi: 10.1152/ajpregu.1992.263.1.R24. [DOI] [PubMed] [Google Scholar]


