Abstract
Activation of the cardiac “sympathetic afferent” reflex (CSAR) has been reported to depress the arterial baroreflex and enhance the arterial chemoreflex via a central mechanism. In the present study, we used single-unit extracellular recording techniques to examine the effects of stimulation of cardiac sympathetic afferents on baro- or chemosensitive neurons in the nucleus tractus solitarius (NTS) in anesthetized rats. Of 54 barosensitive NTS neurons tested for their response to epicardial application of capsaicin (0.4 μg), 38 were significantly (P<0.01) inhibited by 38 % while 16 did not respond. Of 42 NTS chemosensitive neurons tested for their response to capsaicin, 33 were significantly (P<0.01) excited by 47 % while 9 did not respond. In addition, of 12 both barosensitive and chemosensitive NTS neurons tested for capsaicin, 2 were excited, 7 were inhibited, and 3 did not respond. In conclusion, this study indicates that CSAR activation inhibited NTS barosensitive neurons and excited NTS chemosensitive neurons, suggesting that the NTS plays an important role in processing the interactions between these cardiovascular reflexes.
Keywords: cardiovascular reflexes, sympathetic activity, capsaicin, extracellular recording, baro-/chemosensitive neuron
The cardiac “sympathetic afferent” reflex (CSAR) is known to activate the cardiovascular system and is activated by metabolic mediators, myocardial ischemia and cardiac enlargement [1;2]. It has been demonstrated that an enhanced CSAR may be an important factor resulting in abnormalities in sympathetic tone and cardiovascular reflexes such as the arterial baroreflex and the chemoreflex in heart failure [3;4]. The importance of the arterial baroreflex and chemoreflex in controlling cardiovascular and respiratory functions is well established. CSAR activation enhances sympathetic outflow, and also produces a decrease in arterial baroreflex sensitivity as well as an increase in the sensitivity of the arterial chemoreflex [5–7]. Recently, it has been reported from this laboratory that central application of an Angiotensin (Ang II) receptor antagonist completely abolishes the CSAR-induced depression of the baroreflex and enhancement of the chemoreflex, suggesting that the interaction between these cardiovascular reflexes occurs in the central nervous system [5;7].
The nucleus tractus solitarius (NTS) is the site of termination of afferent fibers from arterial baro- and chemoreceptors. Neurons within the NTS are involved in the production of appropriate patterns of the baro- and chemoreflex responses elicited by the activation of these peripheral receptors [8;9]. On the other hand, it has been suggested that cardiac sympathetic afferents project to the dorsal horn of the upper thoracic spinal cord through the stellate ganglia and the sympathetic chain [10;11]. Some ascending fibers from the dorsal horn of the spinal cord terminate in the NTS [12]. Therefore, the NTS has been implicated to be an important area for integrating the central transmission of the CSAR [13;14]. Taken together, it is reasonable to hypothesize that the NTS may be an important region for integrating the processing of interactions between the CSAR and the arterial baro- or chemoreflex. Hence, we investigated the effects of chemical stimulation of cardiac sympathetic afferents by epicardial application of capsaicin on the discharge of baro- or chemosensitive neurons in the NTS, respectively.
All experiments were performed on adult male Sprague-Dawley rats weighing 300–350 g (n = 45) and were approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center and were carried out under the guidelines of the American Physiological Society and the National Institutes of Health Guide of the Care and Use of Laboratory Animals. All data were recorded via a computer-based data acquisition and storage system (ADinstrument Powerlab).
Rats were anesthetized with urethane (800 mg/kg, ip) and α-chloralose (40 mg/kg, ip). The trachea was cannulated and the rats were ventilated artificially with room air supplemented with 100 % oxygen. The right common carotid artery was exposed and the peripheral end catheterized for measurement of arterial pressure (AP) and heart rate (HR). A cannula was introduced retrogradely into the right external carotid artery so that its tip was at the origin of the artery supplying the carotid bodies. The femoral vein was cannulated for intravenous injections. The rats were paralyzed with pancuronium bromide (1 mg/kg, iv). Rats were placed in a stereotaxic frame and the dorsal surface of the medulla was surgically exposed by incising the atlanto-occipital membrane and removing part of the occipital bone and dura. Supplemental doses of α-chloralose (20 mg/kg, iv) were administered to maintain an appropriate level of anesthesia. Body temperature was maintained at 37°C by an animal temperature controller.
The chest was opened through the fourth intercostal space. The pericardium was removed to expose the left ventricle. A piece of filter paper containing capsaicin (3×3 mm, 0.4 μg in 2 μl) was applied to the anterior surface of the left ventricle for stimulating the cardiac sympathetic afferents [5]. The drug was applied for 2 min and then the epicardium was rinsed three times with 10 ml of warm normal saline (37 °C). The time interval of repeated capsaicin was at least 30 min in order to allow the AP and discharge of NTS neuron to return to and stabilize at their control levels. In order to test the barosensitivity of NTS neurons, a vascular occluder was placed around the descending thoracic aorta above the diaphragm and was used to elevate AP by constricting the aorta.
Single-unit extracellular recording was obtained using a single-micropipette (resistance 5–12 MΩ) filled with 0.5 M sodium acetate dissolved in 2 % Pontamine sky blue. The calamus scriptorius was used as a visual landmark for guiding the placement of the electrode. Single-unit recording was restricted to two areas: the chemoreceptor projection site (commissural NTS: 0.2 rostral to 0.3 caudal, 0–0.5 lateral, and 0.3–0.5 deep) [15] and the baroreceptor projection site (dorsal medial NTS: 0.3–0.6 rostral, 0.3–0.5 lateral, and 0.4–0.5 deep) [16]. The spontaneous action potentials were amplified (gain: 1000, 0.1–10 kHz passband) and fed into a window discriminator which generates a standard pulse for each spike. Potentials were visualized on an oscilloscope. The pulse output of the discriminator was then fed into a digital counter/timer whose analog output was proportional to the number of spikes per unit time (1 s). Spontaneously active neurons in the bilateral dorsal medial NTS and the ipsilateral (right) commissural NTS were tested by baroreceptor and chemoreceptor stimulations, respectively. The barosensitive NTS neuron was identified by its excitatory response to a transient AP elevation (20–50 mmHg) in response to aorta occlusion (5–10 s) [17]. The chemoreceptive NTS neurons were identified by their excitatory response to bolus injections of potassium cyanide (KCN) solution (10 μg in 0.1 ml) into the vicinity of the carotid body. Cyanide has been demonstrated to effectively stimulate the arterial chemoreceptors [18]. Although the rats were paralyzed for avoiding brain movement during neuronal recording, the pressor action and bradycardia induced by KCN injection were significantly observed. Some specific NTS neurons that were inhibited either by aorta occlusion or by KCN injection were not further studied in this work. After a neuron of interest was identified, pontamine sky blue was iontophoresed (−15 μA, 20 min) to mark the recording location. At the end of the experiment, the rat brain was removed, frozen, sectioned (50–μm) and stained with neutral red. The dye spots for recording sites were identified according to the atlas [19]. Fig. 1 shows the distributions of all types of tested NTS neurons.
Fig. 1.
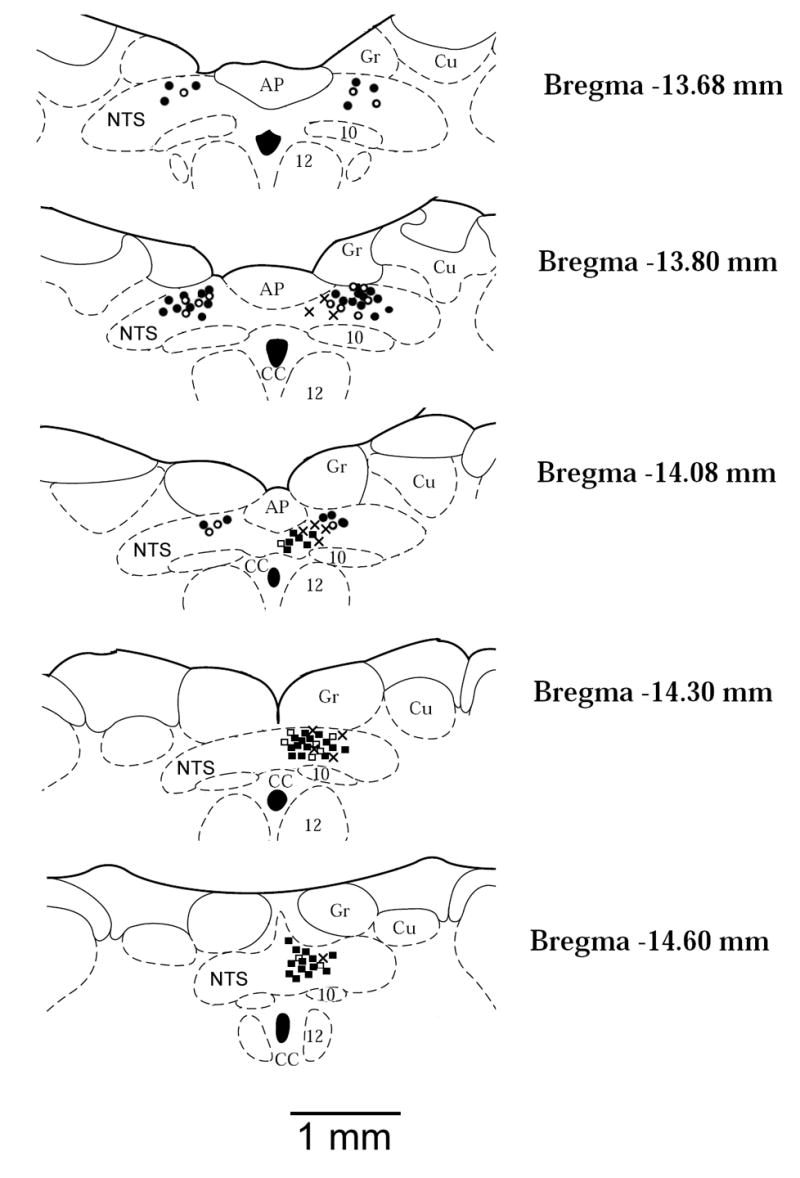
Anatomic locations of recorded neurons plotted on standard coronal sections. Barosensitive neurons in which discharge was decreased (●, 38 sites) or did not respond (○, 16 sites) to epicardial capsaicin; Chemosensitive neurons in which discharge was increased (▪, 33 sites) or did not respond (□, 9 sites) to epicardial capsaicin; Neurons in which discharge was increased by both baroreceptor and chemoreceptor stimulation (×, 12 sites). AP, area postrema; CC, central canal; Cu, cuneate nucleus; Gr, gracile nucleus; NTS, nucleus tractus solitarius; 10, motor nucleus of vagus nerve; 12, nucleus of hypoglossal nerve.
The data are presented as the mean ± SEM. The discharge rate of neurons was averaged during a 30-s control, 5-s (during aorta occlusion), 10-s (after KCN injection), and 1-min (epicardial capsaicin) response periods. The NTS neurons were considered to be responsive if their peak discharge frequency after treatments was changed by at least 30% above the baseline. Comparisons between pre and post-interventions were made by Student’s paired t-test. Differences were considered to be statistically significant when P<0.05.
A total of 54 spontaneous neurons from the dorsal medial NTS, identified as barosensitive by exhibiting increases in their discharges by 102 ± 9 % in response to transient AP elevation (36.4 ± 3.1 mmHg) by aorta occlusion, were studied for their responses to stimulation of cardiac sympathetic afferents. Fig. 2A shows original tracings of a barosensitive NTS neuron in response to capsaicin application. Epicardial application of capsaicin produced significant (P<0.01, n=54) increases in AP (from 92 ± 3 to 109 ± 4 mmHg) and HR (from 354 ± 8 to 396 ± 9 beats/min). Of 54 barosensitive neurons tested for epicardial application of capsaicin, 38 were inhibited by 38 ± 2 % (from 5.3 ± 0.6 to 3.4 ± 0.4 spikes/s, P<0.01) and 16 did not respond (5.2 ± 0.7 vs. 5.3 ± 0.8 spikes/s, P>0.05) (Fig. 2B). The effective time of capsaicin on neuronal discharge lasted about 1 min. In 8 capsaicin-inhibitory barosensitive NTS neurons, epicardial application of 0.9 % normal saline (0.2 μl) did not significantly modify the neuronal discharge (5.8 ± 0.8 vs. 5.2 ± 0.7 spikes/s, P>0.05) (Fig. 2B).
Fig. 2.
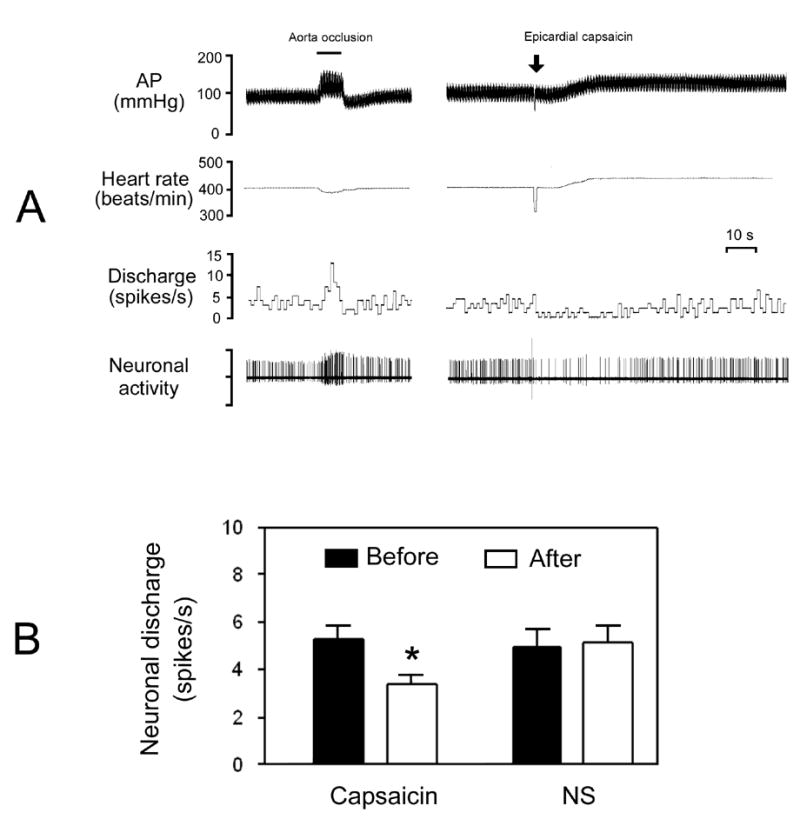
Response of barosensitive neurons in the dorsal medial NTS to epicardial application of capsaicin. A, original tracings showing responses of arterial pressure (AP), heart rate, and discharge of a barosensitive neuron to aorta occlusion and epicardial capsaicin (0.4 μg). B, bar graphs showing the mean data for the discharge of barosensitive NTS neurons before and after epicardial capsaicin (n=38) or normal saline (NS, n=8). *P<0.01 vs. before level.
A total of 42 spontaneous neurons from the commissural NTS were identified as chemosensitive by exhibiting increases in discharge by 73 ± 5 % in response to intra-arterial injection of KCN (10 μg). A transient pressor action (10 ± 2 mmHg) and bradycardia (−12 ± 2 beats/min) were significantly (P<0.01, n = 42) observed following KCN injection. Fig. 3A shows a representative response of a chemosensitive NTS neuron to epicardial application of capsaicin. Of 42 chemosensitive neurons tested for capsaicin, 33 were excited by 47 ± 3 % (from 4.4 ± 0.4 to 6.3 ± 0.5 spikes/s, P<0.01) and 9 did not respond (4.6 ± 0.7 vs. 4.8 ± 0.8 spikes/s, P>0.05) (Fig. 3B). Usually, the excitatory effect of capsaicin on chemosensitive neurons persisted about 1 min. In control tests, in 6 capsaicin-sensitively chemosensitive NTS neurons, epicardial application of normal saline (0.2 μl) did not significantly modify the discharge rate (4.6 ± 0.9 vs. 4.7 ± 0.8 spikes/s, P>0.05) (Fig. 3B).
Fig. 3.
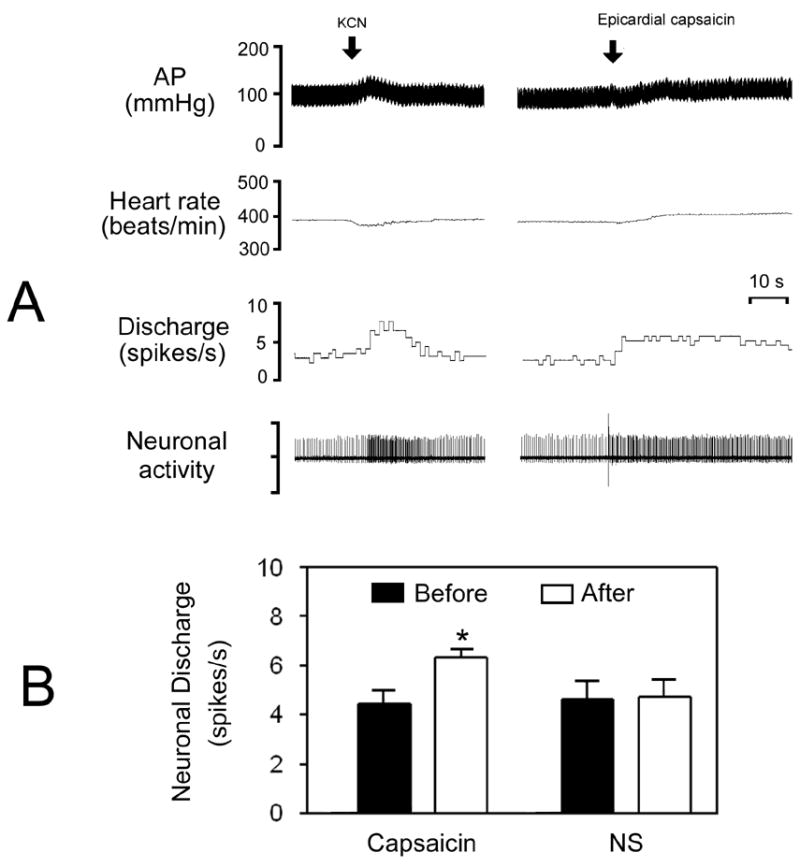
Response of chemosensitive neurons in the commissural NTS to epicardial application of capsaicin. A, original tracings showing the arterial pressure (AP), heart rate, and discharge of a NTS neuron in response to intra-arterial injection of potassium cyanide (KCN, 10 μg) and epicardial capsaicin (0.4 μg). B, bar graphs showing the mean data for discharge of chemosensitive NTS neurons before and after epicardial capsaicin (n=33) or normal saline (NS, n=6). *P<0.01 vs. before level.
Interestingly, we also found that another 12 neurons (basal activity: 6.3 ± 1.0 spikes/s) from the medial NTS (5 units) and the commissural NTS (7 units) were sensitive to stimulation of both baroreceptors and chemoreceptors. During application of capsaicin, a majority of these neurons (7/12, 2 from the medial NTS and 5 from the commissural NTS) were inhibited by 45 %, 2 were excited by 63 %, and 3 did not respond.
It is well known that the arterial baroreflex and chemoreflex are very important mechanisms for controlling autonomic outflow. The NTS receives convergent inputs from baroreceptor afferents, chemoreflex afferents and cardiac sympathetic afferents [12;13;15], and also plays a crucial role in integrating these cardiovascular reflexes [20]. So we chose the NTS as the target region where the interactions between the CSAR and the baro- or chemoreflex may occur. In the present study, we found that CSAR activation significantly inhibited the discharge of 38 of the 54 (70 %) barosensitive NTS neurons. We believe that the CSAR is effectively activated by epicardial application of capsaicin. Similar to our previous study [5], it was found that epicardial capsaicin significantly increased AP and HR. Moreover, vagus afferent stimulation of capsaicin was excluded because the decreases in AP and HR were not observed in the present study. In addition, we suggest that the inhibitory effect of capsaicin on NTS neurons is not due to baroreflex activation because AP elevation by CSAR activation will produce an excitation of NTS barosensitive neurons. It is well known that NTS barosensitive neurons, which receive input from the peripheral baroreceptors, excite the caudal ventrolateral medulla (CVLM) neurons via a glutamatergic projection, whereas the CVLM neurons inhibit the RVLM vasomotor neurons via a GABAergic projection [8;21]. The RVLM has been recognized as a critical site for generating sympathetic outflow and controlling baroreflex function [8]. Therefore, it is logical to assume that the inhibitory effect of CSAR activation on the NTS barosensitive neurons would increase sympathetic outflow and decrease the baroreflex function. Therefore, this new finding contributes to our understanding of the mechanism responsible for CSAR-induced sympathetic overactivity and baroreflex impairment. However, little is known concerning the mechanism(s) by which stimulation of cardiac sympathetic afferents inhibits the barosensitive NTS neurons. It has been reported that excitatory amino acids are involved in synaptic transmission of the CSAR in the NTS [14]. The electrophysiological evidence further shows that a group of neurons exists within the NTS which can be excited by CSAR activation [13]. Therefore, it is possible that the barosensitive NTS neurons may not directly receive cardiac sympathetic afferent input. It is possible that NTS neurons, which receive the cardiac sympathetic afferents, are interneurons that release inhibitory neurotransmitters. CSAR-induced excitation of this inhibitory interneuron may produce an inhibition of NTS barosensitive neurons, which is consistent with CSAR activation resetting resting discharge and barosensitivity. The central pathway of the interaction between baroreceptor afferents and cardiac sympathetic afferents is outlined in Fig. 4. There is substantial evidence that GABA may be the inhibitory neurotransmitter in the NTS. For example, iontophoretic application of GABA markedly reduced or abolished the responses of NTS neurons to stimulation of baroreceptors [22]. An inhibitory interneuron mechanism in the NTS has become a well accepted notion to explain baroreflex resetting under some conditions [23].
Fig. 4.
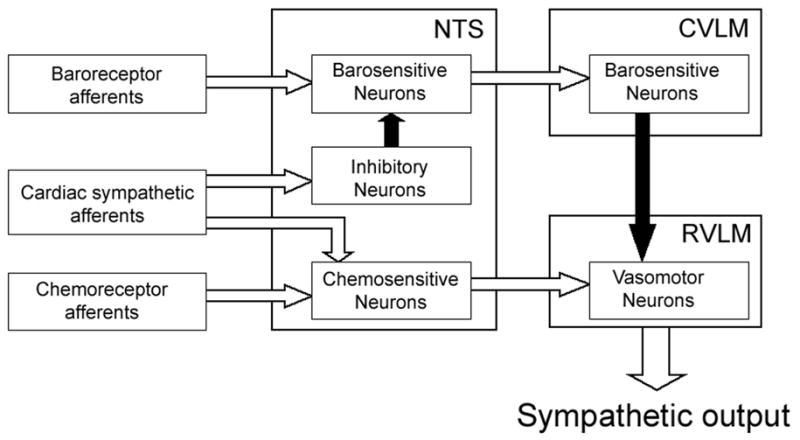
A schematic representation of the pathway for the proposed synaptic interactions between cardiac sympathetic afferents and arterial baro- or chemoreceptor afferents in the NTS. Filled arrows, inhibitory pathways; open arrows, excitatory pathways, CVLM, caudal ventrolateral medulla; RVLM, rostral ventrolateral medulla.
On the other hand, we found that stimulation of cardiac sympathetic afferents produced an increase in discharge rate of 33 of 42 (79%) chemosensitive neurons in the commissural NTS. The commissural NTS has been reported to be a termination site for peripheral chemoreceptor afferents [15], and also receive excitatory inputs from cardiac sympathetic afferents [14]. Moreover, the existence of neurons in the commissural NTS with direct projections to the RVLM has been well documented [24]. This projection includes monosynaptic connections with vasomotor neurons in the RVLM which, in turn, project monosynaptically to preganglionic sympathetic neurons in the spinal cord [24]. Therefore, the direct sympatho-excitatory pathway from the NTS chemoreceptor neurons to the RVLM has been documented to play an important role in mediating sympatho-excitatory reflexes such as the arterial chemoreflex [15;25]. Furthermore, the neurons within the commissural NTS convey a tonic excitatory drive of chemoreceptor origin to the RVLM neurons [26;27]. As indicated in Fig. 4, it is suggested that excitation of chemosensitive NTS neurons by CSAR activation probably produces increases in sympathetic output and arterial chemoreflex sensitivity. The evidence from this study may be very important for interpreting the phenomena of enhanced sympathetic output and chemoreflex function induced by CSAR activation.
In this work, we did not further determine which neurotransmitters in the NTS mediate the interactions between these cardiovascular reflexes. Ang II has been indicated to be a strong candidate for integrating the interactions between these cardiovascular reflexes [4;28]. For example, microinjection of Ang II into the NTS attenuates the arterial baroreflex and enhances the arterial chemoreflex [29;30]. Importantly, the evidence from this laboratory has shown that central Ang II antagonism not only normalized the augmented CSAR in heart failure, but also completely abolished the CSAR-induced blunted baroreflex and augmented chemoreflex [5;6]. More recently, we reported that blockade of NTS Ang II receptors effectively prevented the enhanced chemoreflex sensitivity evoked by CSAR activation [7]. However, the precise mechanism(s) of neurotransmitter transmission for mediating the interactions between these cardiovascular reflexes within the NTS require further experiments.
In addition, 12 specific NTS neurons were recorded in the present study to be sensitive to both baroreceptor and chemoreceptor stimulations. We did not confirm the effects of epicardial capsaicin on these NTS neurons and could not identify the roles of these neurons in mediating the interactions between the CSAR and the baro- or chemoreflex. It has been demonstrated that some NTS neurons simultaneously receive many sensory inputs [31]. However, little is known concerning the roles of these neurons in integrating cardiovascular function.
In summary, stimulation of cardiac sympathetic afferents significantly decreased the basal discharge of barosensitive NTS neurons and increased the basal discharge of chemosensitive NTS neurons, suggesting that the NTS plays an important role in processing the interactions between the CSAR and the baro- or chemoreflex. Because of the acute nature of the experiments in the present study, it is not known if these interactions at the NTS level exist in more chronic pathological conditions such as heart failure.
Acknowledgments
This study was supported by NIH grants RO-1 HL 077691 and PO-1 HL62222.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Longhurst JC, Tjen ALS, Fu LW. Cardiac sympathetic afferent activation provoked by myocardial ischemia and reperfusion. Mechanisms and reflexes. Ann N Y Acad Sci. 2001;940:74–95. doi: 10.1111/j.1749-6632.2001.tb03668.x. [DOI] [PubMed] [Google Scholar]
- 2.Malliani A, Pagani M, Pizzinelli P, Furlan R, Guzzetti S. Cardiovascular reflexes mediated by sympathetic afferent fibers. J Auton Nerv Syst. 1983;7:295–301. doi: 10.1016/0165-1838(83)90082-6. [DOI] [PubMed] [Google Scholar]
- 3.Wang W, Ma R. Cardiac sympathetic afferent reflexes in heart failure. Heart Fail Rev. 2000;5:57–71. doi: 10.1023/A:1009898107964. [DOI] [PubMed] [Google Scholar]
- 4.Zucker IH, Pliquett RU. Novel mechanisms of sympatho-excitation in chronic heart failure. Heart Fail Monit. 2002;3:2–7. [PubMed] [Google Scholar]
- 5.Gao L, Zhu Z, Zucker IH, Wang W. Cardiac sympathetic afferent stimulation impairs baroreflex control of renal sympathetic nerve activity in rats. Am J Physiol Heart Circ Physiol. 2004;286:H1706–H1711. doi: 10.1152/ajpheart.01097.2003. [DOI] [PubMed] [Google Scholar]
- 6.Gao L, Zucker IH, Schultz HD, Wang W. Augmented input from cardiac sympthetic afferents enhances the chemoreceptor reflex in HF rats. FASEB. 2005;19:A1288. [Google Scholar]
- 7.Gao L, Pan YX, Wang WZ, Li YL, Schultz HD, Zucher IH, Wang W. Cardiac sympathetic afferent stimulation augments the aterial chemoreceptor reflex in anesthetized rats. J Appl Physiol. 2006 doi: 10.1152/japplphysiol.00681.2006. (In Press) [DOI] [PubMed] [Google Scholar]
- 8.Spyer KM. Central nervous mechanisms contributing to cardiovascular control. J Physiol. 1994;474:1–19. doi: 10.1113/jphysiol.1994.sp019997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vardhan A, Kachroo A, Sapru HN. Excitatory amino acid receptors in commissural nucleus of the NTS mediate carotid chemoreceptor responses. Am J Physiol. 1993;264:R41–R50. doi: 10.1152/ajpregu.1993.264.1.R41. [DOI] [PubMed] [Google Scholar]
- 10.Cervero F. Sensory innervation of the viscera: peripheral basis of visceral pain. Physiol Rev. 1994;74:95–138. doi: 10.1152/physrev.1994.74.1.95. [DOI] [PubMed] [Google Scholar]
- 11.Kuo DC, Oravitz JJ, DeGroat WC. Tracing of afferent and efferent pathways in the left inferior cardiac nerve of the cat using retrograde and transganglionic transport of horseradish peroxidase. Brain Res. 1984;321:111–118. doi: 10.1016/0006-8993(84)90686-3. [DOI] [PubMed] [Google Scholar]
- 12.Menetrey D, Basbaum AI. Spinal and trigeminal projections to the nucleus of the solitary tract: a possible substrate for somatovisceral and viscerovisceral reflex activation. J Comp Neurol. 1987;255:439–450. doi: 10.1002/cne.902550310. [DOI] [PubMed] [Google Scholar]
- 13.Tjen AL, Bonham A, Longhurst J. Interactions between sympathetic and vagal cardiac afferents in nucleus tractus solitarii. Am J Physiol. 1997;272:H2843–H2851. doi: 10.1152/ajpheart.1997.272.6.H2843. [DOI] [PubMed] [Google Scholar]
- 14.Li DP, Averill DB, Pan HL. Differential roles for glutamate receptor subtypes within commissural NTS in cardiac-sympathetic reflex. Am J Physiol Regul Integr Comp Physiol. 2001;281:R935–R943. doi: 10.1152/ajpregu.2001.281.3.R935. [DOI] [PubMed] [Google Scholar]
- 15.Chitravanshi VC, Sapru HN. Chemoreceptor-sensitive neurons in commissural subnucleus of nucleus tractus solitarius of the rat. Am J Physiol. 1995;268:R851–R858. doi: 10.1152/ajpregu.1995.268.4.R851. [DOI] [PubMed] [Google Scholar]
- 16.Sundaram K, Murugaian J, Watson M, Sapru H. M2 muscarinic receptor agonists produce hypotension and bradycardia when injected into the nucleus tractus solitarii. Brain Res. 1989;477:358–362. doi: 10.1016/0006-8993(89)91427-3. [DOI] [PubMed] [Google Scholar]
- 17.Li DP, Pan HL. Responses of neurons in rostral ventrolateral medulla to activation of cardiac receptors in rats. Am J Physiol Heart Circ Physiol. 2000;279:H2549–H2557. doi: 10.1152/ajpheart.2000.279.5.H2549. [DOI] [PubMed] [Google Scholar]
- 18.Barros RC, Bonagamba LG, Okamoto-Canesin R, de Oliveira M, Branco LG, Machado BH. Cardiovascular responses to chemoreflex activation with potassium cyanide or hypoxic hypoxia in awake rats. Auton Neurosci. 2002;97:110–115. doi: 10.1016/s1566-0702(02)00050-4. [DOI] [PubMed] [Google Scholar]
- 19.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 3. Academic Press; New York: 1998. [Google Scholar]
- 20.Machado BH, Mauad H, Chianca Junior DA, Haibara AS, Colombari E. Autonomic processing of the cardiovascular reflexes in the nucleus tractus solitarii. Braz J Med Biol Res. 1997;30:533–543. doi: 10.1590/s0100-879x1997000400015. [DOI] [PubMed] [Google Scholar]
- 21.Aicher SA, Kurucz OS, Reis DJ, Milner TA. Nucleus tractus solitarius efferent terminals synapse on neurons in the caudal ventrolateral medulla that project to the rostral ventrolateral medulla. Brain Res. 1995;693:51–63. doi: 10.1016/0006-8993(95)00660-i. [DOI] [PubMed] [Google Scholar]
- 22.Bennett JA, McWilliam PN, Shepheard SL. A gamma-aminobutyric-acid-mediated inhibition of neurones in the nucleus tractus solitarius of the cat. J Physiol. 1987;392:417–430. doi: 10.1113/jphysiol.1987.sp016788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Potts JT. Inhibitory neurotransmission in the nucleus tractus solitarii: implications for baroreflex resetting during exercise. Exp Physiol. 2006;91:59–72. doi: 10.1113/expphysiol.2005.032227. [DOI] [PubMed] [Google Scholar]
- 24.Otake K, Nakamura Y, Ezure K. Projections from the commissural subnucleus of the solitary tract onto catecholamine cell groups of the ventrolateral medulla. Neurosci Lett. 1993;149:213–216. doi: 10.1016/0304-3940(93)90774-f. [DOI] [PubMed] [Google Scholar]
- 25.Ross CA, Ruggiero DA, Reis DJ. Projections from the nucleus tractus solitarii to the rostral ventrolateral medulla. J Comp Neurol. 1985;242:511–534. doi: 10.1002/cne.902420405. [DOI] [PubMed] [Google Scholar]
- 26.Koshiya N, Guyenet PG. NTS neurons with carotid chemoreceptor inputs arborize in the rostral ventrolateral medulla. Am J Physiol. 1996;270:R1273–R1278. doi: 10.1152/ajpregu.1996.270.6.R1273. [DOI] [PubMed] [Google Scholar]
- 27.Guyenet PG, Koshiya N. Working model of the sympathetic chemoreflex in rats. Clin Exp Hypertens. 1995;17:167–179. doi: 10.3109/10641969509087063. [DOI] [PubMed] [Google Scholar]
- 28.Zucker IH, Schultz HD, Li YF, Wang Y, Wang W, Patel KP. The origin of sympathetic outflow in heart failure: the roles of angiotensin II and nitric oxide. Prog Biophys Mol Biol. 2004;84:217–232. doi: 10.1016/j.pbiomolbio.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Matsumura K, Averill DB, Ferrario CM. Angiotensin II acts at AT1 receptors in the nucleus of the solitary tract to attenuate the baroreceptor reflex. Am J Physiol. 1998;275:R1611–R1619. doi: 10.1152/ajpregu.1998.275.5.R1611. [DOI] [PubMed] [Google Scholar]
- 30.Paton JF, Kasparov S. Differential effects of angiotensin II on cardiorespiratory reflexes mediated by nucleus tractus solitarii - a microinjection study in the rat. J Physiol 521 Pt. 1999;1:213–225. doi: 10.1111/j.1469-7793.1999.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boscan P, Paton JF. Nociceptive afferents selectively modulate the cardiac component of the peripheral chemoreceptor reflex via actions within the solitary tract nucleus. Neuroscience. 2002;110:319–328. doi: 10.1016/s0306-4522(01)00585-1. [DOI] [PubMed] [Google Scholar]


