Summary
Several naturally-occurring mutations in human luteinizing hormone receptors (LHR) at position 578 are associated with constitutive activation of the receptor. To determine whether human LHRs that signal in the absence of ligand are self-associated, fluorescence resonance energy transfer (FRET) between receptors was evaluated. Values for FRET between wild type LHR in the absence of ligand were less than 1% and increased significantly to over 11% after exposure to hCG. Constitutively active receptors exhibited 11-15% FRET efficiency in the absence of hormone and these values did not change with hCG treatment. A large fraction of constitutively active LHR-D578H receptors were also associated with so-called plasma membrane rafts. Disruption of these membrane microdomains reduced FRET efficiency but did not affect signaling through cAMP. Thus, in the absence of ligand, constitutively-active receptors are self-associated and located in high buoyancy membrane fractions, both characteristics of the hormone-treated wild type receptor.
Keywords: Luteinizing hormone receptor, plasma membrane rafts, signaling, fluorescence resonance energy transfer
Introduction
Functional luteinizing hormone (LH) receptors are critical to fertility in both males and females. In females, the LH receptor is found on granulosa and thecal cells in the follicle and on luteal cells. In males, the receptor is found on Leydig cells. Binding of LH from the anterior pituitary results in signaling cascades leading to follicle maturation, steroidogenesis or spermatogenesis. Mutations in the human luteinizing hormone (hLH) receptor aspartic acid residue at position 578 are associated with constitutive activation of Gs by the receptor [1] as well as with naturally-occurring pathologies, such as Familial Male-limited Precocious Puberty (FMPP) and Leydig cell adenomas [2].
Although details of G protein-coupled receptor signaling through intracellular mediators are increasingly well-characterized, membrane events involved in signaling are less understood including receptor interactions such as receptor dimer or oligomerization or interactions with other membrane proteins. Several lines of evidence suggest that functional LH receptors, i.e., receptors that have bound hormone and are actively transducing signal, are associated within large molecular weight structures following the binding of hormone. Electron micrographs of LHR on rat granulosa cells show large clusters of receptors that form only after binding of hormone [3] as does immunofluorescent labeling of rat receptors in granulosa cells [4]. Large clusters of wild type rat LH receptors tagged with green fluorescent protein (LHR-GFP) also form within minutes following binding of either LH or hCG to receptors on viable cells [5]. These clusters may reflect aggregation of receptors cis-activated by ligand or aggregated receptors in which ligand-occupied receptors have trans-activated nearby unliganded neighbors [6]. The presence of receptors in physically large structures is also suggested by lateral diffusion studies of hormone-treated LH receptors on luteal cells from sheep and rat in which most LH receptors were laterally immobile (reviewed in [7].
The LH receptor within these membrane clusters appears to be self-associated. Rat LH receptors become self-associated upon binding of either LH or hCG [7] and when desensitized in plasma membrane preparations from porcine granulosa cells [8]. Tao et al. [9] have used immunoprecipitation methods to show that some human LH receptors stably expressed in 293 cells exist as receptor dimers or oligomers and that the relative amounts of these receptor structures increases upon binding of hCG.
Receptor self-association may also be accompanied by a redistribution of the LH receptor within the plasma membrane. Upon binding of ligand, rat LH receptors partition into high buoyancy membrane fractions that can be isolated via density gradient centrifugation [7]. Because of their high lipid content, these specialized membrane microdomains or rafts are found in membrane fractions with low density and “float” in sucrose gradients. The outer leaflet of the raft membrane is enriched with sphingolipids and cholesterol as well as glycosylphosphatidyl-inositol (GPI)-anchored proteins and can limit the lateral diffusion of specific membrane proteins [10]. In addition to sequestering receptors, such domains may serve “signaling platforms” for a diverse group of signaling molecules [11] as well as G protein-coupled receptors such as the rat LH receptor [12] and the gonadotropin releasing hormone (GnRH) receptor [13]. The presence of membrane microdomains with higher affinity for activated, signaling receptors than the bulk membrane could also explain, at least in part, why receptor clustering occurs within minutes and, upon microscopic inspection, involves the movement of diffusely distributed LH receptors into discrete membrane locations [5]. In single particle tracking experiments of rat LH receptors on viable cells, the size of compartments accessed by hCG-treated receptors is reduced by over 60% [12]. Thus, the question raised by these various observations is whether constitutively-active hLHRs, but not wild type receptors, are self-associated in the absence of hormone, are restricted in their motions within the plane of the membrane and localized in membrane rafts involved in receptor-mediated signaling.
Materials and Methods
Materials
Dulbecco’s modified Eagle medium containing high glucose was purchased from Irvine Scientific, Santa Ana, CA. Geneticin was purchased from GIBCO, Grand Island, NY. Non-essential amino acids were purchased from Sigma-Aldrich, St. Louis, MO. Fetal bovine serum (FBS) was purchased from Invitrogen (Carlsbad, CA). hCG was purchased from Research Diagnostics Inc. (Flanders, NJ). Methyl-β-cyclodextrin (MβCD) and the FLAG vector were purchased from Sigma-Aldrich (St. Louis, MO). CFP and YFP vectors were purchased from Clontech. Vectors containing human LHR (LHR-wt) receptors or receptors with mutations D578G, D578H or D578Y were gifts from Dr. Andrew Shenker. Intracellular cAMP was measured using a TiterFluor cAMP EIA kit obtained from Assay Designs, Ann Arbor, MI.
Preparation and maintenance of CHO cells expressing visible fluorescent proteins (VFP) or epitope tags
Stable CHO cell lines expressing wild type human LHR receptors or receptors with mutations, D578G, D578H or D578Y, associated with constitutive activation, were coupled to one of the visible fluorescent proteins, either enhanced cyan fluorescent protein (CFP) or enhanced yellow fluorescent protein (YFP), at their C terminus using N-terminal Protein Fusion Vectors cECFP-Ca (6900-1) and pEYFP-C1 (6006-1), respectively. To prepare these cell lines, vectors were constructed for LHR-wt-CFP and -YFP, LHR-D578G-CFP and -YFP, LHR-D578H-CFP and -YFP, and LHR-D578Y-CFP and -YFP as previously described for transfection of rat LHR coupled to GFP [5]. CHO cells in 60 mm dishes at 40-80% confluence were transfected with 5μg DNA in 20μL lipofectamine. DNA was added as either a single vector or, to accomplish co-transfection of two vectors, at a 1:3 ratio of CFP:YFP. After 3-4 weeks, clones expressing both CFP and YFP were selected using fluorescence microscopy. To determine whether LHRs partitioned into membrane rafts, stable CHO cell lines were prepared expressing either LHR-wt or LHR-D578H coupled to the FLAG epitope at the receptor N terminus and were maintained in CHO cell medium [7].
Imaging analysis of FRET using fluorescence dequenching
To evaluate the effects of hCG treatment on energy transfer efficiency, flasks containing 3-4 × 106cells were selected. The medium was discarded and cells were removed from the flask using PBS containing 5mM EDTA, washed with 12mls of PBS, and spun down. The cell pellet was resuspended in 500 μL of PBS alone or in PBS containing 100nM hCG. The cells were then incubated at 37°C for 1 hour, washed once and resuspended in PBS for FRET measurements. Because aggregation of rat LH receptors is visible within minutes [5]and receptors clusters do not dissociate for several hours [14], it is likely that the extent of LH receptor self-association is relatively stable on the time-scale of these experiments. FRET between hLHR-CFP and hLHR-YFP was evaluated on individual cells by measuring the intensity of the plasma membrane localized fluorescence donor CFP in the presence and absence of a fluorescence acceptor YFP [15]. More intense signals from CFP, the fluorescence donor, after photobleaching of YFP, the fluorescence acceptor, were indicative of energy transfer from fluorescence donor to acceptor. For this donor-acceptor pair, the Förster r0is calculated to be 56Å [16]and energy transfer occurs to a measurable extent only when the donor and acceptor are separated by distances less than about 100Å. FRET measurements were made using a Zeiss Axiovert 135 microscope or a Zeiss Axiovert 200m microscope, Omega Optical filter sets for imaging of CFP and YFP and Metamorph software from Universal Imaging. Before photobleaching YFP, CFP and YFP were imaged separately using a Princeton Instruments1300YHS ICCD camera. Imaging the cell involved careful focusing through the entire cell and selection of an image plane that demonstrated “ring-like” fluorescence at the cell’s outermost plasma membrane. FRET measurements were made only on fluorescence emitted from the anular image region containing the plasma membrane. After photobleaching YFP for 5 minutes using a mercury arc lamp source and an Omega Optical XF1074 filter, YFP and CFP were imaged again. Five minute exposure to 525nm light was sufficient to bleach essentially all YFP signal. The intensity of CFP signals before and after YFP photobleaching was then compared. After subtracting the background signal from each image and correcting for the small extent of donor bleaching during the bleaching of acceptor, energy transfer efficiency was calculated using %E= (donor fluorescence after photobleaching-donor fluorescence before photobleaching/donor fluorescence after photobleaching) × 100.
Isolation of plasma membrane rafts
To evaluate the localization of LH receptors in membrane fractions with high buoyancy, stable cell lines were prepared that expressed either LHR-wt or LHR-D578H coupled to the FLAG epitope on their N-terminus as has been described for rat LHR-wt [7]. Cells were incubated with either 100nM hCG or PBS for 1 hour at 37°C prior to cell lysis. As previously described [7], 1 mL cell lysate was combined with 1ml of 80% sucrose and the sample was layered at 40% sucrose within a discontinuous sucrose gradient from 10-80%. After centrifugation at 175,000× g for 20 hours at 4°C, fractions were collected from the top of the gradient downward and an aliquots from each fraction were diluted 1:1 in Laemmli SDS buffer. After separation of proteins using SDS-PAGE and transfer to nitrocellulose, the LH receptor was identified using anti-FLAG M2 monoclonal antibody (Sigma-Aldrich, St. Louis, MO). In some experiments, cells were pretreated for 1 hr at 37°C with 1% MβCD in serum free DMEM medium prior to incubation with hCG or PBS.
Single particle tracking of FLAG-LHR-wt receptors on individual cells
Lateral dynamics and the size of domains accessed by individual FLAG-LHR-wt were evaluated using single-particle tracking methods as described by Kusumi and coworkers [17] and Smith et al. [12]. 40 nm nanogold particles were conjugated with the lowest possible concentration of anti-FLAG monoclonal antibody (mAb) and then incubated with CHO cells expressing FLAG-LHR-wt receptors at concentrations typically less 15 μg/mL to produce 1-4 gold particles per cell. The trajectories for individual gold particles were segmented into domains by calculation of statistical variance in particle position over times using a procedure similar to that developed by a number of investigators [18-20]. Results were analyzed to yield the domain size, the residence time for each particle and the effective macroscopic diffusion constants as described by Saxton [20].
Results
hLHR-wt become self-associated and translocate into membrane rafts following treatment with hormone
We used fluorescence dequenching of the fluorescence donor and imaging methods to examine conditions in which wild type human LHR were self-associated. This FRET method has a number of advantages, the most important being that all measurements of fluorescence emission from the fluorescence donor are accomplished on the same cell. To perform these experiments, CHO cells were stably cotransfected with both CFP- and YFP-coupled LH receptors. Stable transfection of LH receptors is reported to decrease immature or misfolded hLHR and increase cell membrane expression [9]. We imaged CFP and YFP fluorescence separately using fluorescence filter sets for these visible fluorescent proteins that minimized the fluorescence contribution from CFP when imaging YFP [15] and that, in subsequent steps, permitted photobleaching of YFP only. Following photobleaching, each cell was reimaged using the same filter sets. As shown in Figure 1, after photobleaching of YPP, an increase in CFP dequenching and thus a brighter CFP image was obtained when there was energy transfer between the visible fluorescence proteins.
Figure 1.

Photobleaching of the fluorescence acceptor in evaluation of FRET. This figure shows representative images from a single cells before and after photobleaching of YFP. As shown in the lower right panel, photobleaching of YFP eliminates essentially all YFP signal and increases the CFP signal as a result of CFP dequenching. In this examine, energy transfer efficiency was 24%.
We used two methods to analyze data from these studies (Figure 2). In both methods, background fluorescence was subtracted from the four images obtained in each individual experiment. This involved selection of a cell-free site on the complete image and subtraction of the average intensity of that site from the complete image. This typically produced images of cells on a dark background. The first method used for analysis of fluorescence donor intensity averaged fluorescence intensity from the entire cell before and after photobleaching to calculate energy transfer efficiency. Figure 2 shows donor fluorescence from a representative cell before and after photobleaching of the fluorescence acceptor. We compared this method with one in which only fluorescence from the periphery of the cell was used. In the example shown in Figure 2, the average intensity before photobleaching from the entire cell as compared to “plasma membrane only” was reduced from 1530 cps to 487 cps. After photobleaching, values increased to 1753 cps and 560 cps. Energy transfer efficiency (%E) was not statistically different (12.7% and 12.9%, respectively) using these two methods for analysis of FRET. More generally, examining only the plasma membrane of each of the cells lines used for FRET analysis produced a decrease in the mean and S.D. of 2.3 ± 1.4% for %E (data not shown) which was not significant while reducing the overall number of photon counts used to evaluate FRET.
Figure 2.
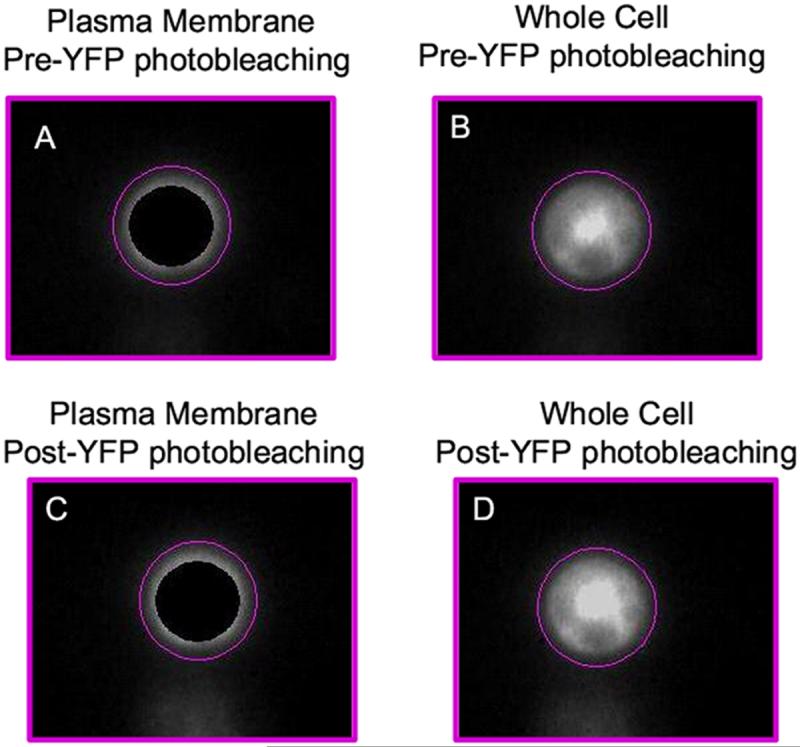
A comparison of FRET efficiencies from a whole cell and from a portion of the plasma membrane. Two methods were used to evaluate the extent of energy transfer efficiency. Panels A and C show donor fluorescence from periphery of the cell before and after acceptor photobleaching. Panels B and D show donor fluorescence from entire cell before and after acceptor photobleaching. In this example, the values for energy transfer efficiency (%E) were 12.7% and 12.9%, respectively.
A summary of results using the whole cell imaging method is shown in Table I. In the absence of ligand, values for energy transfer efficiency between LHR-wt receptors were, on average, less than 1%. Energy transfer efficiency increased significantly to 11.5 ± 1.2 % following exposure of LHR-wt to 100 nM hCG. Since energy transfer occurs to a measurable extent only when the CFP (donor) and YFP (acceptor) are separated by distances less than about 100 Å[16], FRET results indicate that wild type human LHRs, if occupied by a functional ligand, undergo receptor self-association.
Table I.
Efficiency of Fluorescence Energy Transfer between Wild Type LH receptors and Constitutively Active Receptors
| Cell Line | Ligand | Treatment | %Efficiency | n |
|---|---|---|---|---|
| LHR-wt | none | none | 0.8 ± 1.3 | 17 |
| 100 nM hCG | none | 11.5 ± 1.2 | 24 | |
| LHR-DG | none | none | 15.3 ± 1.9 | 11 |
| 100 nM hCG | none | 11.1 ± 2.5 | 14 | |
| LHR-DH | none | none | 13.8 ± 1.6 | 39 |
| 100 nM hCG | none | 13.0 ± 1.3 | 23 | |
| none | 1% mβCD, 45 min | 8.0 ± 1.4 | 15 | |
| LHR-DY | none | none | 11.7 ± 2.2 | 14 |
| 100 nM hCG | none | 14.1 ± 4.3 | 11 |
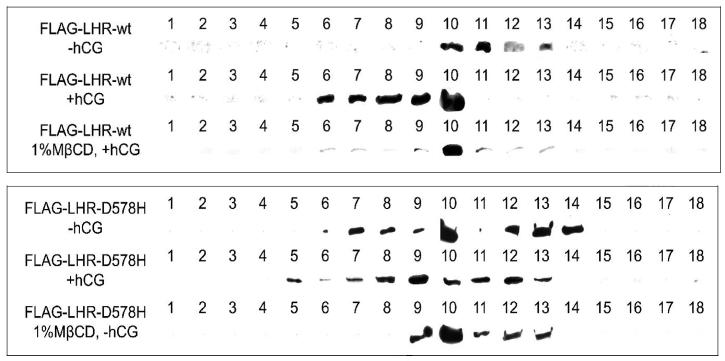
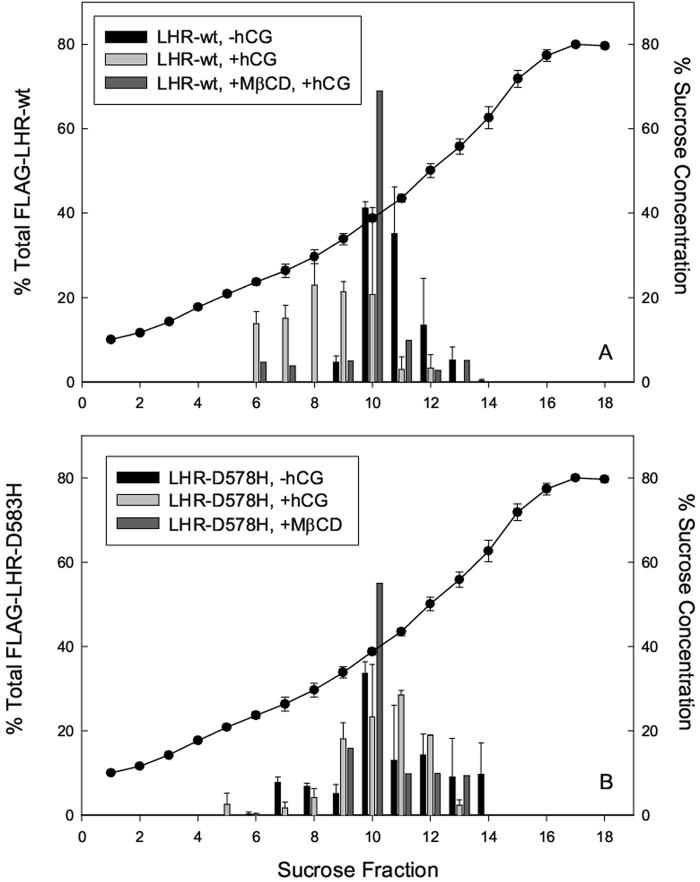
LHR-wt receptors also translocated from the bulk plasma membrane into high buoyancy membrane fractions (rafts) following ligand treatment (Figure 3 and upper panel of Figure 4). Following isopycnic centrifugation of plasma membrane fractions from CHO cells, over 95% of unoccupied receptors were found fractions containing 39-56% sucrose. About 75% of hCG-treated FLAG-hLHR-wt consistently appeared in sucrose fractions containing 24-34% sucrose and thus had “floated” to lower sucrose densities during centrifugation. The remaining receptors were found in fractions containing higher sucrose concentrations. To further demonstrate the presence of hormone-treated LH receptors within rafts, we treated cells with 1% MβCD, a cholesterol-sequestering reagent that is efficient in removing cholesterol from the plasma membranes of live cells and that disrupts raft structure (Figure 3 and the upper panel of Figure 4). At low concentrations MβCD extracts membrane cholesterol by placing it in a central non-polar cavity of cyclic oligomers of glucopyranoside in 1,4 glycosidic linkages [21] without affecting cell viability (data not shown). Pretreatment of CHO cells with MβCD followed by exposure to 100 nM hCG reduced the relative number of receptors in fractions containing less than 34% sucrose from 75% to 13%, presumably by disrupting membrane microdomains.
Figure 3.
Identification of FLAG-tagged receptors in membrane fractions with high buoyancy. Representative western blots obtained from cell samples separated on sucrose density gradients demonstrate that FLAG-LHR-wt receptors (upper images) are localized in high density membrane fractions before exposure to hCG and appear in fractions containing lower sucrose concentrations after treatment of CHO cells with 100 nM hCG. Disruption of plasma membrane rafts by 1% MβCD membrane prevented translocation of FLAG-LHR-wt into low density sucrose fractions. Images in the lower panel demonstrate that a constitutively active human LH receptors LHR-D578H appear in low density membrane fractions both in the absence and presence of hCG. Disruption of plasma membrane rafts by extraction of cholesterol from the plasma membrane causes a redistribution of receptors into lower density sucrose fractions.
Figure 4.
Evaluation of raft localization for LHR-wt and LHR-F578H. The relative amounts of either LHR-wt or LHR-D578H in each sucrose fraction were evaluated after preparation of western blots using densitometry. Results shown are the mean and standard error of the mean (S.E.M.) for at least 2 separate experiments. The sucrose concentration in each fraction obtained from a sucrose gradient following centrifugation was evaluated in five separate experiments using a Baush and Lomb refractometer together with a standard curve. Because, for any given fraction, the sucrose concentration did not vary appreciably from experiment to experiment, the average sucrose concentration for five representative experiments is shown.
Constitutively-active human LHR are self-associated in the absence of hormone
In contrast to wild type human LH receptors, constitutively active receptors in the absence of hormone exhibited relatively high values for fluorescence energy transfer efficiency that ranged from about 11-15% (Table I). Hormone treatment had no statistically significant effect on FRET values. Following treatment of cells with 100 nM hCG, energy transfer between LHR-D578Y increased from 11.7 ± 2.2 % to 14.1 ± 4.2% while energy transfer efficiency for cells expressing LHR-D578H decreased slightly. The absence of any statistically significant effect suggests that there was little, if any, overall change in the extent of receptor association when constitutively active receptors were occupied by ligand.
MβCD treatment disrupts plasma membrane rafts and reduces the extent of energy transfer between FLAG-LHR-D578H receptors but does not affect cAMP signaling
To examine localization of LHR-D578H in rafts, the receptor was epitope-tagged on the N terminus using FLAG. Western blots of sucrose gradient fractions obtained from isopycnic ultracentrifugation showed approximately 20-25% of FLAG-LHR-D578H associated with sucrose fractions containing 24-34% sucrose both before and after binding of hCG (Figure 4, lower panel). As expected, MβCD treatment shifted the constitutively active receptor to lower buoyancy membrane fractions which was consistent with disruption of membrane rafts (Figures 3 and 4, lower panel).
Because it has been suggested that plasma membrane rafts may concentrate receptors and other plasma membrane molecules necessary for the transduction of a productive ligand-mediated signal, we examined whether disruption of membrane rafts reduced or eliminated cAMP signaling by FLAG-LHR-D578H cells. Interestingly, there was no decrease in cell signaling via cAMP following MβCD treatment (Table II) suggesting that localization of LHRs to rafts may not be required for receptor signaling. There was, however, a significant decrease in the extent of receptor self association from an average of 14% to 8% in MβCD-untreated cells (Table I).
Table II.
cAMP responsiveness of cells expressing FLAG-LHR-wt and FLAG-LHR-D578H1
| Cell Line | Treatment | Fold Increase over basal cAMP levels | n |
|---|---|---|---|
| LHR-wt | None | 1.0a | 9 |
| LHR-wt | 100 nM hCG | 1.6 ± 0.1b | 9 |
| LHR-D578H | None | 2.1 ± 0.3b | 9 |
| LHR-D578H | 1% MβCD | 2.9 ± 0.6b | 6 |
CHO cells expressing LHR-wt were assayed for cAMP expression following treatment with 100 nM hCG or following pretreatment of cells expressing LHR-D578H with MβCD. Results are expressed as the mean of the relative increase in cAMP ± S.E.M. compared to basal levels of cAMP in untreated CHO cells expressing LHR-wt receptors.
Means with different superscripts differ (p<0.03).
Means with different superscripts differ (p<0.03).
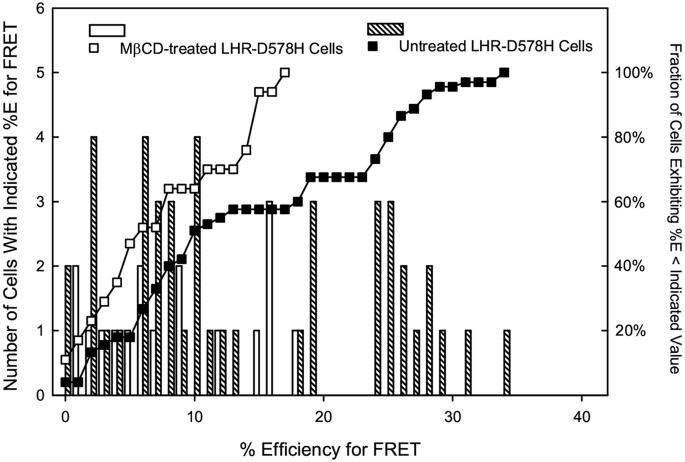
A more detailed analysis of this decrease in FRET efficiency is presented in Figure 5 which shows individual FRET values for both untreated and MβCD-treated cells. The efficiency of energy transfer for untreated and MβCD-treated cells varied on a cell-to-cell basis over a fairly large range. When LHR-D578H cells were treated with MβCD, higher values for FRET efficiency were absent as reflected in the left shift in accumulated FRET values. One explanation for this result is that there are two populations of self-associated, constitutively-active receptors on LHR-D578H cells with more extensive receptor self-association occurring in membrane rafts. The persistence of self-associated receptors in the bulk membrane following cell treatment with MβCD may be sufficient to maintain adenylate cyclase activation and elevated levels of cAMP in cells.
Figure 5.
Effects of MβCD on the distribution of FRET values. Individual FRET measurements for either untreated CHO cells expressing LHR-D578H or cells treated with MβCD were plotted. In the absence of MβCD, energy transfer efficiency ranged from 3% to 36%. Following treatment of cells with MβCD, only energy transfer efficiency was, on average lower, and high extents of energy transfer were no longer observed.
Single particle tracking of hCG-occupied FLAG-LHR-wt receptors demonstrates “trapping” of receptors in small membrane compartments
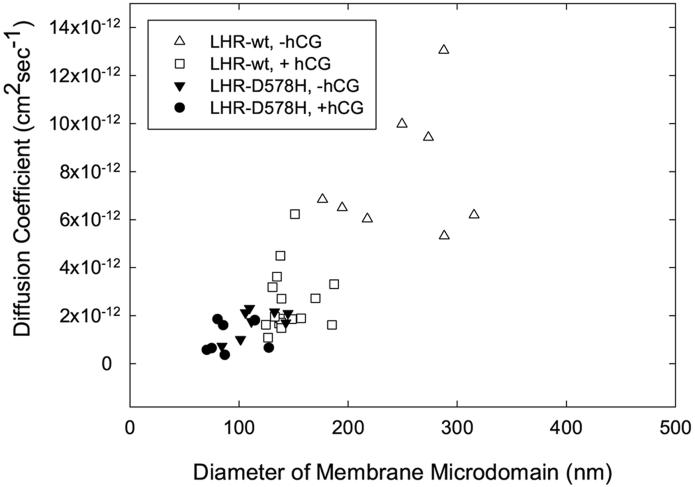
To independently assess the localization of hCG-treated FLAG-LHR-wt in membrane compartments, single particle tracking methods were used. This technique identifies individual LH receptors on the surface of viable cells and tracks their motions over approximately two minutes. The image of a 40 nm gold particle attached to an individual receptor can be identified visually on video obtained from each experiment and the motions of its centroid can be quantitatively described. hCG treatment reduced the average diameter of compartments containing human FLAG-LHR-wt approximately 2-fold from 309 ± 131 nm to 146 ± 119 nm as well as the diffusion coefficient for receptors within compartments (Figure 6 and Table III). For both unoccupied and hormone-treated FLAG-LHR-D578H, the compartments containing receptors were small with domain diameters, Lr, of 114 ± 22 nm and 94 ± 33 nm, respectively. Diffusion coefficients within these small compartments (0.2 × 10-11cm2sec-1 and 0.1 × 10- 11cm2sec-1, respectively) were comparable to those obtained for hCG-treated FLAG-LHR-wt (0.3 × 10- 11cm2sec-1).
Figure 6.
Single particle tracking of individual FLAG-LHR-wt and FLAG-LHR-D578H receptors labeled with gold-conjugated anti-FLAG antibody. The compartment size and diffusion coefficient for the LH receptor within that compartment were calculated as described in Methods and Materials. Results shown are from CHO cells expressing untreated FLAG-LHR-wt receptors (△), FLAG-LHR-wt receptors treated with hCG after labeling of receptors with gold-conjugated anti-FLAG antibody (□), untreated CHO cells expressing FLAG-LHR-D578H (▼) or cells expressing FLAG-LHR-D578H that were treated with hCG after labeling of receptors with gold-conjugated anti-FLAG antibody. Data presented in this figure were obtained for 8 particles on individual cells.
Table III.
Tracking of individual anti-FLAG-gold particles bound to LHR-wt or LHR-D578H on CHO cells.
| Cell line | Hormone | Number of particles analyzed | Number of domains/ 2 min trajectory | D0-1a (10-11cm2sec-1) | D = Lr2/4tb (10-11cm2sec-1) | Timec (sec) | Domain Diameter(Lr)c |
|---|---|---|---|---|---|---|---|
| LHR-wt | none | 8 | 4 ± 1 | 5.1 ± 2.1 | 1.3 ± 1.1 | 27 ± 9 | 309 ± 131 |
| LHR-wt | hCG | 8 | 4 ± 1 | 1.7 ± 0.8 | 0.3 ± 0.1 | 25 ± 11 | 146 ± 19 |
| LHR-D578H | none | 8 | 4 ± 1 | 0.7 ± 0.3 | 0.2 ± 0.1 | 23 ± 11 | 114 ± 22 |
| LHR-D578H | hCG | 8 | 4 ± 1 | 0.7 ± 0.1 | 0.1 ± 0.1 | 19 ± 8 | 94 ± 33 |
D0-1: Diffusion coefficient of the first 2 points [19]
D presents the diffusion coefficient within a domain as described by Saxton [20].
The average diameter of an individual domain was calculated as described by Daumas et al. [19] and Murase et al. [18].
Average time for residence within a domain
Discussion
In addition to exhibiting unregulated signaling, constitutively-active LH receptors have characteristics of hormone-treated, actively signaling wild type human receptors and rat LH receptors [12]. These receptors are self-associated, present in small membrane compartments on viable cells and isolated within plasma membrane rafts. The extent of receptor self-association is characteristic of wild type rat [14] and human LH receptors treated with a high concentration of hCG with values for energy transfer efficiency ranging from 11-15%. The LHR-D578H receptor also appears to be distributed into sucrose fractions with densities comparable to those in which hormone-treated rat [12] or human LHR-wt receptors are found.
We find no evidence for extensive self-association of the LHR-wt receptor on the viable cells used in these studies prior to binding of ligand. This is in contrast to reports by Tao et al. [9] and Urizar et al. [22]. Whether this is because of differences in the experimental approaches used by these investigators or other factors such as receptor density is difficult to determine. However, in our hands, results with human LH receptors are consistent with those seen for rat LH receptors [14] using a related fluorescence method to evaluate FRET on intact, viable cells. Moreover, the evaluation of single particle tracking of individual LH receptors also suggests that extensive aggregation of the receptor has not occurred prior to hormone binding. Although lateral diffusion measurements are only weakly correlated with the size of the diffusing 9 structure, it appears that prior to hormone treatment, wild-type LH receptors are diffusing freely in the membrane over large distances while hormone-treated receptors are significantly restricted in their ability to move laterally in the plane of the membrane. The restriction of LH receptor motions may be due to extensive receptor self association, aggregation of the receptor into large structures containing other proteins, receptor anchoring by, for example, cytoskeletal components, or confinement of the receptor by membrane architectural features such as rafts.
Unlike constitutively active LHR-D578H receptors, human wild type receptors undergo translocation into rafts upon binding of ligand. In addition to rat and human LH receptors, there are a number of examples of plasma membrane receptors that are transiently associated with rafts during signaling. As examples, the β-adrenergic receptor becomes transiently associated with lipid rafts together with nitric oxide synthase and adenylate cyclase [23]and requires the raft environment for adenylate cyclase activity by Gs. The Type I Fc, receptor is also transiently associated with lipid rafts and exhibits increased signaling capability in that environment. Baird and coworkers suggest that, although the Type I Fc, receptor appears to be associated with Lyn in the bulk membrane, Lyn has low activity under these conditions [24]. Rather, it is translocation of Type I Fc, receptors together with Lyn into the raft environment together as well as aggregation of the Fc, receptor that promotes the coupling of Type I Fc, receptors with Lyn phosphorylation. There are also examples of constitutive association of proteins with rafts including gonadotropin releasing hormone receptor, a G protein-coupled receptor [13].
Factors driving translocation of either the wild type receptor into membrane rafts or residence of constitutively-active receptors within these structures are not known. Although the possibility remains that factors regulating LH receptor association with rafts differ for wild type and LHR-D578H, these studies indicate that association of LH receptors with rafts does not involve direct interactions between glycosylated hCG and raft components. While binding of hCG to the wild type receptor is necessary for translocation of the receptor into raft domains, the increase in association of the LHR-D578H with raft domains following addition of hormone is small, approximately 6% of the total receptor number. Moreover, studies of an rat LH receptor with a point mutations in the sixth transmembrane domain [12] suggest that these receptors, which exhibit little if any capacity to signal, remain localized in bulk membrane fractions despite binding of hCG.
On the other hand, conformational differences between the wild type and constitutively active receptor may be sufficient to promote constitutive interactions between the receptor and raft components. An appropriate conformation for the constitutively active receptor and acquisition of a similar conformation by the hormone-occupied wild type receptor may increase receptor affinity for raft-associated proteins. It is also possible that a critical receptor conformation is needed for association of ligand-activated receptors with other non-receptor raft components and it is these interactions that result in retention of the receptor in rafts.
Alternatively, structural modification of receptors such as lipidation could cause preferential localization of the receptor in the cholesterol and spingomyelin-rich outer leaflet that defines the raft environment. There is evidence of palmitoylation of the wild type rat LH receptor upon binding of hormone [25] and both the constitutively active human LH receptor and the human wild type receptor contain the palmitoylation motif. Menon and coworkers [26,27] have suggested that rat LH receptor depalmitoylation may be involved in internalization of the receptors: wild type receptors, which are more extensively palmitoylated, are also more rapidly internalized than a less palmitoylated, constitutively-active mutant. Whether palmitoylation is a dynamic process, occurring in response to, for example, binding of ligand, is not known although there are examples of other G-protein coupled receptors for which this process is dynamic [28]. Moreover, palmitoylation of other membrane proteins such as Thy-1 [17] and linker for activation (LAT) of T cells [29] is necessary for retention in low density membrane fractions.
In these studies, a minimum of two events appear to be necessary for signaling by the LH receptor. First, productive signaling by a hormone occupied receptor appears to involve receptor-receptor interactions that produce comparatively high positive values for energy transfer efficiency. Second, migration to the raft environment or retention in small membrane compartments may also play a role in signaling. Although we anticipated that disruption of lipid rafts would impair signaling by constitutively active LH receptors, as it did for rat LHR-wt receptors [12], this was not the case. Rather, receptor-mediated cAMP accumulation by the constitutively-active receptor was not affected by cholesterol depletion although it did reduce the extent of receptor-receptor interaction. Thus, it remains possible that the key event in signaling by the constitutively-active receptor, in contrast to the wild type receptor, is establishing and maintaining receptor interactions rather than their membrane microenvironment.
Acknowledgments
We would like to thank Daniel Gilsdorf for his help with analysis of fluorescence resonance energy transfer results. This work was supported, in part, by NIH ND23236 (D.A.R.) and NSF DBI-0138322 (B.G.B.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Abell AN, McCormick DJ, Segaloff DL. Certain activating mutations within helix 6 of the human lutenizing hormone receptor may be explained by alterations that allow transmembrane regions to activate Gs. Molecular Endocrinology. 1998;12:1857–1869. doi: 10.1210/mend.12.12.0202. [DOI] [PubMed] [Google Scholar]
- [2].G. Liu, L. Duranteau, Carel J-C, J. Monroe, Doyle DA, A. Shenker. Leydig-cell tumors caused by an activating mutation of the gene encoding the luteinizing hormone receptor. The New England Journal of Medicine. 1999;341:1731–1736. doi: 10.1056/NEJM199912023412304. [DOI] [PubMed] [Google Scholar]
- [3].Luborsky JL, Slater WT, Behrman HR. Luteinizing hormone (LH) receptor aggregation: Modification of ferritin-LH binding and aggregation by prostaglandin F2αand ferritin-LH. Endocrinology. 1984;115:2217–2226. doi: 10.1210/endo-115-6-2217. [DOI] [PubMed] [Google Scholar]
- [4].Podestá EJ, Solano AR, Sánchez ML. Luteinizing hormone triggers two opposite regulatory pathways through an initial common event, receptor aggregation. Endocrinology. 1986;119:989–997. doi: 10.1210/endo-119-3-989. [DOI] [PubMed] [Google Scholar]
- [5].Horvat RD, S. Nelson, Clay CM, Barisas BG, Roess DA. Intrinsically fluorescent luteinizing hormone receptor demonstrates hormone-driven aggregation. Biochem. Biophys. Res. Comm. 1999;255:382–385. doi: 10.1006/bbrc.1999.0185. [DOI] [PubMed] [Google Scholar]
- [6].I. Ji, C. Lee, Y. Song, P. Conn, T. Ji. Cis- and trans-activation of hormone receptors: the LH receptor. Molecular Endocrinology. 2002;16:1299–1308. doi: 10.1210/mend.16.6.0852. [DOI] [PubMed] [Google Scholar]
- [7].Roess DA, Smith SML. Self-association and raft localization of functional luteinizing hormone receptors. Biol Reprod. 2003;69:1765–1770. doi: 10.1095/biolreprod.103.018846. [DOI] [PubMed] [Google Scholar]
- [8].M. Hunzicker-Dunn, Barisas BG, J. Song, Roess DA. Membrane organization of luteinizing hormone receptors differs between actively signaling and desensitized receptors. J. Biol. Chem. 2003;278:42744–42749. doi: 10.1074/jbc.M306133200. [DOI] [PubMed] [Google Scholar]
- [9].Tao Y-X, Johnson NB, Segaloff DL. Constitutive and agonist-dependent self-association of the cell surface human lutropin receptor. J. Biol. Chem. 2004;279:5904–5914. doi: 10.1074/jbc.M311162200. [DOI] [PubMed] [Google Scholar]
- [10].M. Edidin. The state of lipid rafts: from model membranes to cells. Annual Review of Biophysics and Biomolecular Structure. 2003;32:257–283. doi: 10.1146/annurev.biophys.32.110601.142439. [DOI] [PubMed] [Google Scholar]
- [11].K. Simons, D. Toomre. Lipid rafts and signal transduction. Nature Reveiws, Molecular Cell Biology. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- [12].Smith SML, Y. Lei, J. Liu, Cahill ME, Hagen GM, B. Cherrington, Barisas BG, Roess DA. (under review) Luteinizing hormone receptors translocate to plasma membrane microdomains followingbinding of human chorionic gonadotropin. [DOI] [PubMed]
- [13].Navratil AM, Bliss SP, Berghorn KA, Haughian JM, Farmerie TA, Graham JK, Clay CM, Roberson MS. Constitutive localization of the gonadotropin-releasing hormone (GnRH) receptor to low density membrane microdomains is necessary for GnRH signaling to ERK. J. Biol. Chem. 2003;278:31593–31602. doi: 10.1074/jbc.M304273200. [DOI] [PubMed] [Google Scholar]
- [14].Horvat RD, Barisas BG, Roess DA. Luteinizing hormone receptors are self-associated in slowly diffusing complexes during receptor desensitization. Molecular Endocrinology. 2001;15:534–542. doi: 10.1210/mend.15.4.0622. [DOI] [PubMed] [Google Scholar]
- [15].J. Llopis, S. Westin, M. Ricote, Z. Wang, Cho CY, R. Kurokawa, Mullen T-M, Rose DW, Rosenfled MG, Tsien RY, Glass CK. Ligand-dependant interactions of coactivators steroid receptor coactivator-1 and peroxisome proliferator-activated receptor binding protein with nuclear hormome receptors can be imaged in live cells and are required for transcription. PNAS. 2000;97:4363–4368. doi: 10.1073/pnas.97.8.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Patterson GH, Piston DW, Barisas BG. Förster distances between green fluorescent protein pairs. Analytical Biochemistry. 2000;284:438–440. doi: 10.1006/abio.2000.4708. [DOI] [PubMed] [Google Scholar]
- [17].C. Dietrich, B. Yang, T. Fujiwara, A. Kusumi, K. Jacobson. Relationship of lipid rafts to transient confinement zones detected by single particle tracking. Biophys J. 2002;82:274–84. doi: 10.1016/S0006-3495(02)75393-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].K. Murase, T. Fujiwara, Y. Umemura, K. Suzuki, R. Iino, H. Yamashita, M. Saito, H. Murakoshi, K. Ritchie, A. Kusumi. Ultrafine membrane compartments for molecular diffusion as revealed by single molecule techniques. Biophys J. 2004;86:4075–4093. doi: 10.1529/biophysj.103.035717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].F. Daumas, N. Destainville, C. Millot, A. Lopez, D. Dean, Salomé L. Confined diffusion without fences of a G protein coupled receptor as revealed by single particle tracking. The Biophysical Journal. 2003;84:356–366. doi: 10.1016/S0006-3495(03)74856-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Saxton MJ. Single-particle tracking: the distribution of diffusion coefficients. Biophys J. 1997;72:1744–1753. doi: 10.1016/S0006-3495(97)78820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].S. Ilangumaran, Hoessli DC. Effects of cholesterol depletion by cyclodextrin on the sphingolipid microdomains of the plasma membrane. Biochemistry Journal. 1998;335:433–40. doi: 10.1042/bj3350433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].E. Urizar, L. Montanelli, T. Loy, M. Bonomi, S. Swillens, C. Gales, M. Bouvier, G. Smits, G. Vassart, S. Costagliola. Glycoprotein hormone receptors: link between receptor homodimerization and negative cooperativity. Embo J. 2005;24:1954–1964. doi: 10.1038/sj.emboj.7600686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ostrom RS, Bundey RA, Insel PA. Nitric oxide inhibition of adenylyl cyclase type 6 activity is dependent upon lipid rafts and caveolin signaling complexes. J Biol Chem. 2004;279:19846–19853. doi: 10.1074/jbc.M313440200. [DOI] [PubMed] [Google Scholar]
- [24].Young RM, D. Holowka, B. Baird. A lipid raft environment enhances Lyn kinase activity by protecting the active site tyrosine from dephosphorylation. J. Biol. Chem. 2003;278:20746–20752. doi: 10.1074/jbc.M211402200. [DOI] [PubMed] [Google Scholar]
- [25].Menon KMJ, Munshi UM, Clouser CL, Nair AK. Regulation of luteinizing hormone/human chorionic gonadotropin receptor expression: a perspective. Biol. Reprod. 2004;70:861–866. doi: 10.1095/biolreprod.103.024471. [DOI] [PubMed] [Google Scholar]
- [26].Bradbury FA, Menon KMJ. Evidence that constitutively active luteinizing hormone/human chorionic gonadotropin receptors are rapidly internalized. Biochemistry. 1999;38:8703–8712. doi: 10.1021/bi990169t. [DOI] [PubMed] [Google Scholar]
- [27].Munshi UM, H. Peegel, Menon KM. Palmitoylation of the lutenizing hormone/human chorionic gonadotropin receptor regulates reseptor interaction with the arrestin-mediated internalization pathway. Eur. J. Biochem. 2001;268:1631–1639. doi: 10.1046/j.1432-1327.2001.02032.x. [DOI] [PubMed] [Google Scholar]
- [28].R. Qanbar, M. Bouvier. Role of palmitoylation/depalmitoylation reactions in G-protein-coupled receptor function. Pharmacological Therapeutics. 2003;97:1–33. doi: 10.1016/s0163-7258(02)00300-5. [DOI] [PubMed] [Google Scholar]
- [29].H. Shogomori, Hammond AT, Ostermeyer-Fay AG, Barr DJ, Feigenson GW, E. London, Brown DA. Palmitoylation and intracellular domain interactions both contribute to raft targeting of linker for activation of T cells. J Biol Chem. 2005;280:18931–18942. doi: 10.1074/jbc.M500247200. [DOI] [PubMed] [Google Scholar]