Abstract
Rice (Oryza sativa L.) transcription factors RF2a and RF2b are bZIP (basic leucine zipper) proteins that interact with, and activate transcription from the RTBV (rice tungro bacilliform virus) promoter. Here we characterize the C-terminal domains of RF2a and RF2b: these domains are rich in glutamine and proline/glutamine, respectively. Affinity pull-down assays demonstrated that the C-terminal domains of RF2a and RF2b can associate to form either homodimers or heterodimers; however, they do not interact with other domains of RF2a or RF2b. Results of in vitro transcription assays using a rice whole-cell extract demonstrate that the C-terminal domains of both RF2a and RF2b activate transcription from the RTBV promoter. In addition, dimerization of the RF2a C-terminal domain is involved in regulating the transcription activation function of RF2a. The predicted helical region within the RF2a C-terminal glutamine-rich domain was determined to be involved in inter-molecular dimerization, and contributed to the regulatory functions of RF2a in these assays.
Keywords: basic leucine zipper protein (bZIP protein), glutamine-rich domain, proline/glutamine-rich domain, protein–protein interaction, rice (Oryza sativa L.), transcription activation
Abbreviations: A domain, acidic domain; bZIP, basic leucine zipper; CaMV, cauliflower mosaic virus; DTT, dithiothreitol; GST, glutathione S-transferase; P domain, proline-rich domain; P/Q domain, proline/glutamine-rich domain; Q domain, glutamine-rich domain; RTBV, rice tungro bacilliform virus; SPR, surface plasmon resonance; TBP, TATA-box-binding protein
INTRODUCTION
RTBV [rice (Oryza sativa L.) tungro bacilliform virus] is a DNA virus and is the causal agent of rice tungro disease. RTBV possesses a single promoter that confers predominant phloem-tissue-specific expression of reporter genes [1,2]. Multiple cis elements have been identified in a fragment of the RTBV promoter referred to as the E fragment, comprising nt −164 to +45 relative to the transcription start site. Among these the Box II element, which includes nt −53 to −39, was shown to be unique and essential for promoter activity [2–5].
Rice bZIP (basic leucine zipper) transcription activators RF2a and RF2b were initially identified by their capacity to bind to Box II, and were subsequently cloned and partially characterized [6–9]. RF2a and RF2b possess sequence features that are known to play significant roles in regulating transcription [10]. RF2a has three putative regulatory domains, namely a P domain (proline-rich domain) and an A domain (acidic domain) N-terminal to the bZIP domain, and a Q domain (glutamine-rich domain) C-terminal of the bZIP domain [6]. RF2b has acidic and serine/alaninerich domains N-terminal to the bZIP domain, and a P/Q domain (proline/glutamine-rich domain) and a short serine-rich stretch C-terminal of the bZIP domain [9]. Results of transient assays in tobacco BY-2 cells showed that the acidic domain of RF2a is essential for activating transcription, while the Q domain exhibits a modest activation effect under certain experimental conditions [8]. in vitro assays showed that the Q domain of RF2a functionally interacts with TBP (TATA-box-binding protein) [8]. Expression of the Q domain linked with the bZIP domain in transgenic tobacco and Arabidopsis plants resulted in stunting of the plants that was different from the phenotype that is caused by expression of the bZIP domain alone [8] (S. Dai and R.N. Beachy, unpublished work). Thus we hypothesized that the Q domain of RF2a plays an important role in regulating gene expression.
Initiation of transcription by RNA polymerase II involves multiple factors that interact in a co-ordinated manner to effectively control RNA synthesis [11–14]. The Q domains of other characterized transcription factors can activate or repress transcription through interactions with general transcription factors, cofactors and other proteins. For example, the Q domain of Sp1 is critical for transcription activation and multimerization of Sp1 [15–17]. Q domains can interact with TBP, cofactors dTAFII110 and hTAFII130 and other proteins and mediate transcription activation through such interactions [18–24]. The P/Q domains were reported to be involved in protein dimerization and transcriptional repression [25,26].
The C-terminal domains of RF2a and RF2b are rich in glutamine; in the present study we have explored their roles in protein–protein interactions and their function in regulating transcription. Affinity pull-down assays and in vitro transcription assays indicated that the Q domain of RF2a forms homodimers, as does the P/Q domain of RF2b. Our studies also revealed that the Q and P/Q domains interact with each other to form heterodimers but did not interact with N-terminal domains of RF2a or RF2b. We also show that the C-terminal domains of RF2a and RF2b are involved in activating transcription of the RTBV promoter in vitro. A subdomain of the Q domain of RF2a is critical in domain dimerization and activation of transcription. Our studies provide insight into the involvement of functional domains in the inter-molecular interaction of bZIP transcription factors, and the interaction's potential impact on transcription regulation.
MATERIALS AND METHODS
Plasmid construction
To construct plasmids that produce full-length or truncated mutants of RF2a fused with GST (glutathione S-transferase), RF2a and deletion mutants fused with GST, the DNA coding sequences were amplified from plasmid p35S:RF2a [29] with primers listed in the supplementary data at http://www.BiochemJ.org/bj/405/bj4050243add.htm). A BamHI restriction site was added to the 5′-end primers. A stop codon and an EcoRI restriction site were added to all 3′-end primers. PCR-amplified fragments were cloned into pGEX-4T-1 (Amersham Biosciences) through BamHI and EcoRI sites to produce GST fusion proteins.
To construct GST fusion protein plasmids with full-length or mutants of RF2b, the DNA coding sequences were amplified from the plasmid pCS:RF2b [9] with primers listed in the supplementary data. A BamHI restriction site was added to the 5′-end primers. A stop codon and an XhoI or EcoRI restriction site were added to 3′-end primers. PCR-amplified fragments were cloned into pGEX-4T-1 through BamHI and XhoI or EcoRI sites to produce GST fusion proteins.
The primers used to amplify full-length GST2a are GSTa1 and GSTa2. The primers for G2aPA are GSTa1 and GSTa3. The primers for G2aQ are GSTa2 and GSTa4. The primers for full-length GST2b are GSTb1 and GSTb5. The primers for GST2bN are GSTb1 and GSTb3. The primers for GST2bC are GSTb5 and GSTb4. G2aQ-h23 was amplified by primers GaC-1 and GSTa2. G2aQ-h3 was amplified by primers GaC-3 and GSTa2. G2aQ-h12 was amplified by primers GSTa4 and GaC-2. G2aQ-h2 was amplified by primers GaC-1 and GaC-2.
For His-tagged RF2b and RF2b truncation proteins, an NdeI restriction site was added to the beginning of 5′-end primers and a stop codon and BamHI restriction site were added to 3′-end primers. PCR-amplified fragments were cloned into pET28a (Novagen) through NdeI and BamHI sites to produce His-tagged proteins. The construction of plasmids to produce His tagged RF2a, 2a-ΔQ, 2a-ΔPΔA and 2a-3Δ has been described [8]. The plasmid containing 2a-ΔAΔQ was constructed by fusion PCR. The primary PCR products were amplified from p35S:RF2a with primer sets RF2a 5′/RF2a(P-B) 3′ and RF2a(P-B) 5′/RF2a 3′ [8]. The primary PCR products were used as templates for the second PCR reaction with primers RF2a 5′/RF2a-ΔQ 3′ [8]. The second run PCR product was cloned into pET28a as described above.
To construct His-tag fusion proteins with deletions in the Q domain of RF2a, fusion PCR strategies were applied. For 2a-h23, the primer sets for primary PCR reactions were RF2a-ΔPΔA/haC-D1 and GaC-1/RF2a3′ [8]; the primer set for the second PCR reaction was RF2a-ΔPΔA/RF2a3′. 2a-h3 was amplified using primers RF2a-ΔPΔA/haC-D2 and GaC-3/RF2a3′ for the primary PCRs, and RF2a-ΔPΔA/RF2a3′ for the second PCR. 2a-h12 was amplified by primers RF2a-ΔPΔA/GaC-2, and cloned into pET28a through NdeI and EcoRI sites. 2a-h2 was amplified from a plasmid encoding 2a-h23, with primers RF2a-ΔPΔA/GaC-2, and cloned into pET28a through NdeI and EcoRI sites. The construction of plasmids to produce His-tagged full-length RF2b has been described [9]. The primers for amplifying 2bΔN are RF2b-131F and RF2b 3′.
Protein purification
Plasmids were transformed into Escherichia coli strain BL21(DE3) pLysS to produce recombinant proteins. His-tagged and GST fusion proteins were purified as described in [8]. In some experiments, protein fragments of RF2a and RF2b were released from the GST fusion proteins using thrombin digestion through its recognition site located between the GST-tag sequence and the target protein.
Affinity pull-down assays
Purified recombinant GST fusion proteins (15 pmol of each) were immobilized on 20 μl of glutathione–agarose beads (Sigma, St. Louis, MO, U.S.A.) by incubating the GST fusion protein with beads at 4°C for 1 h in 300 μl of PBS and 1 mM DTT (dithiothreitol). After protein was bound, the beads were washed three times with 500 μl of PBS containing 1% dry milk and 0.5% Triton X-100. Then, 15 pmol of each purified His-tagged protein was incubated with the beads at 4°C for 1 h in 300 μl of PBS containing 0.5% Triton X-100, followed by five repeats of a washing step with 500 μl of TNT buffer containing 50 mM Tris/HCl (pH 7.6), 500 mM NaCl and 0.5% Triton X-100. The bound proteins were eluted by boiling in SDS/PAGE sample buffer, resolved in SDS/PAGE and visualized by Western-blot analysis with anti-His antibody (Qiagen, Valencia, CA, U.S.A.).
in vitro transcription assays
Rice whole-cell extracts were prepared from rice cv. 02408 suspension culture cells [6]. Extracts were depleted of proteins that bound to Box II cis element by incubating the extract with biotinylated, tandem repeats of Box II sequences immobilized on avidin beads. The template for the reactions was pE:GUS [2] comprising nt −164 to +45 of the RTBV promoter (referred to as the ‘E fragment’) and the uidA open reading frame. Recombinant RF2a, RF2b or their derivatives were pre-incubated with the template and the depleted rice whole-cell extract, at 30°C for 30 min, in transcription buffer consisting of 50 mM Hepes (pH 7.9), 10 mM potassium acetate, 3.75 mM magnesium acetate, 3.75 mM magnesium sulfate, 5 mM EGTA (pH 7.9), 6% (v/v) glycerol, 2 mM DTT and 0.5 unit RNase inhibitor. The final volume was adjusted to be 50 μl. Transcription was initiated by addition of 0.5 mM ribonucleotide mix, and was extended for 9 min. Transcription was terminated with addition of 90 μl of stop buffer consisting of 20 mM EDTA, 0.2 M NaCl, 1% SDS and 250 ng/ml yeast tRNA. Transcripts were extracted with phenol/chloroform and precipitated. Transcripts were then added to a primer extension assay with radiolabelled GUS3′ primer, 5′-GATTTCACGGGTTGGGGTTTCTA-3′, which is complementary to sequences in the GUS coding region, to confirm transcription. The [32P]cDNA was precipitated and resolved in a denaturing sequencing gel and analysed with a Typhoon imager (Molecular Dynamics).
SPR (surface plasmon resonance)
The binding affinities of 2aQ and full-length RF2a were measured using SPR with a BIAcore 2000 instrument (Biacore, Piscataway, NJ, U.S.A.). Anti-GST antibody was immobilized on CM5 sensor chips using the amine coupling kit and the GST capture kit provided by the manufacturer. GST or GST–2aQ fusion protein was then bound to individual flow cells in HBS buffer (pH 7.4) containing 10 mM Hepes, 150 mM NaCl, 3 mM EDTA and 0.005% surfactant P20. Various concentrations of purified full-length RF2a were subsequently injected over each surface at a flow rate of 30 μl/min, for 3 min. After allowing 10 min for protein dissociation, the chip was regenerated with a 1-min injection of 1 M NaCl and 50 mM KOH. The flow cell coupled with GST served as control to account for bulk-shift responses and minor, non-specific interactions, and the baseline was subtracted from the experimental flow cell. The association and dissociation rate constants were determined by global fit analysis with BIA evaluation software 3.2 (Biacore).
RESULTS
Interactions between the C-terminal domains of RF2a and RF2b
bZIP proteins dimerize via the bZIP DNA-binding domains that form the leucine zipper. To determine whether the Q domain of rice bZIP protein RF2a plays a role in homodimer formation, affinity pull-down experiments were carried out using proteins purified from E. coli (see the upper panel of Figure 1A for a diagram of fusion proteins). In these assays GST fusion proteins with full-length RF2a and different functional domains, i.e. the P domain, A domain and Q domain, were purified from E. coli and immobilized on glutathione–agarose beads. His-tagged Q domain in the absence of the P or A domains (2a-ΔPΔA) was used to test interactions with these GST fusion proteins in the pull-down assays. Samples were resolved in SDS/PAGE. The His-tagged Q domain protein was detected by Western-blot analyses using anti-His antibody. As shown in Figure 1(A), the Q domain of RF2a interacts with itself (G2aQ) as well as with full-length RF2a (G2a), but not with the P- and A-rich regions (G2aPA). These results suggest that, in addition to interactions between the bZIP regions in RF2a homodimers, the Q domains are capable of dimerization.
Figure 1. The C-terminal domains of RF2a and RF2b are involved in protein–protein interactions.
(A) His-tagged 2a-ΔPΔA was incubated with GST fusion proteins of full-length RF2a or mutants of RF2a immobilized on glutathione–agarose beads, after which the beads were washed extensively. Samples were released by treating with SDS sample buffer and resolved by Western-blot analysis with anti-His antibody. The first lane contains 2a-ΔPΔA positive control representing an amount equivalent to 10% of the input in each reaction. (B) His-tagged 2bΔN was incubated with immobilized GST fusion protein of full-length RF2b or truncation mutants of RF2b, followed by extensive washing. Samples were resolved by Western-blot analysis with anti-His antibody. The first lane contains 2bΔN positive control representing an amount equivalent to 10% of the input in each reaction. (C) His-tagged RF2a full-length or mutant proteins were incubated with immobilized GST-fused RF2b P/Q domain (G2bC), followed by extensive washing. Samples were resolved by Western-blot analysis with anti-His antibody. (D) Representative BIAcore analysis of interaction of the Q domain of RF2a (2aQ) with full-length RF2a. SPR binding was performed as described in the Materials and methods section. RF2a in the amount of 0 nM (buffer), 2 nM, 5 nM, 12.5 nM, 32 nM, 80 nM and 200 nM was injected subsequently over the surface bound with either GST–2aQ or GST alone. Evaluation of the kinetic parameters was done according to a global fit model. The residual deviation from the 1:1 binding model is listed below the sensorgram. RU, resonance units.
To determine whether the P/Q domain of RF2b is involved in protein–protein interactions in RF2b homodimers, affinity pull-down experiments were performed similar to those described for the Q domain of RF2a. Fusion proteins comprising full-length RF2b, or the N- or C-terminal regions of RF2b, with GST were prepared and immobilized on agarose beads (Figure 1B). His-tagged 2bΔN, a truncated protein in which the N-terminal domains of RF2b were removed, was incubated with the immobilized GST fusion proteins in the pull-down assay. As shown in Figure 1(B), the His-tagged 2bΔN binds to the C-terminal fragment of RF2b (G2bC) as well as to full-length RF2b (G2b), but not to the N-terminal region of RF2b (G2bN). The results suggest that, in addition to interactions between the bZIP domains, the C-terminal P/Q domain is involved in protein–protein interactions in the RF2b homodimer.
Since RF2a and RF2b are capable of forming heterodimers [9], affinity pull-down experiments were conducted to determine whether the C-terminal domains of RF2a and RF2b interact with each other. As shown in Figure 1(C), the P/Q domain of RF2b interacts with the Q domain of RF2a, as well as with full-length RF2a, but not with the N-terminal region of RF2a. Thus the results indicate that the Q regions of RF2a and the P/Q domain of RF2b interact with themselves and with each other. These interactions are likely to contribute to the formation and stability of RF2a and RF2b homodimers and heterodimers.
Kinetics of protein–protein interactions
To quantify the binding affinity of the Q domain of RF2a to full-length RF2a, SPR experiments were carried out on a BIAcore instrument. Purified 2aQ fused with GST (G2aQ) was immobilized on a CM5 sensor chip coated with anti-GST antibody. Various concentrations of full-length RF2a purified from E. coli were used as analytes for real-time interaction measurement (Figure 1D). The results reveal a KD of 8.2 nM with an association rate constant of 6.0±2.8×104 M−1·s−1 (mean±S.D., n=2) and a dissociation rate constant of 4.9±0.2×10−4 s−1 (mean±S.D., n=2), suggesting strong interactions between the RF2a and the Q domain. Similar studies of the interactions between 2aQ and full-length RF2b, as well as between 2bC and full-length RF2b, revealed similar binding affinity (results not shown). There was no evidence from these studies that homodimers and heterodimers have significantly different KD.
Role of the C-terminal domains in activating transcription by RF2a and RF2b
To determine whether interactions between C-terminal regions of RF2a and RF2b play an important role in transcription activation, a rice in vitro transcription assay system was employed. In the assay the E fragment of the RTBV promoter (nt −165 to +45) ligated to uidA gene sequences served as the template. His-tagged full-length RF2a or RF2b were added to the reactions. As previously reported, recombinant RF2a activated transcription from the RTBV promoter in vitro (Figure 2A, left panel). Similar reactions to which RF2b was added confirmed that recombinant RF2b can also activate transcription from the RTBV promoter in vitro (Figure 2A, right panel). This result is in agreement with conclusions of in vivo studies reported previously [9].
Figure 2. Impact of the C-terminal domains on RF2a- and RF2b-mediated transcriptional activation.
(A) C-terminal domains repress RF2a- and RF2b-mediated transcription activation. Left panel: the Q domain of RF2a (2aQ) represses RF2a-mediated activation. in vitro transcription reactions were carried out in the presence of template, depleted extract, 250 ng of RF2a, or 250 ng of RF2a plus an equal molar amount of 2aQ in a 50 μl reaction volume. The relative amount of gene-specific transcript from each reaction was calculated as compared with the reaction without the addition of recombinant proteins, and listed as mean±S.D. (n=3). The P value in lane 2 represents the results of the t test between the values in lane 1 and lane 2; the P value in lane 3 represents the t test between the values in lane 3 and lane 2. Right panel: the C-terminal P/Q domain RF2b represses RF2b mediated activation. RF2b (250 ng) and an equal molar amount of 2bC were used in the experiment. The statistical analysis was carried out as in the left panel. (B) RF2a and RF2b act synergistically to activate transcription. RF2a and RF2b (200 ng of each volume were used in a 50 μl reaction). The relative transcription activation is given as mean±S.D. (n=2). The P values in lane 3 represent the t test between the values in lane 3 and lane 1, and between lane 3 and lane 2, respectively.
Protein samples 2aQ and 2bC were prepared by removing the GST tag from GST fusion proteins G2aQ (Figure 1A) and G2bC (Figure 1B) via trypsin digestion. Purified 2aQ and 2bC were then added to transcription reactions together with RF2a or RF2b to determine their effect, if any, on in vitro transcription. As shown in Figure 2(A), addition of 2aQ (without the bZIP domain) repressed transcription from the RTBV promoter that is stimulated by addition of RF2a (left panel). Similarly, 2bC repressed transcription that was stimulated by RF2b (Figure 2A, right panel). In contrast, adding full-length RF2a and RF2b together in the in vitro assay showed a synergistic effect on transcriptional activation (Figure 2B). We propose that interactions between 2aQ and RF2a or 2bC and RF2b may cause interference of active dimer formation, or form malfunctioning dimers that reduce the activity of RF2a and RF2b. Alternatively, 2aQ and 2bC may bind to and titrate out interacting partners that are required by RF2a- and RF2b-mediated transcription activation.
The Q domain is predicted to contain three helical regions when the HMMSTR/Rosetta program is applied [27,28] (Figure 3A). To characterize its regulatory function and interaction with RF2a, the Q domain of RF2a was further dissected to subdomains. GST fusion proteins were constructed after removing one or more predicted helices from the 2aQ domain. Purified GST fusion proteins (Figure 3B) were used in affinity pull-down assays (as in Figure 1) to determine whether the mutations reduced or enhanced the interaction with RF2a. As shown in Figure 3(C), when the first helix was removed, the remaining fragment (G2aQ-h23) retained its ability to interact with RF2a, as did the second helix (GaQ-h2), and the third helix alone (GaQ-h3). However, peptides that contain helices 1 and 2 (G2aQ-h12) did not interact with RF2a in this assay. These results suggest that helices 2 and 3 contain sequences that are involved in dimerization of the Q domain of RF2a, while helix 1 interferes with interactions between helix 2 and RF2a.
Figure 3. Motifs of RF2a Q domain involved in protein interactions and transcription regulation.
(A) Structural prediction of 2aQ using the HMMSTR/Rosetta server [28]. Arrows indicate positions for subdomain mutation. (B) Diagram of the mutants of 2aQ. The amino acid numbers of predicted helices 1 (amino acids 233–286), 2 (amino acids 286–333) and 3 (amino acids 333–368) correspond to those in (A). (C) His-tagged full-length RF2a was incubated with immobilized GST or GST fusion protein of 2aQ or 2aQ mutants, followed by extensive washes. Samples were resolved by Western-blot analysis with anti-His antibody. (D) Effect of motifs in 2aQ on RF2a mediated transcription activation. Equal molar amounts of RF2a and 2aQ or 2aQ mutants were added to in vitro transcription reactions. The relative amount of gene-specific transcript from each reaction was calculated as compared with the reaction without the addition of recombinant proteins, and listed as mean±S.D. (n=2). The P value represents the t test between the values in each lane and lane 1.
The effects of deletion mutants of 2aQ on in vitro transcription from the RTBV promoter were evaluated. Transcription reactions were conducted with or without addition of one of the 2aQ mutants, in combination with RF2a. As shown in Figure 3(D), addition of 2aQ repressed RF2a-mediated activation of transcription (as in Figure 2). Similar affects were observed when 2aQ-h2, 2aQ-h3 or 2aQ-h23 was added in the reactions. Mutants containing putative helices 1 and 2 (2aQ-h12), which do not bind to RF2a (Figure 3C), did not repress RF2a-mediated transcription activation. These results suggested that binding of 2aQ to RF2a directly correlates to its repression of RF2a-mediated activation of transcription. The deletion mutants of 2aQ also had similar effects on RF2b-mediated transcription activation (results not shown).
The C-terminal domains of RF2a and RF2b activate transcription in vitro
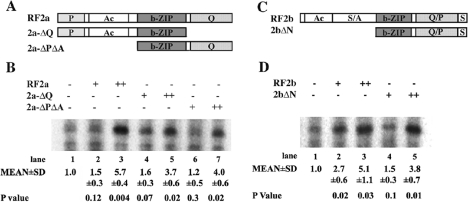
In a previous study using a transient assay in tobacco BY-2 protoplasts, RF2aΔPΔA (i.e. RF2a bZIP region plus the Q domain) did not activate a chimaeric promoter with Box II fused to a minimal CaMV (cauliflower mosaic virus) 35S promoter [8]. However, when the Q domain was fused to 2C7, a synthetic zinc finger DNA-binding domain, the Q domain of RF2a showed modest transcription activation from a chimaeric promoter containing the 2C7-binding site [8]. To gain a better understanding of the role of the Q and P/Q domains in gene expression, we tested mutants of RF2a and RF2b (Figure 4A) in the in vitro transcription system. As shown in Figure 4(B), recombinant protein with both A and P domains but lacking the Q domain (2a-ΔQ) activated transcription; this result recapitulates the observations in transient assays conducted in tobacco BY-2 protoplasts [8]. In addition, the Q domain in the absence of the P or A domains (2a-ΔPΔA) activated transcription in a dose-dependent manner (Figure 4B). Similarly, a mutant of RF2b from which the N-terminal domains were removed (2bΔN; Figure 4C) activates transcription in vitro (Figure 4D). The bZIP domains of RF2a or RF2b alone did not have an effect on transcription (results not shown). All these results indicate that the Q domain of RF2a and the P/Q domain of RF2b can activate transcription under certain circumstances.
Figure 4. The C-terminal domains of RF2a and RF2b activate transcription in vitro.
(A) Diagram of RF2a and various truncation and deletion mutants of RF2a used in the in vitro transcription assays. All proteins possess a His tag at the N-terminus and were purified as described in the text. (B) The function of mutants of RF2a in regulating transcription. For full-length RF2a, 150 ng (+) and 350 ng (++) of protein were added to a 50 μl reaction volume. Truncation or deletion mutants of RF2a were added to the reaction mixtures in equal molar amounts with full-length RF2a. The relative amount of gene-specific transcript from each reaction was calculated as compared with the reaction without the addition of recombinant proteins, and listed as mean±S.D. (n=3). The P value represents the t test between the values in each lane and lane 1. (C) Diagram of full-length RF2b and RF2b C-terminal mutants used in the in vitro transcription assay. (D) The C-terminal proline domain of RF2b activates transcription in vitro. Full-length RF2b [150 ng (+) or 350 ng (++)], or equal molar amounts of 2bΔN were added to a 50 μl reaction volume. The relative amount of gene-specific transcript from each reaction was calculated as compared with the reaction without the addition of recombinant proteins, and listed as mean±S.D. (n=3). The P value represents the t test between the values in each lane and lane 1.
To identify the region of the Q domain of RF2a that is responsible for activating transcription, the deletion mutants of 2aQ (as in Figure 3B) were fused with the bZIP DNA-binding domain of RF2a (Figure 5A), and tested in the in vitro transcription system. As seen in Figure 5, the mutant that includes helices 2 and 3 activates transcription from the RTBV promoter (lane 3), as does the mutant that retains only helix 2 (lane 5). The combined data (i.e. Figures 3 and 5) suggest that helix 2 is involved in both protein–protein interactions and transcription activation. In contrast helix 3 is involved in domain interaction, but has no effect on transcription in these assays.
Figure 5. Effects of motifs of 2aQ on transcription activation.
(A) Diagram of mutants of 2aQ. The 2aQ fragments were fused with the bZIP region, His-tagged at the N-terminus, and purified as described in the text. (B) in vitro transcription assay. Equal molar amounts of His-tagged 2aQ or 2aQ mutants were added to the transcription reactions. The relative amount of gene-specific transcript from each reaction was calculated as compared with the reaction without the addition of recombinant proteins, and listed as mean±S.D. (n=3). The P value represents the t test between the values in each lane and lane 1.
DISCUSSION
The transcription factors RF2a and RF2b have at their C-termini, regions that are enriched in glutamine (Q) or proline and glutamine (P/Q) respectively. We used a rice in vitro transcription system, immuno-affinity pull-down assays and SPR measurements to investigate the protein–protein interactions of the Q and P/Q domains of RF2a and RF2b and their roles in regulating transcription.
Affinity pull-down studies revealed that the Q and P/Q domains of RF2a and RF2b are involved in dimerization and may form homo- and hetero-dimers. Addition of the Q or P/Q domain lacking the bZIP domain to in vitro reactions suppressed the transcription-enhancing activities of RF2a and RF2b. In other studies, we observed different biological effects as a consequence of expressing the bZIP domain of RF2a (i.e. RF2a-3Δ) or the bZIP domain with the Q domain (i.e. RF2a-ΔPΔA) in transgenic tobacco and Arabidopsis plants. Although both caused dwarf plants with delayed flowering, the severity of the phenotypic changes, especially in seed-set, stem and root development, clearly differed [8] (S. Dai and R.N. Beachy, unpublished work). These results suggest that the C-terminal domains of RF2a and possibly RF2b are important for the function of these proteins, and disruption of such interactions is likely to affect their biological functions. Both RF2a-3Δ and RF2a-ΔPΔA are capable of forming dimers with full-length RF2a [29]. Different biological effects caused by expression of RF2a-3Δ and RF2a-ΔPΔA in transgenic plants may indicate differences between the functions of heterodimers comprising RF2a/RF2a-3Δ and RF2a/RF2a-ΔPΔA, although potential effects caused by homodimers of RF2a-3Δ and RF2a-ΔPΔA cannot be ruled out. Dimerization of the C-termini of RF2a and RF2b is presumably important for the function of these proteins in vivo and in vitro, potentially by having an impact on interactions with other regulatory proteins such as general transcription factors and cofactors. A previous study reported that the Q domain of RF2a interacts with rice TBP, an interaction that contributes to transcription activation [8].
Dissection of the Q domain of RF2a identified subdomains that are important for domain dimerization and transcriptional regulation. The study suggests that the predicted helical structure helix 2 of the Q domain is involved in homodimer formation and in interactions between RF2a and RF2b. Furthermore, helix 2 activated transcription when presented in a fusion protein with the RF2a bZIP domain. The result suggests a role of this sequence in protein–protein interaction and activation of transcription by RF2a.
Suppression of the function of RF2a and RF2b by either the Q domain of RF2a or the P/Q domain of RF2b may be caused by formation of malfunctioning dimers that render them unable to bind to DNA targets, or by interfering with interactions with other regulatory proteins that are required for transcription activation. It may also suggest that the dimerization of C-terminal domains of RF2a and RF2b may provide a different molecular scaffold for interactions with different cofactors and/or general transcription factors that result in different specific functions of the homo- and hetero-dimers of the C-terminal domains.
Unlike the acidic domain of RF2a, which is a strong activator of transcription, the Q domain of RF2a showed only moderate activation of gene expression when fused to a synthetic zinc finger DNA-binding domain in transient assays in tobacco BY-2 cells, but the Q domain fused to the RF2a bZIP DNA-binding domain did not activate a chimaeric promoter with Box II fused to a minimal CaMV 35S promoter [8]. However, the present study indicates that the Q domain activates transcription from the RTBV promoter in the rice in vitro transcription assay system. Differences between experimental systems may be a result of the differences between plant species or promoter settings; it may also reflect inherent differences between in vivo and in vitro systems. Similar phenomena have been observed in other studies, and it was previously reported that Q domains function differently in different cells [30–32]. Both Q and P domains have been shown to activate transcription in reconstituted mammalian in vitro transcription assays, but fail to activate transcription in vivo [33,34]. Such differences may also reflect differences in the structure of the cis elements in the reporter genes ([8,35] and the present study). It may also suggest that interactions with other regulatory proteins such as cofactors are important for the function of these domains and the composition of these interacting partners may vary among species. To further understand the mode of action of the transcriptional activation caused by RF2a and RF2b and the functional consensus of these proteins in different plant species, it will be very interesting to identify the interaction partners of the functional domains of these two proteins in both native and heterozygous plant systems.
Online data
Acknowledgments
We thank Dr Maria Isabel Ordiz and Dr Baoxian Wei (Danforth Center) and Dr Qun Zhu (The Du Pont Company) for helpful discussions and other input. This work was supported by the U.S. Department of Energy grant DE-FG02-99ER20355 (to R.N.B.). Y. L. was supported by the NIH (National Institutes of Health, Bethesda, MD, U.S.A.) National Research Service Award for Postdoctoral Fellows F32-AI049672.
References
- 1.Bhattacharyya-Pakrasi M., Peng J., Elmer J. S., Laco G., Shen P., Kaniewska M. B., Kononowicz H., Wen F., Hodges T. K., Beachy R. N. Specificity of a promoter from the rice tungro bacilliform virus for expression in phloem tissues. Plant J. 1993;4:71–79. doi: 10.1046/j.1365-313x.1993.04010071.x. [DOI] [PubMed] [Google Scholar]
- 2.Yin Y., Beachy R. N. The regulatory regions of the rice tungro bacilliform virus promoter and interacting nuclear factors in rice (Oryza sativa L.) Plant J. 1995;7:969–980. doi: 10.1046/j.1365-313x.1995.07060969.x. [DOI] [PubMed] [Google Scholar]
- 3.Yin Y., Chen L., Beachy R. Promoter elements required for phloem-specific gene expression from the RTBV promoter in rice. Plant J. 1997;12:1179–1188. doi: 10.1046/j.1365-313x.1997.12051179.x. [DOI] [PubMed] [Google Scholar]
- 4.He X., Hohn T., Futterer J. Transcriptional activation of the rice tungro bacilliform virus gene is critically dependent on an activator element located immediately upstream of the TATA box. J. Biol. Chem. 2000;275:11799–11808. doi: 10.1074/jbc.275.16.11799. [DOI] [PubMed] [Google Scholar]
- 5.Dai S., Zhang Z., Bick J., Beachy R. N. Essential role of the Box II cis element and cognate host factors in regulating the promoter of rice tungro bacilliform virus. J. Gen. Virol. 2006;87:715–722. doi: 10.1099/vir.0.81488-0. [DOI] [PubMed] [Google Scholar]
- 6.Yin Y., Zhu Q., Dai S., Lamb C., Beachy R. N. RF2a, a bZIP transcriptional activator of the phloem-specific rice tungro bacilliform virus promoter, functions in vascular development. EMBO J. 1997;16:5247–5259. doi: 10.1093/emboj/16.17.5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu Q., Ordiz M. I., Dabi T., Beachy R. N., Lamb C. Rice TATA binding protein interacts functionally with transcription factor IIB and the RF2a bZIP transcriptional activator in an enhanced plant in vitro transcription system. Plant Cell. 2002;14:795–803. doi: 10.1105/tpc.010364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai S., Petruccelli S., Ordiz M. I., Zhang Z., Chen S., Beachy R. N. Functional analysis of RF2a, a rice transcription factor. J. Biol. Chem. 2003;278:36396–36402. doi: 10.1074/jbc.M304862200. [DOI] [PubMed] [Google Scholar]
- 9.Dai S., Zhang Z., Chen S., Beachy R. N. RF2b, a rice bZIP transcription activator, interacts with RF2a and is involved in symptom development of rice tungro disease. Proc. Natl. Acad. Sci. U.S.A. 2004;101:687–692. doi: 10.1073/pnas.0307687100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meshi T., Iwabuchi M. Plant transcription factors. Plant Cell Physiol. 1995;36:1405–1420. [PubMed] [Google Scholar]
- 11.Roeder R. G. The complexities of eukaryotic transcription initiation: regulation of preinitiation complex assembly. Trends Biochem. Sci. 1991;16:402–408. doi: 10.1016/0968-0004(91)90164-q. [DOI] [PubMed] [Google Scholar]
- 12.Yankulov K., Blau J., Purton T., Roberts S., Bentley D. L. Transcriptional elongation by RNA polymerase II is stimulated by transactivators. Cell. 1994;77:749–759. doi: 10.1016/0092-8674(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 13.Martinez E. Multi-protein complexes in eukaryotic gene transcription. Plant Mol. Biol. 2002;50:925–947. doi: 10.1023/a:1021258713850. [DOI] [PubMed] [Google Scholar]
- 14.Levine M., Tjian R. Transcription regulation and animal diversity. Nature. 2003;424:147–151. doi: 10.1038/nature01763. [DOI] [PubMed] [Google Scholar]
- 15.Courey A. J., Tjian R. Analysis of Sp1 in vivo reveals multiple transcriptional domains, including a novel glutamine-rich activation motif. Cell. 1988;55:887–898. doi: 10.1016/0092-8674(88)90144-4. [DOI] [PubMed] [Google Scholar]
- 16.Courey A. J., Holtzman D. A., Jackson S. P., Tjian R. Synergistic activation by the glutamine-rich domains of human transcription factor Sp1. Cell. 1989;59:827–836. doi: 10.1016/0092-8674(89)90606-5. [DOI] [PubMed] [Google Scholar]
- 17.Pascal E., Tjian R. Different activation domains of Sp1 govern formation of multimers and mediate transcriptional synergism. Genes Dev. 1991;5:1646–1656. doi: 10.1101/gad.5.9.1646. [DOI] [PubMed] [Google Scholar]
- 18.Hoey T, Weinzierl R. O., Gill G., Chen J. L., Dynlacht B. D., Tjian R. Molecular cloning and functional analysis of Drosophila TAF110 reveal properties expected of coactivators. Cell. 1993;72:247–260. doi: 10.1016/0092-8674(93)90664-c. [DOI] [PubMed] [Google Scholar]
- 19.Gill G., Pascal E., Tseng Z. H., Tjian R. A glutamine-rich hydrophobic patch in transcription factor Sp1 contacts the dTAFII110 component of the Drosophila TFIID complex and mediates transcriptional activation. Proc. Natl. Acad. Sci. U.S.A. 1994;91:192–196. doi: 10.1073/pnas.91.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saluja D., Vassallo M. F., Tanese N. Distinct subdomains of human TAFII130 are required for interactions with glutamine-rich transcriptional activators. Mol. Cell. Biol. 1998;18:5734–5743. doi: 10.1128/mcb.18.10.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferreri K., Gill G., Montminy M. The cAMP-regulated transcription factor CREB interacts with a component of the TFIID complex. Proc. Natl. Acad. Sci. U.S.A. 1994;91:1210–1213. doi: 10.1073/pnas.91.4.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka M., Clouston W. M., Herr W. The Oct-2 glutamine-rich and proline rich activation domains can synergize with each other or duplicates of themselves to activate transcription. Mol. Cell. Biol. 1994;14:6046–6055. doi: 10.1128/mcb.14.9.6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinto M., Lobe C. G. Products of the grg (Groucho-related gene) family can dimerize through the amino-terminal Q domain. J. Biol. Chem. 1996;271:33026–33031. doi: 10.1074/jbc.271.51.33026. [DOI] [PubMed] [Google Scholar]
- 24.Sweetman D, Smith T, Farrell E. R., Chantry A., Munsterberg A. The conserved glutamine-rich region of chick csal1 and csal3 mediates protein interactions with other spalt family members. Implications for Townes–Brocks syndrome. J. Biol. Chem. 2003;278:6560–6566. doi: 10.1074/jbc.M209066200. [DOI] [PubMed] [Google Scholar]
- 25.Madden S. L., Cook D. M., Morris J. F., Gashler A., Sukhatme V. P., Rauscher F. J., III Transcriptional repression mediated by the WT1 Wilms tumor gene product. Science. 1991;253:1550–1553. doi: 10.1126/science.1654597. [DOI] [PubMed] [Google Scholar]
- 26.Melo E. O., Dhalia R., Martins de Sa C., Standart N., de Melo Neto O. P. Identification of a C-terminal poly(A)-binding protein (PABP)–PABP interaction domain: role in cooperative binding to poly (A) and efficient cap distal translational repression. J. Biol. Chem. 2003;278:46357–46368. doi: 10.1074/jbc.M307624200. [DOI] [PubMed] [Google Scholar]
- 27.Simons K. T., Kooperberg C., Huang E., Baker D. Assembly of protein tertiary structures from fragments with similar local sequences using simulated annealing and Bayesian scoring functions. J. Mol. Biol. 1997;268:209–225. doi: 10.1006/jmbi.1997.0959. [DOI] [PubMed] [Google Scholar]
- 28.Bystroff C., Shao Y. Fully automated ab initio protein structure prediction using I-SITES, HMMSTR and ROSETTA. Bioinformatics. 2002;18:S54–S61. doi: 10.1093/bioinformatics/18.suppl_1.s54. [DOI] [PubMed] [Google Scholar]
- 29.Petruccelli S., Dai S., Carcamo R., Yin Y., Chen S., Beachy R. N. Transcription factor RF2a alters expression of the rice tungro bacilliform virus promoter in transgenic tobacco plants. Proc. Natl. Acad. Sci. U.S.A. 2001;98:7635–7640. doi: 10.1073/pnas.121186398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Emili A., Greenblatt J., Ingles C. J. Species-specific interaction of the glutamine-rich activation domains of Sp1 with the TATA box-binding protein. Mol. Cell. Biol. 1994;14:1582–1593. doi: 10.1128/mcb.14.3.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kunzler M., Braus G. H., Georgiev O., Seipel K., Schaffner W. Functional differences between mammalian transcription activation domains at the yeast GAL1 promoter. EMBO J. 1994;13:641–645. doi: 10.1002/j.1460-2075.1994.tb06302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ponticelli A. S., Pardee T. S., Struhl K. The glutamine-rich activation domains of human Sp1 do not stimulate transcription in Saccharomyces cerevisiae. Mol. Cell. Biol. 1995;15:983–988. doi: 10.1128/mcb.15.2.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gerber H. P., Seipel K., Hofferer M., Hug M., Rusconi S., Schaffner W. Transcriptional activation modulated by homopolymeric glutamine and proline stretches. Science. 1994;263:808–811. doi: 10.1126/science.8303297. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka M., Herr W. Reconstitution of transcriptional activation domains by reiteration of short peptide segments reveals the modular organization of a glutamine-rich activation domain. Mol. Cell. Biol. 1994;14:6056–6067. doi: 10.1128/mcb.14.9.6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Escher D., Bodmer-Glavas M., Barberis A., Schaffner W. Conservation of glutamine-rich transactivation function between yeast and humans. Mol. Cell. Biol. 2000;20:2774–2782. doi: 10.1128/mcb.20.8.2774-2782.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.