Abstract
Haem is used as a versatile receptor for redox active molecules; most notably NO (nitric oxide) and oxygen. Three haem-containing proteins, myoglobin, haemoglobin and cytochrome c oxidase, are now known to bind NO, and in all these cases competition with oxygen plays an important role in the biological outcome. NO also binds to the haem group of sGC (soluble guanylate cyclase) and initiates signal transduction through the formation of cGMP in a process that is oxygen-independent. From biochemical studies, it has been shown that sGC is substantially more sensitive to NO than is cytochrome c oxidase, but a direct comparison in a cellular setting under various oxygen levels has not been reported previously. In this issue of the Biochemical Journal, Cadenas and co-workers reveal how oxygen can act as the master regulator of the relative sensitivity of the cytochrome c oxidase and sGC signalling pathways to NO. These findings have important implications for our understanding of the interplay between NO and oxygen in both physiology and the pathology of diseases associated with hypoxia.
Keywords: cytochrome c oxidase, haem (heme), mitochondria, nitric oxide, redox signalling
Nitric oxide (NO) is an unusual signalling molecule since its biological responses are primarily governed by its chemical reactivity [1]. In fact, the first signalling function of NO was revealed with the discovery that NO serves as a ligand for the haem group in sGC (soluble guanylate cyclase), resulting in increased cGMP production and activation of downstream signalling cascades. However, the story became more complex with the recognition that NO serves as a ligand for other haem proteins such as COX (cytochrome c oxidase) in the mitochondria and haemoglobin in the red blood cell [2,3]. The fact that NO interacts with the haem groups that normally bind oxygen essentially expanded the role of NO in cell signalling from a simple haem ligand to a regulatory modulator of oxygen-sensitive processes [4–6].
Since both COX and sGC frequently co-exist within the same cell, the relative sensitivity of the two pathways to NO should determine the ultimate biological effect. It has been shown that activation of sGC occurs at much lower concentrations of NO than inhibition of COX, but these studies have not addressed the sensitivity of these pathways using endogenous NO formation; nor have they investigated the influence of oxygen on this relative sensitivity in a cellular setting [7]. Using an elegant experimental model that allows the controlled formation of NO from iNOS (inducible NO synthase) in cells that contain both COX and sGC, the authors have compared the response of these two signalling pathways with the concentration of NO measured extracellularly. Several endpoints were selected to assess the activity of sGC and COX. These include production of cGMP and phosphorylation of VASP (vasodilator-stimulated phosphoprotein) for the sGC pathway, and oxygen consumption and AMPK (AMP-activated protein kinase) phosphorylation for the COX pathway. The activation of sGC was essentially independent of the oxygen tension, except at very low oxygen concentrations where oxygen is necessary for NO synthesis by iNOS. In contrast, inhibition of respiration by the interaction of NO with COX remained sensitive to low levels of oxygen, with the inhibition becoming progressively more pronounced as the oxygen levels decreased.
When compared at an oxygen concentration of 30 μM, the authors estimate that sGC is approx. 50 times more sensitive to NO than COX on the basis of cGMP formation and inhibition of oxygen consumption [8]. However, using downstream indicators of the two pathways, VASP phosphorylation (for sGC) and AMPK phosphorylation (for COX) the authors show that sGC is only 10–20-fold more sensitive than COX [8]. Factors intrinsic to this experimental design may explain these apparent differences. For example, the measurements of cGMP formation, VASP phosphorylation and AMPK phosphorylation were performed using adherent cells, whereas the measurement of oxygen consumption occurred in a stirred chamber with detached cells. This difference in the conditions is likely to result in greater oxygenation of cells in suspension than adherent cells in culture, thus decreasing the apparent sensitivity of COX to NO inhibition in the oxygen consumption experiments [8].
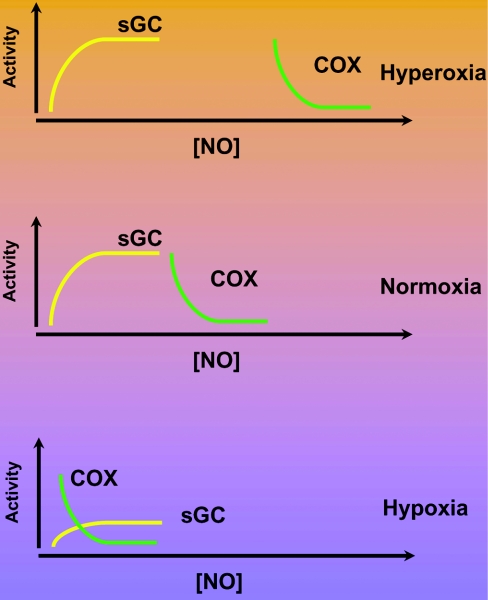
Under physiological conditions, it is important to note that the concentration of 30 μM oxygen used in the present study is hyperoxic for many cell types in complex organs such as the liver, kidney or heart. Nevertheless, these cell culture experiments can yield some interesting insights into how oxygen can act as the master regulator of the relative responsiveness of these two haem-dependent NO-signalling pathways. In Figure 1, we illustrate how extrapolation of the data shown in the paper by Cadenas and co-workers [8] can be used to illustrate this concept. The activities of sGC and COX are shown as a function of increasing NO concentration under conditions of hyperoxia, normoxia and hypoxia. As the conditions move from hyperoxia (approximating those in cell culture) to normoxia, oxygen is not initially limiting for the synthesis of NO, and the relative responsiveness of sGC and COX to NO is well separated along the NO concentration curve. Under conditions of hypoxia, the availability of oxygen for NO synthesis becomes limiting and decreases the overall capacity of sGC to generate cGMP. In contrast, at these low oxygen concentrations, COX becomes much more sensitive to inhibition of respiration by NO. One can speculate that, in hypoxia, inhibiting the main consuming pathway of oxygen in the cell may be an adaptive response to restore the normoxic condition.
Figure 1. Regulation of NO signalling by sGC and COX by oxygen.
The activity of sGC and inhibition of COX is shown schematically as a function of increasing NO concentration at three different levels of oxygen. It is assumed in this Figure that NO is derived from NOSs that also require oxygen for the synthesis of NO. Under the conditions of hyperoxia and normoxia, oxygen is not limiting for NO synthesis. In the case of hypoxia, however, the amount of NO produced will be limited by oxygen, and this then decreases the levels of cGMP that can be synthesized by sGC.
Finally, these studies have potential implications for a number of pathologies where iNOS is induced in tissues subject to stress. The concept that NO and oxygen interact to play a complementary role in the regulation of the concentration gradients of each other has been suggested by a number of authors [9–11]. Interestingly, the intrinsic sensitivity of cellular respiration to inhibition by NO can be modified under stress. For example, the inhibition of respiration by NO is enhanced in liver mitochondria from animals subject to an oxidative stress such as chronic alcohol consumption [12]. The very high levels of NO shown in the present study are capable of inhibiting oxygen consumption by the mitochondria sufficient to decrease ATP synthesis. This can create a condition we have called ‘nitroxia’, which is associated with high levels of NO, oxygen and impaired mitochondrial bioenergetics [5,6]. The highly reducing status of the mitochondrial respiratory chain in the presence of oxygen will then promote superoxide formation. This can then lead to bioenergetic dysfunction through the formation of reactive nitrogen species, such as peroxynitrite, which are capable of damaging proteins and DNA. From a therapeutic perspective, a number of possibilities are suggested by the present study and others in the literature. For example, during ischaemia, low oxygen levels will prevent NO production from NOS; however, NO may still be produced from the reduction of nitrite by both the mitochondrion and myoglobin [13]. This NO may then act through the sGC pathway to suppress the inflammation associated with ischaemia–reperfusion and protect mitochondria from damage due to calcium overload. Clearly, the study of Cadenas and co-workers [8] demonstrates the important role of oxygen in determining the ultimate biological effect of NO. This concept will no doubt become important in dissecting the critical role of the interaction of oxygen and NO in both physiology and numerous disease states, particularly those associated with hypoxia.
References
- 1.Pacher P., Beckman J. S., Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper C. E. Nitric oxide and cytochrome oxidase: substrate, inhibitor or effector? Trends Biochem. Sci. 2002;27:33–39. doi: 10.1016/s0968-0004(01)02035-7. [DOI] [PubMed] [Google Scholar]
- 3.Kim-Shapiro D. B., Schechter A. N., Gladwin M. T. Unraveling the reactions of nitric oxide, nitrite, and hemoglobin in physiology and therapeutics. Arterioscler. Thromb. Vasc. Biol. 2006;26:697–705. doi: 10.1161/01.ATV.0000204350.44226.9a. [DOI] [PubMed] [Google Scholar]
- 4.Moncada S., Erusalimsky J. D. Does nitric oxide modulate mitochondrial energy generation and apoptosis? Nat. Rev. Mol. Cell. Biol. 2002;3:214–220. doi: 10.1038/nrm762. [DOI] [PubMed] [Google Scholar]
- 5.Shiva S., Oh J. Y., Landar A. L., Ulasova E., Venkatraman A., Bailey S. M., Darley-Usmar V. M. Nitroxia: the pathological consequence of dysfunction in the nitric oxide-cytochrome c oxidase signaling pathway. Free Radical Biol. Med. 2005;38:297–306. doi: 10.1016/j.freeradbiomed.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 6.Xu W., Charles I. G., Moncada S. Nitric oxide: orchestrating hypoxia regulation through mitochondrial respiration and the endoplasmic reticulum stress response. Cell Res. 2005;15:63–65. doi: 10.1038/sj.cr.7290267. [DOI] [PubMed] [Google Scholar]
- 7.Bellamy T. C., Griffiths C., Garthwaite J. Differential sensitivity of guanylyl cyclase and mitochondrial respiration to nitric oxide measured using clamped concentrations. J. Biol. Chem. 2002;277:31801–31807. doi: 10.1074/jbc.M205936200. [DOI] [PubMed] [Google Scholar]
- 8.Shiva S., Brookes P. S., Patel R. P., Anderson P. G., Darley-Usmar V. M. Nitric oxide partitioning into mitochondrial membranes and the control of respiration at cytochrome c oxidase. Proc. Natl. Acad. Sci. U.S.A. 2001;98:7212–7217. doi: 10.1073/pnas.131128898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodríguez-Juárez F., Aguirre A., Cadenas S. Relative sensitivity of soluble guanylate cyclase and mitochondrial respiration to endogenous nitric oxide at physiological oxygen concentration. Biochem. J. 2007;405:223–231. doi: 10.1042/BJ20070033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brookes P. S., Kraus D. W., Shiva S., Doeller J. E., Barone M. C., Patel R. P., Lancaster J. R., Jr, Darley-Usmar V. Control of mitochondrial respiration by NO·, effects of low oxygen and respiratory state. J. Biol. Chem. 2003;278:31603–31609. doi: 10.1074/jbc.M211784200. [DOI] [PubMed] [Google Scholar]
- 11.Thomas D. D., Liu X., Kantrow S. P., Lancaster J. R., Jr The biological lifetime of nitric oxide: implications for the perivascular dynamics of NO and O2. Proc. Natl. Acad. Sci. U.S.A. 2001;98:355–360. doi: 10.1073/pnas.011379598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venkatraman A., Shiva S., Wigley A., Ulasova E., Chhieng D., Bailey S. M., Darley-Usmar V. M. The role of iNOS in alcohol-dependent hepatotoxicity and mitochondrial dysfunction in mice. Hepatology. 2004;40:565–573. doi: 10.1002/hep.20326. [DOI] [PubMed] [Google Scholar]
- 13.Shiva S., Huang Z., Grubina R., Sun J., Ringwood L. A., MacArthur P. H., Xu X., Murphy E., Darley-Usmar V. M., Gladwin M. T. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ. Res. 2007;100:654–661. doi: 10.1161/01.RES.0000260171.52224.6b. [DOI] [PubMed] [Google Scholar]