Abstract
Repetitive hormone-induced changes in concentration of free cytoplasmic Ca2+ in hepatocytes require Ca2+ entry through receptor-activated Ca2+ channels and SOCs (store-operated Ca2+ channels). SOCs are activated by a decrease in Ca2+ concentration in the intracellular Ca2+ stores, but the molecular components and mechanisms are not well understood. Some studies with other cell types suggest that PLC-γ (phospholipase C-γ) is involved in the activation of receptor-activated Ca2+ channels and/or SOCs, independently of PLC-γ-mediated generation of IP3 (inositol 1,4,5-trisphosphate). The nature of the Ca2+ channels regulated by PLC-γ has not been defined clearly. The aim of the present study was to determine if PLC-γ is required for the activation of SOCs in liver cells. Transfection of H4IIE cells derived from rat hepatocytes with siRNA (short interfering RNA) targeted to PLC-γ1 caused a reduction (by approx. 70%) in the PLC-γ1 protein expression, with maximal effect at 72–96 h. This was associated with a decrease (by approx. 60%) in the amplitude of the ISOC (store-operated Ca2+ current) developed in response to intracellular perfusion with either IP3 or thapsigargin. Knockdown of STIM1 (stromal interaction molecule type 1) by siRNA also resulted in a significant reduction (approx. 80% at 72 h post-transfection) of the ISOC amplitude. Immunoprecipitation of PLC-γ1 and STIM1, however, suggested that under the experimental conditions these proteins do not interact with each other. It is concluded that the PLC-γ1 protein, independently of IP3 generation and STIM1, is required to couple endoplasmic reticulum Ca2+ release to the activation of SOCs in the plasma membrane of H4IIE liver cells.
Keywords: phospholipase C (PLC), short interfering RNA (siRNA), stromal interaction molecule type 1 (STIM1), store-operated Ca2+ channel (SOC)
Abbreviations: BCA, bicinchoninic acid; [Ca2+]cyt, free cytoplasmic Ca2+; CRAC, Ca2+ release-activated Ca2+; DAG, diacylglycerol; DAPI, 4′,6-diamidino-2-phenylindole; diC8-PIP2, 1,2-dioctanoyl-sn-glycero-3-[phosphoinositol-4,5-bisphosphate]tri-ammonium salt; DMEM, Dulbecco's modified Eagle's medium; ER, endoplasmic reticulum; FBS, foetal bovine serum; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HRP, horseradish peroxidase; ICRAC, CRAC current; IP3, inositol 1,4,5-trisphosphate; ISOC, store-operated Ca2+ current; KRH, Krebs/Ringer/Hepes; MEM, minimal essential medium; OAG, 1-oleoyl-2-acetyl-sn-glycerol; PIP2, phosphoinositol-4,5-bisphosphate; PLC-γ, phospholipase C-γ; SH3, Src homology 3; siRNA, short interfering RNA; SOC, store-operated Ca2+ channel; STIM1, stromal interaction molecule type 1; TBST, Tris-buffered saline and 0.1% Tween-20; TRP, transient receptor potential; TRPC, TRP canonical
INTRODUCTION
SOCs (store-operated Ca2+ channels) and receptor-activated Ca2+ channels in the plasma membrane provide major routes for agonist-initiated Ca2+ entry in hepatocytes and many other non-excitable cells [1]. The activation of SOCs is initiated when the concentration of Ca2+ in the intracellular Ca2+ stores falls below a certain level. Physiologically, release of Ca2+ from the stores and activation of SOCs occurs in response to hormones binding to the cell surface receptors. This activates PLC (phospholipase C), which in turn generates IP3 (inositol 1,4,5-trisphosphate), a ligand for IP3 receptor Ca2+-release channels in the ER (endoplasmic reticulum) [1]. Recent observations suggest that the CRAC (Ca2+ release-activated Ca2+) channel, which is the best characterized SOC, is composed, at least in part, of Orai1 [also known as CRACM1 (CRAC modulator 1)] polypeptides that interact with STIM1 (stromal interaction molecule type 1), a polypeptide which probably senses the decrease in Ca2+ in the ER. It is not yet clear, however, if Orai1 and STIM1 mediate store-operated Ca2+ entry in all cell types, and if they comprise the entire mechanism [2–4].
Previous studies have led to the suggestion that the PLC-γ protein is required as a molecular component of agonist-initiated Ca2+ entry mechanism, independent of its role in the generation of IP3 [5–7]. Using RNA interference it has been shown that depletion of either PLC-γ1 or PLC-γ2 in PC12 and A7r5 cells inhibited Ca2+ entry in response to agonists [5]. This Ca2+ entry could be restored by the expression of a lipase-inactive mutant of PLC-γ2, suggesting that PLC-γ might have a role unrelated to its production of IP3 in agonist-initiated Ca2+ entry [5]. Store-operated Ca2+ entry, activated using either ionomycin or thapsigargin to deplete Ca2+ in the ER, was unaffected by the knockdown of PLC-γ in these cells. In contrast, PLC-γ1 knockdown in keratinocytes using antisense cDNA inhibited the activation of Ca2+ entry in response to depletion of Ca2+ stores with thapsigargin [7]. In both cases, the type of the Ca2+ entry channel that has been affected by the knockdown of PLC-γ is not known, although evidence has been presented to indicate that PLC-γ1 interacts with several members of the TRP (transient receptor potential) family, including TRPC3 (TRP canonical 3) [7,8].
In primary hepatocytes, and cell lines derived from hepatocytes, the ISOC (store-operated Ca2+ current) can be activated by the binding of hormones to G-protein or tyrosine kinase coupled receptors, or by depletion of intracellular Ca2+ stores by IP3 or thapsigargin [9]. The resulting current exhibits essentially the same properties as ICRAC (CRAC current) studied in haematopoietic cells, and is unlikely to be mediated by any of the known members of the TRP family [10,11]. It is currently not known if ICRAC in blood cells or ISOC in hepatocytes is regulated in any way by PLC-γ. In the present study, we used RNA interference to knockdown PLC-γ1, whole-cell patch clamping to measure ISOC, and fura 2 to measure [Ca2+]cyt (free cytoplasmic Ca2+), to investigate the role of PLC-γ1 in the mechanism by which ISOC is activated in the H4IIE rat hepatoma cell line, derived from rat hepatocytes. The results show that a reduction of the expression of the PLC-γ1 is associated with a decrease in thapsigargin-induced Ca2+ entry and a reduction in the amplitude of ISOC, without affecting Ca2+ release from the stores. This reduction in ISOC amplitude was observed regardless of how the stores where depleted: by intercellular perfusion with 20 μM IP3 or by the SERCA (sarcoplasmic/endoplasmic reticulum Ca2+-ATPase) inhibitor thapsigargin. Furthermore, we investigated the requirement for STIM1 in the activation of the ISOC, and the possible interaction between STIM1 and PLC-γ1 in H4IIE cells. Although siRNA (short interfering RNA)-mediated knockdown of STIM1 resulted in almost complete ablation of the ISOC, immunoprecipitation experiments showed that STIM1 and PLC-γ1 are unlikely to interact with each other. It is concluded that PLC-γ1 is required in the process by which a decline in Ca2+ concentration in the ER leads to the activation of SOCs, but this role of PLC-γ1 is not related to its enzymatic activity.
MATERIALS AND METHODS
Materials
Double-stranded siRNAs and HiPerFect were purchased from Qiagen (Doncaster, Victoria, Australia). DMEM (Dulbecco's modified Eagle's medium), L-glutamine and MEM (minimal essential medium) non-essential amino acids were from Invitrogen (Mount Waverley, Victoria, Australia). FBS (foetal bovine serum) was from JRH Biosciences (Brooklyn, Victoria, Australia). Rabbit anti-PLC-γ1 antibody (sc-81), rabbit anti-PLC-γ2 antibodies (sc-407 and sc-9015), mouse monoclonal anti-PLC-γ2 antibody (sc-5283), rabbit anti-GAPDH (glyceraldehyde-3-phosphate dehydrogenase) antibody (sc-25778) and goat anti-rabbit IgG–HRP (horseradish peroxidase)-conjugate (sc-2004) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). Donkey anti-mouse IgG–HRP-conjugate (610-703-124) was from Rockland (Gilbertsville, PA, U.S.A.). FITC-conjugated goat anti-rabbit IgG antibody (ab6717) was from Abcam (Cambridge, U.K.). diC8-PIP2 (1,2-dioctanoyl-sn-glycero-3-[phosphoinositol-4,5-bisphosphate]tri-ammonium salt) was purchased from Avanti Polar Lipids (Alabaster, AL, U.S.A.). All other chemicals, including Nonidet P40, deoxycholate, Tween-20, protease inhibitor cocktail and BCA (bicinchoninic acid) protein assay kit, were from Sigma (Castle Hill, NSW, Australia).
Cell culture
H4IIE (ATCC: CRL 1548) cells were cultured at 37°C in 5% (v/v) CO2 in air in complete DMEM [DMEM supplemented with 100 μM MEM non-essential amino acids, 2 mM L-glutamine and 10% (v/v) FBS].
siRNA design
The 21-nucleotide double-stranded PLCγ1-01 siRNA was designed using the algorithm developed by Reynolds et al. [12], and the specificity checked by BLAST search against the NCBI (National Center for Biotechnology Information) database. The RNA sense sequence of rat PLCγ1-01 siRNA was 5′-CCGAGAGGAUCGUAUAUCAdTdT-3′ (nucleotide positions 501–519 relative to the start codon of rat PLCγ1; GenBank accession no. NM_013187), where dT is 2′-deoxythymidine. The RNA sense sequence of rat STIM1-01 siRNA was 5′-GAAGCUGCGUGACGAGAUCdTdT-3′ (nucleotide positions 851–869 relative to the start codon of rat STIM1; GenBank accession no. XM_341896) and was the rat equivalent sequence of the human siRNA huSTIM1-1414 described in Roos et al. [13]. The RNA sense sequence of the negative control siRNA was 5′-UUCUCCGAACGUGUCACGUdTdT-3′. The negative or non-silencing control siRNA had no significant sequence homology to any known rat gene. siRNAs were resuspended and annealed according to the manufacturer's instructions to a stock concentration of 20 μM. Both unlabelled- and AlexaFluor-546-labelled negative control siRNA were synthesized.
Transfection and immunofluorescence microscopy
On the day prior to transfection, H4IIE cells were seeded at 5–10% confluence on glass coverslips in 35-mm-diameter Petri dishes containing 1.5 ml of complete DMEM. On the day of transfection, 92 μl of DMEM, 8 μl of 20 μM siRNA and 15 μl of HiPerFect reagent were mixed and incubated at room temperature (21°C) for 5 min. Subsequently, 500 μl of complete DMEM was added to make the final transfection medium, which was then added dropwise to the H4IIE cells, resulting in a final siRNA concentration of 75 nM. The cells were incubated at 37°C for 72–96 h and then processed for immunofluorescence microscopy. Coverslips with transfected H4IIE cells were washed three times with PBS and then fixed in cold methanol at −20°C for 5 min. Fixed cells were washed three times with PBS and blocked with 20% (v/v) FBS in PBS for 15 min at room temperature. The cells were then incubated with rabbit anti-PLC-γ1 antibody (diluted 1:100 in diluent buffer [PBS containing 0.1% Tween-20]) or mouse anti-STIM1 (diluted 1:100 in dilutent buffer) for 3 h and washed three times with diluent buffer. The cells were then incubated with FITC-conjugated goat anti-rabbit IgG (diluted 1:500 in diluent) or AlexaFluor-488-conjugated goat anti-mouse IgG (diluted 1:2000 in diluent) for 1 h, washed three times with PBS and the coverslips mounted on slides in 50% (v/v) glycerol in PBS (pH 8.4) or in ProLong Gold antifade, supplemented with 200 nM DAPI (4′,6-diamidino-2-phenylindole).
Immunofluorescence was visualized using an Olympus BX50 microscope equipped with a U-MNIBA filter (excitation wavelength: 470–490 nm; emission wavelength: 515–550 nm), UplanApo 60×/0.90 or UplanFl 100×/1.30 oil immersion objectives, SPOT RT colour camera and Spot software version 4.0.1 (Diagnostic Instruments, Sterling Heights, MI, U.S.A.).
Transfection for Western blotting
H4IIE cells were seeded at approx. 15% confluence in 25 cm2 culture flasks in 3.5 ml of complete DMEM. Subsequently, 77.3 μl of DMEM, 22.7 μl of 20 μM siRNA and 37.5 μl of HiPerFect reagent were mixed and incubated at room temperature for 5 min. The transfection mixture was then added dropwise to the H4IIE cells, resulting in a final siRNA concentration of 125 nM. The cells were incubated at 37°C for 72–96 h and then cell extracts were prepared.
Preparation of cell extracts and protein quantification
H4IIE cells cultured in 25 cm2 flasks were harvested at the indicated time points after transfection and the protein was extracted according to a modification of the method described by Su et al. [14]. Briefly, adherent cells were rinsed with PBS, harvested in PBS supplemented with 1.8 mM EDTA and the cell pellets resuspended and lysed by vortexing in ice-cold 40 μl of lysis buffer [50 mM Tris/HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% (v/v) Nonidet P-40, 0.25% sodium deoxycholate, 0.1% SDS and 0.1% protease inhibitor cocktail (Sigma)]. After incubation for 10 min on ice, the lysates were centrifuged at 15000 g at 4°C for 10 min and the supernatants retained. The protein concentration was determined using the BCA protein assay.
Western blotting
Protein samples (1 μg) were resolved by SDS/PAGE (7.5% gels) and transferred on to nitrocellulose membranes. The membranes were incubated for 1–2 h at room temperature in blocking buffer [TBST (Tris-buffered saline and 0.1% Tween-20) containing 5% (w/v) non-fat milk powder]. The membranes were then probed with primary antibody (rabbit anti-PLCγ1 antibody diluted 1:400; or rabbit anti-GAPDH antibody diluted 1:500) in blocking buffer at room temperature for 2 h. The membranes were then washed three times with TBST, followed by incubation of the membranes with secondary antibody (goat-anti-rabbit IgG–HRP-conjugate diluted 1:2000 in blocking buffer) at room temperature for 1 hour. The membranes were then washed 3 times with Tris-buffered saline solution containing 0.1% (v/v) Tween-20 and bands were visualized by enhanced chemiluminescence and autoradiography film. The autoradiographs were scanned using a GS-800 densitometer (Bio-Rad Laboratories, Hercules, CA, U.S.A.) and the band intensities quantified using Quantity One software version 4.3.1 (Bio-Rad Laboratories). Membranes to be re-probed were stripped at 50°C for 30 min in stripping solution [100 mM 2-mercaptoethanol, 62.5 mM Tris/HCl, pH 6.8, and (w/v) 2% SDS] and then washed in TBST.
Immunoprecipitation
Near confluent H4IIE cells were harvested, washed and then resuspended in either complete DMEM only or in complete DMEM supplemented with 1 μM thapsigargin and incubated at room temperature for 5 min. Cell lysates were then prepared using either Ca2+-free (EGTA-containing) lysis buffer [50 mM Tris/HCl, pH 7.4, 150 mM NaCl, 5 mM EGTA, 1% (v/v) Nonidet P-40, 0.25% sodium deoxycholate, 0.1% SDS and 0.1% protease inhibitor cocktail] or Ca2+-supplemented (5 mM free Ca2+) lysis buffer in which EGTA of the Ca2+-free lysis buffer was replaced with 5 mM CaCl2. Cell lysates (100 μg aliquots) and each lysis buffer were mixed with either 1 μg of the precipitating antibody (anti-PLCγ1 or anti-STIM1) or PBS only (as a negative control) and incubated at 4°C on a shaking platform for 1 h. Protein A/G plus agarose (20 μl) was added, the mixtures were incubated at 4°C on a shaking platform for 2 h and the immunoprecipitates were collected by centrifugation (15000 g for 10 min at 4°C) and washed three times with the corresponding cell lysis buffer. The immunoprecipitates were resolved by SDS/PAGE, transferred on to nitrocellulose and the proteins detected as described in the Western blotting section. For STIM1-immunoprecipitated cell extracts, the Western blot primary and secondary antibodies were rabbit anti-PLCγ1 (1:400 dilution) and goat antirabbit IgG–HRP-conjugate (1:2000 dilution) respectively. For PLCγ1-immunoprecipitated cell extracts, the Western blot primary and secondary antibodies were mouse anti-STIM1 (1:200 dilution) and donkey anti-mouse IgG–HRP-conjugate (1:1000 dilution) respectively.
Measurements of [Ca2+]cyt using fura 2
To estimate [Ca2+]cyt H4IIE cells grown on glass coverslips were loaded at room temperature for 30 min with 5 μM fura 2/AM (acetoxymethyl ester) in KRH (Krebs/Ringer/Hepes) buffer containing 0.02% pluronic acid. The KRH buffer contained 136 mM NaCl, 4.7 mM KCl, 1.3 mM CaCl2, 1.25 mM MgCl2, 10 mM glucose and 10 mM Hepes (pH 7.4). After 30 min of de-esterification period, the fluorescence emission of fura 2 was imaged at 340 and 380 nm by selecting regions of interest (ROI) under 40× objective of a Nikon TE300 epifluorescence microscope in conjunction with a Sutter DG-4/OF wavelength switcher, Omega XF04 filter set for fura 2, Photonic Science ISIS-3 ICCD camera and UIC Metafluor software, as described previously [15]. Values of fluorescence ratio were converted into values of [Ca2+]cyt using an in situ calibration method and a Kd value of 224 for the Ca2+–fura-2 complex [16]. For determination of the initial rate of increase in [Ca2+]cyt, data were standardized by assigning the baseline fluorescence observed before [Ca2+]cyt re-addition a value of 1, in order to compare [Ca2+]cyt values from different cells. Initial rates were determined by fitting linear regression functions to the standardized [Ca2+]cyt data, for changes starting immediately after [Ca2+]ext re-addition. [Ca2+]cyt plateau was determined by difference of maximum [Ca2+]cyt levels after [Ca2+]ext re-addition minus the ones before [Ca2+]ext re-addition.
Patch clamping
Whole-cell patch clamping was performed at room temperature using a computer-based patch-clamp amplifier (EPC-9, HEKA Electronics, Germany) and PULSE software (HEKA Electronics). The bath solution contained: 140 mM NaCl, 4 mM CsCl, 10 mM CaCl2, 2 mM MgCl2, 10 mM glucose and 10 mM Hepes (pH 7.4). The internal solution contained (mM): 130 mM Cs glutamate, 5 mM CaCl2, 5 mM MgCl2, 1 mM MgATP, 10 mM EGTA and 10 mM Hepes (pH 7.2). The calculated internal free Ca2+ concentration was about 100 nM (EQCAL, Biosoft, Cambridge, U.K.). Depletion of the intracellular Ca2+ stores was achieved by addition of either 20 μM IP3 (Amersham) or 2 μM thapsigargin (Sigma) to the internal solution. Patch pipettes were pulled from borosilicate glass and fire-polished; pipette resistance ranged between 2 and 4 MΩ. Series resistance, for which no compensation was made, did not exceed 10 MΩ. In order to monitor the development of ISOC, voltage ramps between −138 and +102 mV were applied every 2 s, starting immediately after achieving the whole-cell configuration. Acquired currents were filtered at 2.7 kHz and sampled at 10 kHz. All voltages shown have been corrected for the liquid junction potential of −18 mV between the bath and electrode solutions (estimated by JPCalc [17]). The holding potential was −18 mV throughout. The EPC9 amplifier compensated cell capacitance automatically.
Data analysis
Curve fitting and linear regression analysis were performed with GraphPad Prism 4. Data are presented as means±S.E.M. Statistical significance was assessed using Student's t test. Differences between means were considered significant at P<0.05.
RESULTS
Activation of ISOC in H4IIE cells is inhibited by U73122
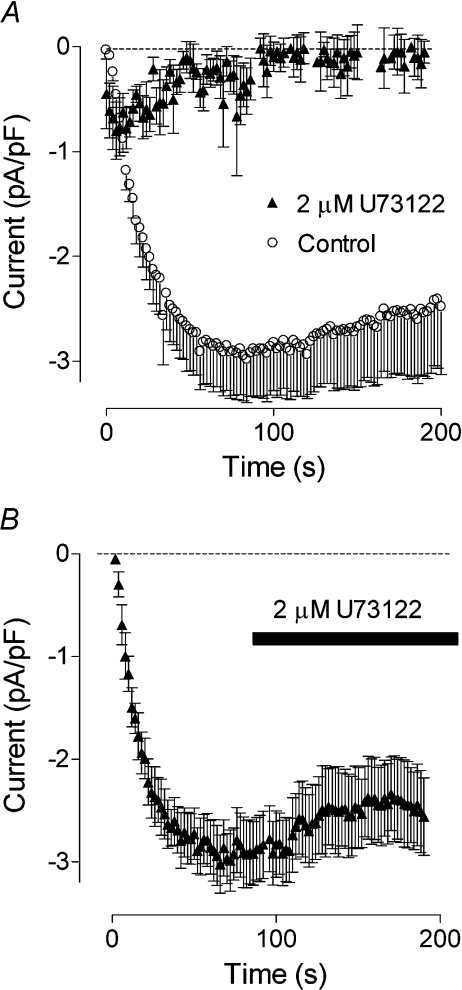
We have shown previously that depletion of intracellular Ca2+ stores in H4IIE liver cells and hepatocytes activates ISOC with characteristics similar to that of ICRAC described in haematopoietic cell lines [10,11]. To investigate a possible role of PLC in the mechanism of ISOC activation, H4IIE cells were pre-incubated with U73122, a widely used PLC inhibitor (2 μM in the bath for 5 min). In these cells IP3 (20 μM applied through the patch pipette) failed to activate any current (Figure 1A). Application of U73122 to the bath after ISOC had fully developed had little effect on current amplitude, suggesting that U73122 did not directly block the current, but had probably affected the mechanism of ISOC activation. Similar results were obtained using thapsigargin (1μM) to activate ISOC (results not shown). U73343, an inactive analogue of U73122, had no effect on the development of ISOC in H4IIE cells (results not shown).
Figure 1. Effect of the PLC inhibitor U73122 on the ISOC in H4IIE cells.
(A) Pre-incubation of H4IIE cells with 2 μM of U73122 for 2–5 min inhibited activation of ISOC by intracellular perfusion with 20 μM IP3 (closed symbols, n=6). For comparison, activation of ISOC by IP3 is shown in untreated cells (open symbols, n=4).(B) Application of U73122 to the bath after the ISOC had fully developed had no effect on its amplitude. Each point represents the amplitude of ISOC at −118 mV taken from voltage ramps from −138 to 102 mV, applied every 2 s.
H4IIE cells express only one isoform of PLC-γ
The results above implied that PLC is required for activation of ISOC in liver cells. It has been suggested previously that different isoforms of PLC-γ might be involved in regulation of SOCE in different cell types. Using protein extracts from H4IIE cells and Western blotting it was found that H4IIE cells express PLC-γ1 with an approximate molecular mass of 150 kDa (results not shown). Another isoform, PLC-γ2, could not be detected in H4IIE cells by Western blot analysis using three different anti-PLCγ2 antibodies, whereas each of these antibodies detected PLCγ2 in MCF-7 cells (results not shown).
Reduction of PLC-γ1 expression by RNA interference
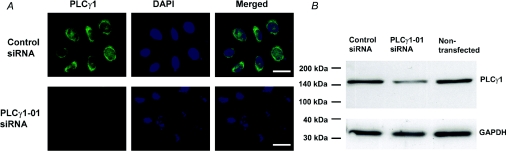
To investigate the role of PLCγ1 in the activation of the ISOC, RNA interference (reviewed in [18]) was used to specifically silence PLCγ1 expression. H4IIE cells were transfected with PLC-γ1-01 siRNA and the reduction in PLCγ1 expression was assessed by Western blotting and immunofluorescence. H4IIE cells transfected with siRNA targeted against PLC-γ1 showed a substantial decrease in PLC-γ1-specific immunofluorescence at 48 h post transfection, with maximum reduction at 72–96 h post transfection (Figure 2A). The reduction in the PLC-γ1 protein levels in transfected cells was confirmed by Western blotting (Figure 2B). Densitometry analysis showed 60–70% knockdown of PLC-γ1 at 72–96 h post transfection (64±4%; n=4).
Figure 2. Knockdown of PLC-γ1 in H4IIE cells by a specific siRNA.
(A) Immunofluorescence microscopy of PLC-γ1 (green) in cells transfected for 96 h with negative control siRNA (first row) and PLC-γ1-01 siRNA (second row). Second column shows staining of the cells nuclei with DAPI (blue), and third column shows merged images. Results are representative of four individual experiments. Bar=20 μm. (B) Reduction of the PLC-γ1 protein as determined by Western blotting. Expression of GAPDH served as a loading control. Molecular mass markers are indicated on the left-hand side of the Figure. Results are representative of four individual experiments.
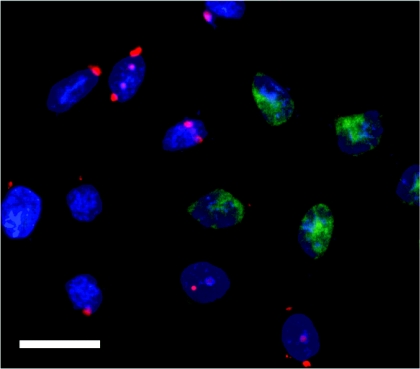
As we could not achieve a complete knockdown of PLC-γ1, negative control siRNA labelled with Alexa Fluor-546 was used to ascertain the level of transfection with siRNA. In most experiments 70–80% of H4IIE cells had detectable levels of fluorescent siRNA at 72–96 h post transfection, suggesting that the maximal achievable level of transfection of H4IIE cells is approx. 80%. Co-transfection of AlexaFluor-546-labelled negative control siRNA and PLC-γ1-01 siRNA showed that the knockdown of PLC-γ1 in individual cells correlated with the presence of the fluorescent siRNA in these cells (Figure 3).
Figure 3. Immunofluorescence microscopy analysis of siRNA transfection efficiency.
H4IIE cells were co-transfected with 38 nM unlabelled PLC-γ1-01 siRNA and 38 nM AlexaFluor-546-labelled negative control siRNA. At 96 h after transfection the cells were fixed, immunostained with anti-PLC-γ1 antibody, followed by incubation with FITC-conjugated secondary antibody and then the nuclei were counterstained with DAPI (blue). H4IIE cells co-transfected with PLC-γ1-01 siRNA (unlabelled) and AlexaFluor-546-labelled negative control siRNA (red) show reduced PLC-γ1 expression (green) compared with neighbouring non-transfected cells. Bar=20 μm.
Decrease in PLCγ1 expression is associated with decreased ISOC and Ca2+ entry
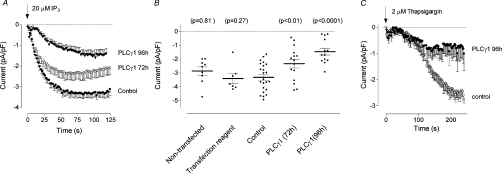
In H4IIE cells transfected with negative control siRNA, intracellular perfusion with 20 μM IP3 activated ISOC with an average maximal amplitude of −3.3±0.21 pA/pF at −100 mV (n=23) (Figures 4A and 4B). The amplitude and the time course of development of ISOC in cells transfected with control siRNA were not different from those in non-transfected cells and cells treated only with the transfection reagent (Figure 4B). Since the magnitude and time course of development of ISOC recorded from cells transfected with control siRNA for 72 and 96 h were the same, these results were pooled and used as a control for both time points. Only cells containing fluorescently labelled siRNA were used for patch clamping. Transfection with siRNA against PLC-γ1 reduced the average maximal amplitude of ISOC measured at −100 mV to −2.3±0.31 pA/pF (n=15) and −1.46±0.24 pA/pF (n=13) at 72 h and 96 h post transfection respectively (Figures 4A and 4B). In addition, at 96 h post transfection, the time required for ISOC to develop to half maximal amplitude increased from 14±2 s in control cells to 26±3 s in cells transfected with siRNA against PLC-γ1. Similarly, when thapsigargin was used to activate ISOC, the average maximal amplitude of the current was reduced from −2.5±0.21 pA/pF (n=17) in cells transfected with control siRNA to −0.7±0.31 pA/pF (n=8) in cells transfected for 96 h with siRNA targeted against PLC-γ1 (Figure 4C).
Figure 4. Effect of PLC-γ1 knockdown on ISOC in H4IIE cells.
(A) Development of ISOC in response to intracellular IP3 in H4IIE cells treated with either control siRNA or PLC-γ1-01 siRNA for 72 and 96 h. (B) Scatter plot of the data presented in panel (A) and the data obtained on non-transfected cells and cells treated with transfection reagent only at 90 s after establishing whole cell. The P values are relative to the Control (cells transfected with control siRNA). (C) Activation of ISOC by intracellular application of thapsigargin in H4IIE cells treated with either control siRNA or PLC-γ1-01 siRNA for 96 h.
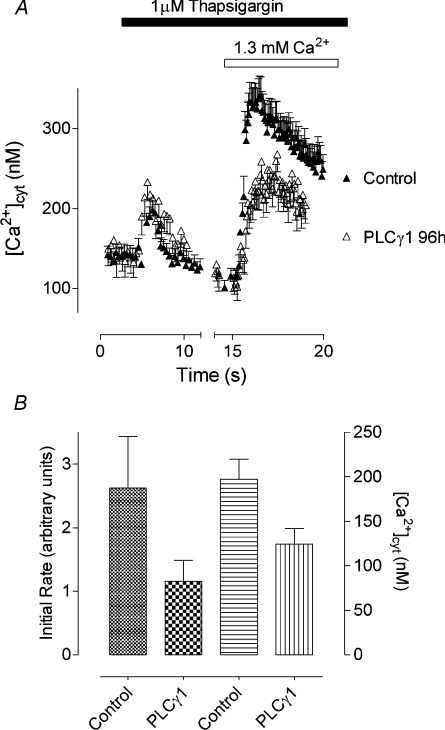
The results obtained in patch-clamping experiments were confirmed by using fura 2 to measure changes in [Ca2+]cyt. In the absence of extracellular Ca2+, the amounts of Ca2+ released from intracellular stores by thapsigargin in cells transfected with control siRNA and PLC-γ1-01 siRNA were similar (Figure 5A). However, upon re-addition of Ca2+ to the bath, the initial rate of the increase in [Ca2+]cyt and the maximum value of [Ca2+]cyt were substantially reduced in cells transfected with PLC-γ1-01 siRNA, compared with cells transfected with control siRNA, indicating a reduction in Ca2+ entry (Figure 5).
Figure 5. Effect of PLC-γ1 knockdown on thapsigargin-induced Ca2+ entry in H4IIE cells.
(A) Reduction of thapsigargin-induced Ca2+ entry in H4IIE cells treated with PLC-γ1-01 siRNA (open symbols) compared with cells treated with control siRNA (closed symbols), at 96 h post-transfection. The results are shown as means±S.E.M. for 15–20 cells; n=4. (B) Reduction of the initial rate and the peak concentration of thapsigargin-induced Ca2+ entry by PLC-γ1 knockdown in four separate experiments (96 h post-transfection). The results are shown as means±S.E.M. for four independent experiments.
Effect of PIP2 and DAG (diacylglycerol) on ISOC
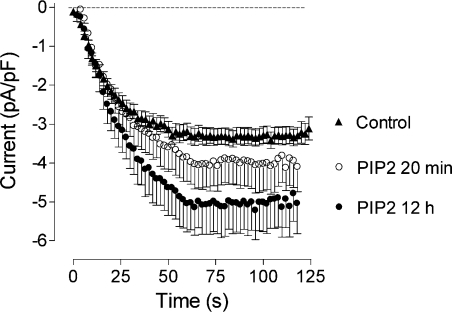
One consequence of the knockdown of PLC-γ1 could be an increase in the concentration of PIP2, the substrate for PLC-γ in the plasma membrane. PIP2 is known to regulate the activity of a wide range of plasma membrane channels [19], but its effects on ISOC in liver cells have not been investigated previously. To establish if the decrease in amplitude of ISOC in cells lacking PLC-γ1 could have resulted from an increase in the concentration of PIP2, we performed patch clamping of cells pre-incubated with exogenous diC8PIP2, a synthetic PIP2 [20,21]. When applied to the bath solution for 10–20 min, diC8PIP2 did not cause a significant change in the amplitude of ISOC (−3.9±0.6 pA/pF, n=9, compared with −3.3±0.21 pA/pF, n=23; P=0.23) activated by intracellular perfusion with IP3. Application of diC8PIP2 to the culture medium for 12–24 h resulted in a significant increase (approx. 50%; P<0.001) of the amplitude of the ISOC (−5.0±0.7 pA/pF, n=5) (Figure 6).
Figure 6. Dependence of the ISOC in H4IIE cells on PIP2.
Development of the ISOC in H4IIE cells transfected with control siRNA for 72–96 h and treated with 10 μM PIP2 for either 20 min or overnight. Each point represents the amplitude of ISOC at −118 mV taken from voltage ramps from −138 to 102 mV, applied every 2 s.
The knockdown of PLC-γ1 could also result in a decrease in the concentration of DAG, a product of PLC activity in the plasma membrane. Ca2+ entry activated by DAG has been shown to contribute to receptor-activated Ca2+ entry in some cell types [22,23]. To investigate the role of DAG in the observed decrease in ISOC caused by knockdown of PLC-γ1, the effect of a DAG analogue, OAG (1-oleoyl-2-acetyl-sn-glycerol), on the activation of ISOC was investigated. Neither acute application of 100 μM OAG to the bath solution after ISOC had fully developed, nor prolonged incubation (up to 1 h) of H4IIE cells with 100 μM OAG had any effect on the ISOC amplitude (results not shown).
Activation of ISOC is inhibited by RNA interference of STIM1
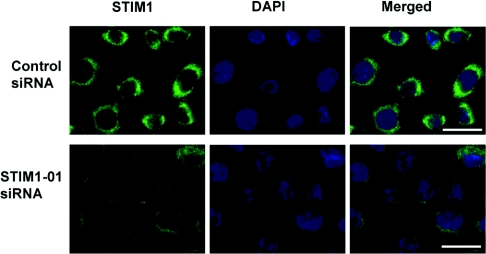
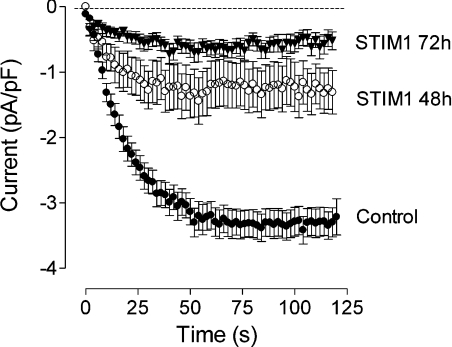
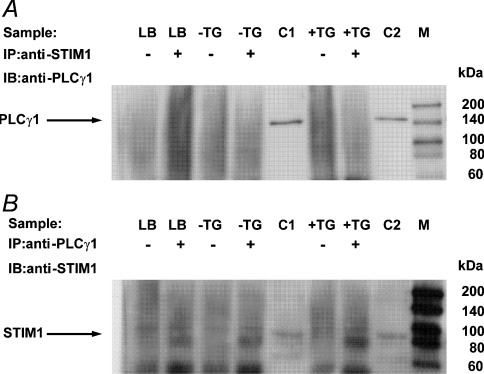
To investigate further the mechanism of PLC-γ1 involvement in regulation of the ISOC in H4IIE cells, we explored the possibility that PLC-γ1 interacts with STIM1, a protein required for activation of ICRAC in haematopoietic cells [2,3]. Western blotting indicated that H4IIE cells express STIM1 with an approximate molecular mass of 85 kDa (results not shown). H4IIE cells did not appear to express the STIM2 isoform, as no 105 kDa band was observed, although the anti-STIM1 antibody employed is known to cross-react with STIM2 [24]. To investigate the role of STIM1 in ISOC activation, H4IIE cells were transfected with STIM1-01 siRNA and the reduction in STIM1 expression was assessed by immunofluorescence microscopy. Maximal reduction in expression was observed at 72–96 h after transfection (Figure 7). siRNA mediated knockdown of STIM1 reduced average maximal amplitude of the ISOC from −3.3±0.22 pA/pF (n=23) to −1.25±0.34 pA/pF (n=13) and −0.62±0.17 pA/pF (n=25) at 48 h and 72h post transfection respectively (Figure 8). These results confirmed that STIM1 is required for activation of ISOC in liver cells. The possible interaction between endogenous PLC-γ1 and STIM1 in H4IIE cells was investigated by immunoprecipitation. As the interaction between PLC-γ1 and STIM1 could be transient and occur only during activation of the store-operated Ca2+ entry pathway, or could be Ca2+-dependent, the experiment was conducted on thapsigargin-treated and untreated cells as well as in the presence and absence of Ca2+ (Figure 9). No interaction between endogenous PLC-γ1 and STIM1 in H4IIE cells was observed in either thapsigargin-treated or untreated cells in the presence (Figure 9) or absence (results not shown) of free Ca2+.
Figure 7. Knockdown of STIM1 in H4IIE cells by specific siRNA.
Immunofluorescence microscopy of STIM1 (green) in cells transfected for 96 h with negative control siRNA (first row) and STIM1-01 siRNA (second row). Second column shows staining of the cells nuclei with DAPI (blue) and the third column shows the merged images. Results are representative of three individual experiments. Bar=20 μm.
Figure 8. Effect of STIM1 knockdown on ISOC in H4IIE cells.
Development of ISOC in response to intracellular IP3 in H4IIE cells treated with either control siRNA or STIM1-01 siRNA for 48 and 72 h. Each point represents the amplitude of ISOC at −118 mV taken from voltage ramps from −138 to 102 mV, applied every 2 s.
Figure 9. Immunoprecipitation of STIM1 and PLC-γ1.
(A) Cell lysates (100 μg) or lysis buffer only (LB) were immunoprecipitated (IP) with anti-STIM1 antibody (+) [or PBS as a negative control (−)] and the immunoprecipitates were analysed by immunoblotting (IB) with anti-PLCγ1 antibody. H4IIE cells were either untreated (−TG) or treated (+TG) with 1 μM thapsigargin and cell lysates were prepared using Ca2+-supplemented (5 mM free Ca2+) lysis buffer. Positive controls included non-immunoprecipitated lysates (10 μg) from cells untreated (C1) or treated (C2) with 1 μM thapsigargin. No STIM1-immunoprecipitated 150 kDa PLC-γ1 band was observed in either the untreated or thapsigargin treated cell lysates. Molecular mass markers (M) are indicated to the right-hand side of the Figure. The results are representative of two independent experiments. (B) Cell lysates (100 μg) or lysis buffer only (LB) were immunoprecipitated (IP) with anti-PLC-γ1 antibody (+) [or PBS (−)] and the immunoprecipitates were analysed by immunoblotting (IB) with anti-STIM1 antibody. No PLCγ1-immunoprecipitated 85 kDa STIM1 band was observed in either the untreated or thapsigargin-treated cell lysates. The results are representative of two independent experiments. The band at approx. 75 kDa present in the IP(+) samples, including the lysis buffer only sample, is most probably non-denatured anti-PLCγ1 immunoprecipitating antibody. The faint band at approx. 95 kDa present in both IP(−) and IP(+) samples, including the lysis buffer only samples, is most probably an artefact due to the Protein A/G Plus agarose beads.
DISCUSSION
Several studies have reported previously that PLC-γ regulates Ca2+ entry in different cell types. As all of these studies used fluorescent Ca2+ probes to measure Ca2+ entry, the types of channels that are regulated by PLC-γ could not be easily established. Nevertheless, using overexpression and/or ablation of expression it was concluded that the likely targets for PLC-γ regulation are different members of the TRP family [25]. The demonstration of a direct interaction of PLC-γ with TRPC1, TRPC2, TRPC3 and TRPM7 supported this notion (for a review see [25]).
In the present study, we demonstrate for the first time that the ablation of expression of PLC-γ1 causes a substantial decrease in Ca2+ entry through SOCs in rat liver cells, which have characteristics similar to those of CRAC channels present in haematopoietic cell lines [10,11]. Accumulated evidence suggests that CRAC channels expressed in haematopoietic cells lines are not encoded by any known member of the TRP family. Moreover, it has recently been clearly demonstrated that the probable molecular component of the pore-forming subunit of the CRAC channel is the Orai1/CRACM1 protein [3,4,13,26]. Considering that the properties of ISOC in liver cells are very similar to those of ICRAC it is also very likely that Orai1, or a highly homologous protein, forms a part of the SOC in liver cells.
Thus the present results indicate a role for PLC-γ1 in the mechanism by which a SOC, similar or identical to the CRAC channel, is activated. Since a saturating concentration of IP3 (20 μM) was used to activate ISOC in the patch clamping experiments, it is unlikely that any reduction in the cytoplasmic concentration of endogenous IP3, due to ablation of PLC-γ1, could explain the observed inhibition of ISOC. The possibility of indirect regulation of ISOC by PLC-γ1 through a decrease in the concentration of DAG, as a consequence of ablation of PLC-γ1, is also unlikely, since the application of 100 μM OAG had no effect on ISOC. The fact that a similar reduction of the amplitude of ISOC in PLC-γ1 knockdown cells was detected when the current was activated by thapsigargin instead of IP3, and that no change was observed in the ability of thapsigargin to induce the release of Ca2+ from intracellular stores, suggests that the effect of PLC-γ1 knockdown on ISOC is not due to alterations in either passive Ca2+ leak from the ER or Ca2+ release through IP3 receptors. Furthermore, the effects of ablation of PLCγ1 are unlikely to be due to a subsequent accumulation of PIP2 in the plasma membrane. If this were the case, addition of exogenous synthetic PIP2 would be expected to inhibit the current (cf. PLC-γ1 ablation), but it actually augmented ISOC. Overall these results suggest that the role of PLC-γ1 in the mechanism of activation of the SOCs does not involve its catalytic activity.
The conclusion that PLC-γ1 has a role in the activation of SOCs in H4IIE liver cells unrelated to its catalytic activity is similar to conclusions reached in several other studies. These include experiments in PC12 and A7r5 cells, which employed siRNA targeted against PLC-γ1 and transient transfection with a lipase-inactive mutant of PLC-γ2 [5], and experiments employing the PLC inhibitor U73122 in hepatocytes [27], keratinocytes [7], DT40-B lymphocytes and RBL cells [6]. In the last mentioned study, it was shown that U73122 inhibits the activation of CRAC channels, while not affecting the channel directly, and that this role of PLC is unrelated to IP3 and to IP3 receptors [6] (cf. the results of the present study). Taken together, these results indicate that the PLC-γ protein is required for the activation of SOCs in many different cell types.
Functional effects of PLC-γ, independent of its catalytic activity, suggest that this enzyme may also act as a molecular scaffold [28]. PLC-γ polypeptides include several protein–protein interaction domains including an N-terminal PH (pleckstrin homology) domain, two SH2 (Src homology 2) domains, one SH3 (Src homology 3) domain and a C-terminal C2 domain [25,29]. Evidence that the SH3 domain, which is involved in the interaction of PLC-γ with the actin cytoskeleton [30], is required for agonist- and thapsigargin-activated Ca2+ entry in PC12 and human embryonic kidney 293 T3-65 cells was obtained using a mutant in which the SH3 domain was deleted [5]. Moreover, there is evidence to indicate that PLC-γ1 can interact with various Ca2+-permeable channels including TRPC1 [7], TRPC2 [31], TRPC3 [5] and TRPM7 [32]. Interestingly, TRPC1 channels that have been shown to interact with PLC-γ1 also interact with STIM1 protein, which acts as a Ca2+ sensor in the activation of CRAC channels [33,34].
Activation of CRAC channels depends on a precise spatial organization between STIM1 and CRACM1/Orai1 proteins and may depend on numerous factors that are currently not known [2]. The mechanism by which PLC-γ1 regulates SOCs in H4IIE cells could involve the interaction of PLC-γ1 with one or more of the proteins involved directly in the activation pathway itself, such as STIM1 and CRACM1/Orai1. A drastic reduction of the ISOC amplitude by the siRNA-mediated knockdown of STIM1 in H4IIE cells suggested that in these cells STIM1 also plays a role of the ER Ca2+ sensor, similar to the role it plays in T-lymphocytes [13]. However, immunoprecipitation experiments did not show a direct interaction between STIM1 and PLC-γ1. Therefore it is unlikely that PLC-γ1 is required for the activation of the ISOC due to its interaction with STIM1. The possibility that PLC-γ1 interacts with Orai1 in H4IIE cells still remains and will be investigated in a separate study. Alternatively, PLC-γ may regulate SOCs indirectly by interacting with the part of the actin cytoskeleton that is involved in the formation and maintenance of regions of the putative junctional ER in close proximity to the plasma membrane where interaction between STIM1 and CRACM1/Orai1 occur [2].
It can be concluded from the present study that PLCγ1 is required for the activation of SOCs, which are similar or identical to CRAC channels. However, the precise role of PLC-γ in the mechanism of activation of the SOCs remains to be established.
Acknowledgments
This work was supported by the Australian Research Council. We thank Dr Michael Roberts (University of Adelaide) for valuable comments on the manuscript.
References
- 1.Bird G. S., Aziz O., Lievremont J. P., Wedel B. J., Trebak M., Vazquez G., Putney J. W., Jr Mechanisms of phospholipase C-regulated calcium entry. Curr. Mol. Med. 2004;4:291–301. doi: 10.2174/1566524043360681. [DOI] [PubMed] [Google Scholar]
- 2.Luik R. M., Wu M. M., Buchanan J., Lewis R. S. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER–plasma membrane junctions. J. Cell Biol. 2006;174:815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peinelt C., Vig M., Koomoa D. L., Beck A., Nadler M. J. S., Koblan-Huberson M., Lis A., Fleig A., Penner R., Kinet J. P. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nat. Cell Biol. 2006;8:771–773. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prakriya M., Feske S., Gwack Y., Srikanth S., Rao A., Hogan P. G. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 5.Patterson R. L., van Rossum D. B., Ford D. L., Hurt K. J., Bae S. S., Suh P. G., Kurosaki T., Snyder S. H., Gill D. L. Phospholipase C-γ is required for agonist-induced Ca2+ entry. Cell. 2002;111:529–541. doi: 10.1016/s0092-8674(02)01045-0. [DOI] [PubMed] [Google Scholar]
- 6.Broad L. M., Braun F. J., Lievremont J. P., Bird G. S., Kurosaki T., Putney J. W., Jr Role of the phospholipase C–inositol 1,4,5-trisphosphate pathway in calcium release-activated calcium current and capacitative calcium entry. J. Biol. Chem. 2001;276:15945–15952. doi: 10.1074/jbc.M011571200. [DOI] [PubMed] [Google Scholar]
- 7.Tu C. L., Chang W., Bikle D. D. Phospholipase Cγ1 is required for activation of store-operated channels in human keratinocytes. J. Invest. Dermatol. 2005;124:187–197. doi: 10.1111/j.0022-202X.2004.23544.x. [DOI] [PubMed] [Google Scholar]
- 8.van Rossum D. B., Patterson R. L., Sharma S., Barrow R. K., Kornberg M., Gill D. L., Snyder S. H. Phospholipase Cγ1 controls surface expression of TRPC3 through an intermolecular PH domain. Nature. 2005;434:99–104. doi: 10.1038/nature03340. [DOI] [PubMed] [Google Scholar]
- 9.Rychkov G. Y., Litjens T., Roberts M. L., Barritt G. J. ATP and vasopressin activate a single type of store-operated Ca2+ channel, identified by patch-clamp recording, in rat hepatocytes. Cell Calcium. 2005;37:183–191. doi: 10.1016/j.ceca.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Litjens T., Harland M. L., Roberts M. L., Barritt G. J., Rychkov G. Y. Fast Ca2+-dependent inactivation of the store-operated Ca2+ current (ISOC) in liver cells: a role for calmodulin. J. Physiol. 2004;558:85–97. doi: 10.1113/jphysiol.2004.065870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rychkov G., Brereton H. M., Harland M. L., Barritt G. J. Plasma membrane Ca2+ release-activated Ca2+ channels with a high selectivity for Ca2+ identified by patch-clamp recording in rat liver cells. Hepatology. 2001;33:938–947. doi: 10.1053/jhep.2001.23051. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds A., Leake D., Boese Q., Scaringe S., Marshall W. S., Khvorova A. Rational siRNA design for RNA interference. Nat. Biotechnol. 2004;22:326–330. doi: 10.1038/nbt936. [DOI] [PubMed] [Google Scholar]
- 13.Roos J., DiGregorio P. J., Yeromin A. V., Ohlsen K., Lioudyno M., Zhang S. Y., Safrina O., Kozak J. A., Wagner S. L., Cahalan M. D., et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su X., Mancuso D. J., Bickel P. E., Jenkins C. M., Gross R. W. Small interfering RNA knockdown of calcium-independent phospholipases A2 β or γ inhibits the hormone-induced differentiation of 3T3-L1 preadipocytes. J. Biol. Chem. 2004;279:21740–21748. doi: 10.1074/jbc.M314166200. [DOI] [PubMed] [Google Scholar]
- 15.Chen J., Barritt G. J. Evidence that TRPC1 (transient receptor potential canonical 1) forms a Ca2+-permeable channel linked to the regulation of cell volume in liver cells obtained using small interfering RNA targeted against TRPC1. Biochem. J. 2003;373:327–336. doi: 10.1042/BJ20021904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 17.Barry P. H. JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. J. Neurosci. Methods. 1994;51:107–116. doi: 10.1016/0165-0270(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 18.Hannon G. J. RNA interference. Nature. 2002;418:244–251. doi: 10.1038/418244a. [DOI] [PubMed] [Google Scholar]
- 19.Suh B. C., Hille B. Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Curr. Opin. Neurobiol. 2005;15:370–378. doi: 10.1016/j.conb.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H., Craciun L. C., Mirshahi T., Rohacs T., Lopes C. M. B., Jin T., Logothetis D. E. PIP2 activates KCNQ channels, and its hydrolysis underlies receptor-mediated inhibition of M currents. Neuron. 2003;37:963–975. doi: 10.1016/s0896-6273(03)00125-9. [DOI] [PubMed] [Google Scholar]
- 21.Li Y., Gamper N., Hilgemann D. W., Shapiro M. S. Regulation of Kv7 (KCNQ) K+ channel open probability by phosphatidylinositol 4,5-bisphosphate. J. Neurosci. 2005;25:9825–9835. doi: 10.1523/JNEUROSCI.2597-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sydorenko V., Shuba Y., Thebault S., Roudbaraki M., Lepage G., Prevarskaya N., Skryma R. Receptor-coupled, DAG-gated Ca2+-permeable cationic channels in LNCaP human prostate cancer epithelial cells. J. Physiol. 2003;548:823–836. doi: 10.1113/jphysiol.2002.036772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venkatachalam K., Zheng F., Gill D. L. Regulation of canonical transient receptor potential (TRPC) channel function by diacylglycerol and protein kinase C. J. Biol. Chem. 2003;278:29031–29040. doi: 10.1074/jbc.M302751200. [DOI] [PubMed] [Google Scholar]
- 24.Williams R. T., Senior P. V., Van Stelenburg L., Layton J. E., Smith P. J., Dziadek M. A. Stromal interaction molecule 1 (STIM1), a transmembrane protein with growth suppressor activity, contains an extracellular SAM domain modified by N-linked glycosylation. Biochim. Biophys. Acta. 2002;1596:131–137. doi: 10.1016/s0167-4838(02)00211-x. [DOI] [PubMed] [Google Scholar]
- 25.Patterson R. L., van Rossum D. B., Nikolaidis N., Gill D. L., Snyder S. H. Phospholipase C-γ: diverse roles in receptor-mediated calcium signaling. Trends Biochem. Sci. 2005;30:688–697. doi: 10.1016/j.tibs.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Soboloff J., Spassova M. A., Tang X. D., Hewavitharana T., Xu W., Gill D. L. Orai1 and STIM reconstitute store-operated calcium channel function. J. Biol. Chem. 2006;281:20661–20665. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- 27.Berven L. A., Barritt G. J. Evidence obtained using single hepatocytes for inhibition by the phospholipase-C inhibitor U73122 of store-operated Ca2+ inflow. Biochem. Pharmacol. 1995;49:1373–1379. doi: 10.1016/0006-2952(95)00050-a. [DOI] [PubMed] [Google Scholar]
- 28.Irvin B. J., Williams B. L., Nilson A. E., Maynor H. O., Abraham R. T. Pleiotropic contributions of phospholipase C-γ1 (PLC-γ1) to T-cell antigen receptor-mediated signaling: reconstitution studies of a PLC-γ1-deficient Jurkat T-cell line. Mol. Cell. Biol. 2000;20:9149–9161. doi: 10.1128/mcb.20.24.9149-9161.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rebecchi M. J., Pentyala S. N. Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol. Rev. 2000;80:1291–1335. doi: 10.1152/physrev.2000.80.4.1291. [DOI] [PubMed] [Google Scholar]
- 30.McBride K., Rhee S., Jaken S. Immunocytochemical localization of phospholipase C-γ in rat embryo fibroblasts. Proc. Natl. Acad. Sci. U.S.A. 1991;88:7111–7115. doi: 10.1073/pnas.88.16.7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tong Q., Chu X., Cheung J. Y., Conrad K., Stahl R., Barber D. L., Mignery G., Miller B. A. Erythropoietin-modulated calcium influx through TRPC2 is mediated by phospholipase Cγ and IP3R. Am. J. Physiol. Cell. Physiol. 2004;287:C1667–C1678. doi: 10.1152/ajpcell.00265.2004. [DOI] [PubMed] [Google Scholar]
- 32.Runnels L. W., Yue L., Clapham D. E. The TRPM7 channel is inactivated by PIP2 hydrolysis. Nat. Cell Biol. 2002;4:329–336. doi: 10.1038/ncb781. [DOI] [PubMed] [Google Scholar]
- 33.Huang G. N., Zeng W. Z., Kim J. Y., Yuan J. P., Han L. H., Muallem S., Worley P. F. STIM1 carboxyl-terminus activates native SOC, ICRAC and TRPC1 channels. Nat. Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- 34.Lopez J. J., Salido G. M., Pariente J. A., Rosado J. A. Interaction of STIM1 with endogenously expressed human canonical TRP1 upon depletion of intracellular Ca2+ stores. J. Biol. Chem. 2006;281:28254–28264. doi: 10.1074/jbc.M604272200. [DOI] [PubMed] [Google Scholar]