Abstract
GRP94 (glucose-regulated protein of 94 kDa) is a major luminal constituent of the endoplasmic reticulum with known high capacity for calcium in vivo and a peptide-binding activity in vitro. In the present study, we show that Ca2+ regulates the ability of GRP94 to bind peptides. This effect is due to a Ca2+-binding site located in the charged linker domain of GRP94, which, when occupied, enhances the association of peptides with the peptide-binding site in the N-terminal domain of the protein. We further show that grp94−/− cells are hypersensitive to perturbation of intracellular calcium and thus GRP94 is important for cellular Ca2+ storage.
Keywords: calcium store, chaperone, endoplasmic reticulum (ER), glucose-regulated protein of 94 kDa (GRP94), heat-shock protein (HSP)
Abbreviations: ABTS, 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid; BiP, immunoglobulin heavy-chain-binding protein; ER, endoplasmic reticulum; ES, embryonic stem; GRP94, glucose-regulated protein of 94 kDa; HRP, horseradish peroxidase; HSP90, heat-shock protein 90; N34–355, N-terminal 34–355 amino acids; Ni-NTA, Ni2+-nitrilotriacetate; Tg, thapsigargin; VSV, vesicular stomatitis virus
INTRODUCTION
GRP94 (glucose-regulated protein of 94 kDa), also known as gp96 (glycoprotein 96), is an ER (endoplasmic reticulum) molecular chaperone that belongs to the HSP90 (heat-shock protein 90) class of stress proteins. It is known to affect the biosynthesis of several secreted and membrane-bound proteins, such as immunoglobulins [1] or Toll-like receptors [2], but the list of known GRP94-chaperoned proteins is not extensive and the specificity of GRP94 in protein recognition is thought to be restricted [3]. Similar to other members of the HSP90 class, the association of GRP94 with its chaperoned proteins can be inhibited with the polycyclic compounds geldanamycin and radicicol, which bind to the N-terminal domain of the protein [4]. Apart from its activity in vivo, GRP94 has long been known to be a peptide-binding protein [5,6]. Its ability to bind many different peptides has been exploited to augment immune responses. When injected into mice, GRP94-bound peptides are transferred on to MHC class I proteins that then activate peptide-specific T-cells [7–9]. We have mapped a peptide-binding site in the N-terminal domain of GRP94, distinct from the geldanamycin/radicicol-binding pocket, and have shown that binding of peptides to this N-terminal site accounts for the immunological activity of GRP94 [10–12]. The peptide-binding activity in vitro may serve as a surrogate assay for the chaperone activity in vivo, although the relationship between these activities still needs to be determined conclusively.
GRP94 has been linked extensively to cellular Ca2+ homoeostasis [13]. One of the defining characteristics that GRP94 shares with other ER stress proteins is that its expression is induced via a transcriptional feedback loop [14] when cells are treated with Ca2+ ionophores [15,16]. GRP94 binds Ca2+ and is one of approximately six luminal proteins that serve as the major Ca2+ buffers of the ER [13,17–19]. Purified GRP94 is predicted to bind 28 mol of Ca2+/mol of protein by one estimate [13] and 16–20 mol/mol by another [19], with a few high-affinity (Kd 1–5 μM) binding sites and the rest being lower-affinity sites (Kd∼600 μM). In these respects, GRP94 resembles two other ER chaperones, calreticulin and calnexin [20,21]. Calreticulin and GRP94 are calculated to provide 30 μM each of Ca2+ storage capacity. Together, the Ca2+-binding proteins in the ER provide a large Ca2+ storage capacity [22] that enables the ER to accumulate high levels of free and bound Ca2+ to supply Ca2+ for release in response to a variety of metabolic demands. Despite the apparent redundancy of ER Ca2+-binding proteins, each of them may play a unique role. For example, the ER of calreticulin-deficient cells can store much less total Ca2+, although the luminal free Ca2+ concentration is unchanged [23]. No unique role in Ca2+ homoeostasis has been ascribed to GRP94 to date, which provided one impetus for the present study.
None of the Ca2+-binding sites of GRP94 have been mapped yet, and importantly, it is not known how the Ca2+ binding activity affects the other activities of the chaperone. In previous work, we demonstrated that several of the biological properties of the full-length protein reside within the segment encoding amino acids 34–355 [10–12], and that amino acids 356–802 can be deleted without affecting these activities. The fragment N34–355 (N-terminal 34–355 amino acids) of GRP94 allows binding of nucleotides, peptides, radicicol or geldanamycin, and it also contains the binding site(s) for the CD91 receptor and SR-A (scavenger receptor type A)[12]. Therefore, the ability of GRP94 to augment peptide presentation and activation of T-cells is contained fully within this segment [12]. N34–355 contains most of the N-terminal domain of GRP94 which is divided into two lobes [24], the helical lobe that contains the nucleotide-binding site [10,11] and the β-sheet lobe that contains the peptide-binding site [11]. In addition, N34–355 contains the first charged domain of GRP94 (amino acids 273–307), to which no distinct activity has so far been ascribed.
Aside from the high-capacity of GRP94 for Ca2+, the only evidence to date for a functional role for Ca2+ binding is that, in the presence of ATP, Ca2+ stimulates the release of GRP94, protein disulfide isomerase, ERp72, calreticulin and p50 from columns of unfolded protein [17]. In the present study we have demonstrated that the N-terminal portion of GRP94 contains at least one high-affinity Ca2+-binding site in the charged linker domain, the occupancy of which is important because it regulates the peptide-binding activity of the chaperone.
MATERIALS AND METHODS
Cells
ES (embryonic stem) cell lines were established from blastocysts flushed at embryonic day 3.5 from pregnant mice. Line 42.1 is from a wild-type C57/B6 embryo, whereas line 14.1 is from a grp94−/− embryo. They were grown in endotoxin-free DMEM (Dulbecco's modified Eagle's medium), supplemented with antibiotics, 20% (v/v) fetal calf serum (HyClone), non-essential amino acids and ESGRO® (Chemicon International), either on feeder layers of irradiated mouse embryonic fibroblasts or on gelatin-coated plates. As described elsewhere (S. Wanderling, O. Ostrovsky and Y. Argon, unpublished work), these cells were capable of differentiation. The phenotype of the line 14.1 cells is due to lack of GRP94 and not to secondary adaptation, because it can be complemented by re-expression of GRP94 (S. Wanderling, O. Ostrovsky and Y. Argon, unpublished work). Transformed cells lines were established from these ES cells by immortalization with hTERT (human telomerase reverse transcriptase). Briefly, line 42.1 and line 14.1 cells were transfected with the plasmid pGRN145 (A.T.C.C.), hygromycin-resistant colonies were selected, re-cloned and expression of GRP94 was verified by Western blotting. The cell lines were capable of growing without feeder cell layers on uncoated tissue-culture plastic dishes and grew well at serum concentrations of 10% or less.
Cell viability assays
Cells that were attached and excluded Trypan Blue were considered live and were counted and evaluate over several doubling times. In some experiments, viable cells were determined by the XTT [2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide] diphenyltetrazolium bromide assay (Roche), according to the manufacturer's instructions. Enzymatic values were converted into absolute cell numbers by calibration with known numbers of ES cells.
Recombinant proteins
N1–355
The construct for expression of N1–355 in insect cells and the purification procedure have been described previously [10]. Recombinant N1–355 contained an N-terminal His6 tag followed by the first 355 amino acids of the mature GRP94 sequence (out of 802) and a C-terminal ER-targeting signal KDEL.
N34–355
The sequence coding for the first 33 amino acids of GRP94 was deleted by PCR cloning. The resultant PCR product was inserted into the pQE-3OXa vector (Qiagen) using BamHI and XmaI to add a His6 tag followed by a Factor Xa recognition sequence at the N-terminus. The plasmid was transformed into M15 Escherichia coli, which were allowed to grow to mid-exponential phase and then incubated with 1 mM IPTG (isopropyl β-D-thiogalactoside) for 4 h at 28°C to induce protein expression. Bacteria were harvested and lysed in 1% NP40 (Nonidet P40) (Sigma Chemicals) in 20 mM phosphate buffer (Na2HPO4 and NaHPO4), pH 7.2, containing 500 mM NaCl and 20 mM imidazole. N34–355 was purified from the detergent lysates by affinity chromatography on Ni-NTA (Ni2+-nitrilotriacetate) columns (Qiagen), according to the manufacturer's instructions. Bound proteins were eluted by use of an imidazole gradient from 20–500 mM in 20 mM phosphate buffer, pH 7.2, containing 20 mM NaCl. The fractions containing N34–355 were purified further on a Mono-Q column (Amersham). Pooled protein fractions were dialysed, concentrated and stored at −80°C in 25 mM Hepes, pH 7.2, 110 mM potassium acetate, 20 mM NaCl and 1 mM magnesium acetate, containing 10–20% (w/v) sucrose (buffer A−Ca2+), or in the same buffer containing 0.1 mM CaCl2 (buffer A).
Cleavage with thrombin
In order to perform thrombin cleavage, 5 μg of either N34–355 or BSA in 25 μl of buffer A were incubated in the presence of 1 unit of thrombin per μg of protein at 37°C for 2 h. The reactions were terminated by addition of EDTA to a final concentration of 5 mM. The digests were resolved by SDS/PAGE.
Peptides
The VSV (vesicular stomatitis virus) 8 peptide, RGYVYQGL, is derived from the VSV N protein, peptide A is a 15-mer (KRQIYTDLEMNRLGK) derived from the G protein of VSV and the peptide SIINFEKL is derived from ovalbumin. The peptides were synthesized at the University of Chicago Peptide Protein Core Facility, purified by HPLC on a C18 column (Waters) and verified by MS. Stock solutions were prepared in DMSO and stored at −80°C. Peptide concentrations were determined by a BCA (binchoninic acid) protein assay (Pierce). Where indicated, peptides were iodinated by use of the IodoBead method (Pierce) and unincorporated iodine was removed by passage over a short Dowex AG1 ×8 column. The specific radioactivity of the peptides was routinely 2×1014–1×1015 c.p.m./mol.
Peptide-binding assays
Two types of peptide-binding assays were used. The solution binding assay was as described previously [10]. In brief, recombinant proteins were incubated with iodinated peptide under saturating conditions and radioactivity associated with protein–peptide complexes was measured after separation of free peptide over spin columns containing P10 beads (Bio-Rad) in buffer A. Iodinated peptide without protein was used as a background control for spin-column separation.
The second assay was a solid-phase plate-binding assay. The 96-well plates (Costar 9017 medium binding, Corning) were coated with a saturating concentration of VSV8. The recombinant proteins (8 μg/ml) were heat-shocked for 10 min at 50°C, added at 100 μl/well and allowed to bind for 30 min. Binding was quantified by use of a HRP (horseradish peroxidase)-conjugated-rabbit anti-His6 antibody (Clontech). After addition of ABTS [2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid)] (Roche) colour development was monitored at 415 nm with a BioTek plate reader. Since both N1–355 and N34–355 normally reached saturation at input levels of 7 or 10 μg/ml respectively, the A415 was defined as 1 and all data points were normalized to it. Inhibition by 300 μM radicicol (Sigma; stock solution in DMSO) was used as a specificity control.
Direct binding of calcium
Direct binding of Ca2+ to N34–355 was measured by using 45Ca2+ as a tracer. N34–355 that had been purified in the absence of Ca2+ was incubated with increasing concentrations of 45Ca2+ for 1–16 h. Equilibrium binding was observed after 2 h. The protein was then separated from free Ca2+ by binding to Ni-NTA beads and the protein-associated radioactivity was counted in a scintillation counter. Background binding of 45Ca2+ to the beads was subtracted and specific binding was calculated on the basis of the known specific ratioactivity.
45Ca2+ overlays
Blots were washed four times with overlay buffer (10 mM imidazole, 70 mM KCl and 5 mM MgCl2, pH 6.8), incubated with 1.5 μCi/ml 45Ca2+ in overlay buffer for 1 h, washed with 50% (v/v) ethanol for 5 min, exposed to a phosphorimager screen for 48 h and developed using a Typhoon imager (GE Heathsystems).
Quantification of 9G10 binding
N34–355 (500 ng/well) was immobilized on Ni-NTA plates (Qiagen) overnight. After washing, the wells were incubated in buffer containing Ca2+ or EGTA as indicated, followed by incubation with a monoclonal anti-GRP94 antibody, 9G10 (StressGen). Binding of the antibody was detected using a HRP-conjugated anti-rat IgG antibody and the ABTS substrate. Colour development was monitored at 415 nm.
Tryptophan fluorescence
Intrinsic tryptophan fluorescence of either N34–355 (2.5 μM) or N1–355 (1 μM) was measured using a JASCO fluorimeter. Each protein in buffer A was untreated or treated with EGTA (1 mM), radicicol (300 μM) or heat-shocked at 50°C for 10 min. Excess unbound ligand was removed by spin-column chromatography, the proteins were excited at 295 nm and their emission spectra were monitored between 300 to 450 nm. The fluorescence values were buffer-corrected and normalized to the protein concentration in each sample.
CD
Recombinant N34–355, which had been purified and stored either in complete buffer A or in Ca2+-free buffers, were desalted into 10 mM Mops, pH 7.0, at a final concentration of 2.8 μM protein. CD spectra of the protein solution were measured using a JASCO 810 spectrometer at 25°C in 0.1-cm-path-length cuvettes. All spectra were buffer-corrected.
RESULTS
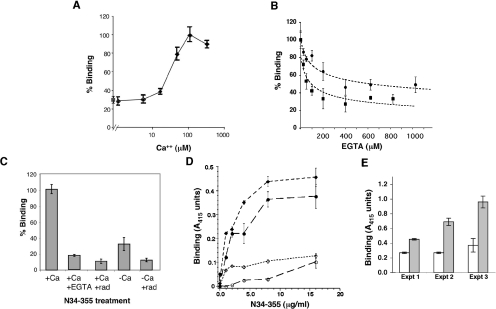
Given that the peptide-binding activity of GRP94 is important for its function in augmenting immune responses, we first investigated whether this activity is regulated by Ca2+. Two short versions of GRP94, designated N1–355 and N34–355, were produced in baculovirus and bacterial expression systems respectively, and used for peptide-binding studies. Both versions contain the N-terminal domain with its peptide-binding site [10,11] and the nucleotide-binding site, as well as the charged linker domain. The main peptide used was the octamer VSV8, the major antigenic determinant of VSV. When N1–355 was used to bind VSV8 in solution, binding was promoted by increased concentrations of Ca2+, with maximal binding activity at approx. 100 μM Ca2+ (Figure 1A). Similarly, when N34–355 was used in solution binding with VSV8 (see Figure 5 below), more peptide was bound in the presence of Ca2+. In a solid-phase binding assay, increasing concentrations of EGTA progressively inhibited peptide binding with maximal inhibition occuring in the presence of 400 μM EGTA (Figure 1B). Importantly, the level of peptide binding in the absence of Ca2+ was similar to the level of peptide binding in the presence of radicicol, a well-studied inhibitor of chaperone function (Figure 1A–1C). The binding of N34–355 to two other peptides, the 15-mer peptide A [10] and the ovalbumin-derived octamer SIINFEKL [8], was similarly sensitive to Ca2+, as shown in Figure 1(D). Both these peptides were demonstrated previously to bind full-length GRP94 in a saturable fashion, and in the cases of VSV8 and peptide A, to cross-inhibit each other, indicating that they presumably bind to the same site [6,10]. Therefore the dependence of peptide-binding on Ca2+ is a property of the protein and not as a result of the peptide or the assay format.
Figure 1. Ca2+ facilitates peptide binding.
(A) N1–355 expressed in Sf9 cells (3.6 μM) was incubated with excess 125I-labelled VSV8 in the presence of increasing Ca2+ concentrations (◆). Protein was separated from free peptide using Biogel P-10 spin columns and the protein-associated radioactivity determined by γ-radiation counting. Values were normalized to the level of peptide binding in the presence of 100 μM Ca2+. ■, The level of binding in the presence of radicicol (300 μM), given as a reference point for a drug-inhibited chaperone [11]. (B) Inhibition of peptide binding by Ca2+ chelation. Progressively higher concentrations of EGTA were added to a standard binding reaction of recombinant chaperones (8 μg/ml) in buffer A (containing 100 μM Ca2+) to VSV8-coated microtitre plates. ●, Experimental data expressed as the means±S.D. of peptide binding of N1–355 produced in insect cells. ■, Binding data of N34–355 produced in bacteria. After the proteins were added to the plates, they were heat-shocked at 50°C for 10 min and binding of the proteins was then allowed to proceed for 30 min at room temperature. Binding was quantified by ELISA with antibody against His6 N-terminal tag, as described in Materials and methods section, normalized to the binding of each protein in the absence of EGTA and is shown as the percentage of maximal binding. Results shown are representative of several experiments with different batches of recombinant proteins. Differences in the extent of Ca2+ sensitivity are not due to the source of the protein (bacterial compared with insect cells), but to the quality of individual protein preparations. (C) Two batches of N34–355 were purified from bacteria and one was stored in buffer A with 100 μM Ca2+ (+Ca), whereas the other was stored in Ca2+-free buffer A (−Ca). Each was heat-shocked at 50°C for 10 min and allowed to bind for 30 min at a concentration of 8 μg/ml to VSV8-coated ELISA plates in the presence or absence of EGTA or radicicol (rad). The binding of N34–355 to the peptide was quantified as above. (D) Ca2+-sensitive binding of two other peptides. Microtitre plates were coated with peptide A (◆, ◇) or the ovalbumin-derived peptide SIINFEKL (●, ○). Different concentrations of recombinant N34–355 protein, purified in the absence of Ca2+, was incubated with the peptide-coated plates either in the presence of 100 μM Ca2+ (●, ◆) or in the absence of Ca2+(○, ◇). Binding was quantified with anti-His6 antibody. (E) Rescue of peptide binding by addition of Ca2+. A plate-binding assay was performed as described in (B)–(D), in Ca2+-free buffer A (open bars), and in parallel in buffer A that was supplemented with 100 μM Ca2+ (closed bars). Three separate experiments are shown, with two different preparations of N34–355 (experiments 1 and 2) and one preparation of N1–355 (experiment 3).
Figure 5. Ca2+ regulates the association of peptide with GRP94.
(A) Time course of the binding reaction. N34–355 (3.6 μM) in buffer A was incubated at 50°C for 10 min and then incubated with 125I-labelled VSV8 in solution for the times indicated. At the end of each time point, the chaperone was separated from the free peptide by spin-column chromatography and its γ-radiation counted. The quantity of chaperone-bound peptide was determined from the known specific radioactivity of 125I-labelled VSV8. ◆, Binding in the presence of 100 μM Ca2+. ◇, Binding in the absence of Ca2+. (B) Time course of 125I-labelled VSV8 binding to N34–355 at room temperature without heat shock. The extent of complex formation was determined by γ-radiation counting as in (A). ♦, Binding in the presence of 100 μM Ca2+. ◇, Binding in the absence of Ca2+. (C) Inhibition of peptide binding by increasing concentrations of EGTA when present during the binding reaction (□) or during the dissociation phase (●). Binding of N34–355 to the peptide was determined using the peptide-coated plate assay, with the protein quantified with anti-His6 antibody and developed with the substrate ABTS. ■, The inhibition of peptide binding by radicicol. (D) Dose binding of intact N34–355 to VSV8-coated plates (untreated N34–355, ●) or after cleavage with thrombin (N34–355+Th, □). The plate assay was used as in (C) above.
In further support of the regulation of activity by Ca2+, when recombinant N34–355 protein was purified in Ca2+-containing buffers, it displayed superior peptide-binding activity that was inhibited in the presence of EGTA. However, when the recombinant protein was purified in Ca2+-free buffers, it had a much lower peptide-binding activity (Figure 1C). This was a reversible deficiency: when Ca2+ was added back to N34–355 that had been purified in the absence of Ca2+ or to EGTA-treated protein, peptide binding was restored (Figure 1E), maximal activity was regained in the presence of 100 μM Ca2+. Addition of Mg2+ did not have a similar effect (results not shown). This shows that the effect of EGTA treatment on N34–355 was via its action as a Ca2+ chelator.
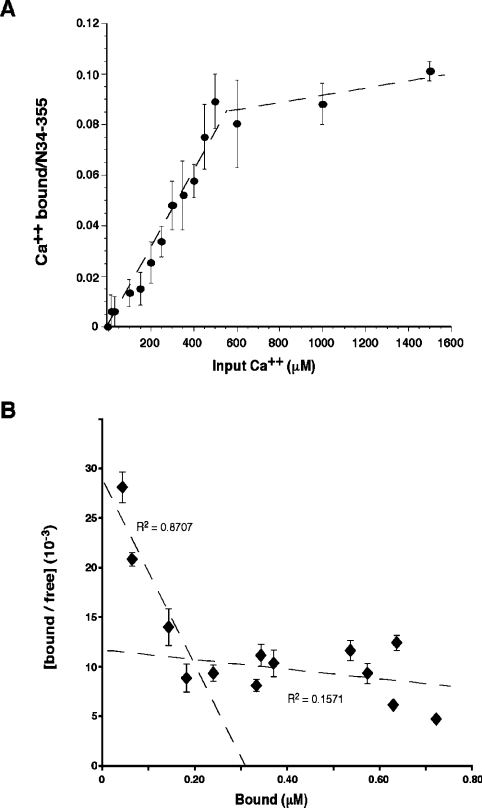
To demonstrate directly that Ca2+ binds to the truncated GRP94 construct, we incubated the protein with various concentrations of 45Ca2+ of known specific activity and measured the protein-bound radioactivity at equilibrium (Figure 2A). Binding of Ca2+ to the protein was saturable at >500 μM input Ca2+. Scatchard analysis of the binding isotherm showed biphasic behaviour (Figure 2B). The higher-affinity binding is consistent with one binding site per molecule of N34–355 and an estimated Kd of 110±50 μM. This value is within the physiological range for fluctuations of free Ca2+ in the ER. The low-affinity binding represents multiple binding sites with an average dissociation constant of 1.4±0.6 mM.
Figure 2. Direct binding of Ca2+ to N34–355.
(A) Recombinant N34–355 (3.6–6.9 μM per reaction) that had been purified in Ca2+-free buffer was incubated with increasing concentrations of 45Ca2+ at known specific activities for 4 h at room temperature. Each sample was then passed over Ni-NTA beads to separate bound complexes from free Ca2+, and the radioactivity associated with N34–355 was measured by scintillation counting. The background-corrected radioactivity was used to calculate the amount of Ca2+ bound to the protein. The results shown are the means±S.E.M. for three experiments. (B) Scatchard plot of the binding results in (A).
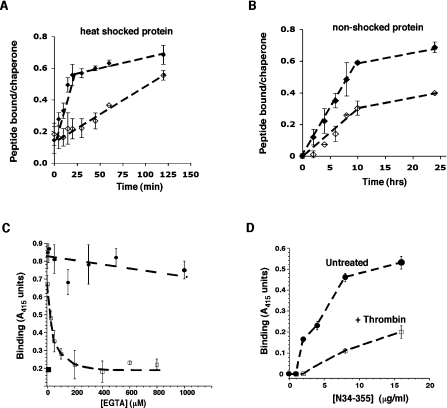
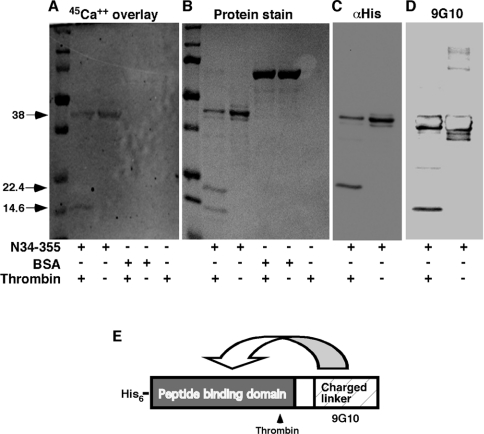
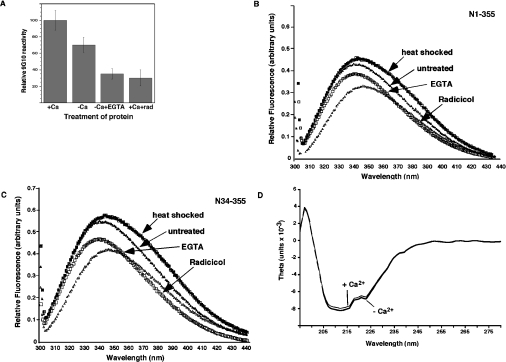
Since no Ca2+-binding sites have yet been mapped on to GRP94, we aimed to localize the Ca2+-binding site within N34–355 using the blot overlay technique. As shown in Figure 3, when blots containing N34–355 were incubated with 45Ca2+, direct binding of Ca2+ to the protein could be visualized. This binding was specific, since no Ca2+ binding to either BSA or other proteins was detected. Digestion of the protein with thrombin, which had one potential cleavage site after Arg222, generated two fragments of 22.4 kDa and 14.6 kDa (Figure 3B). When the radioactive 45Ca2+ overlay technique was used on thrombin-cleaved N34–355, the radioactivity was clearly associated with the smaller thrombin fragment and not with the larger one (Figure 3A). As we have shown previously [11], the 22.4 kDa thrombin fragment encompasses the N-terminal His6 tag, the nucleotide-binding site and, importantly, the peptide-binding site. The 14.6 kDa fragment, on the other hand, contains the C-terminus and the 9G10 monoclonal antibody epitope (Figure 3D). We conclude that the Ca2+ site, which affects peptide binding, maps to the charged linker domain of GRP94.
Figure 3. Localization of Ca2+ binding to the charged linker domain.
N34–355 was digested with thrombin (1 unit/μg of protein), which cleaves after Arg222. The digest was resolved by SDS/PAGE and blotted on to nitrocellulose membranes. BSA was treated and analysed in parallel, as a specificity control. (A) The blot was probed with 45Ca2+ (1.5 μCi/ml) and developed by phosphoimaging, showing 45Ca2+ binding to the smaller thrombin fragment, as well as to the full-length N34–355. (B) Coomassie Brilliant Blue staining of the gel, showing the two thrombin fragments as well as the undigested N34–355 (or BSA). (C) Probing the blot with anti-His, to detect the N-terminal His6 tag [see (E)] identifies the larger thrombin fragment as the N-terminal one. (D) Probing the blot with anti-GRP94 monoclonal antibody 9G10, thee epitope for which is in the charged linker domain [see (E)], identifies the smaller thrombin fragment as the C-terminal one. (E) Diagram of the two domains of N34–355, the location of the thrombin cleavage site and the affinity tag. The putative interactions between the Ca2+-binding and peptide-binding domains are shown by the arrow.
Since the epitope for the anti-GRP94 monoclonal antibody, 9G10, maps to the same charged domain [10,25], we tested whether Ca2+ binding affects the accessibility of the 9G10 epitope. Full binding of the antibody to N34–355 was obtained in the presence of ≥100 μM Ca2+, but antibody binding was reduced if N34–355 was treated with EGTA or if N34–355 was purified under Ca2+-free conditions (Figure 4A). Since the 9G10 epitope is conformational in nature and binding was abolished when GRP94 was inhibited with radicicol or geldanamycin ([10] and Figure 4A), these results are consistent with a Ca2+-induced conformational change in the charged linker domain. This conformational change seems to be localized, as there is no significant change in the mobility of N34–355 when analysed by blue native gel electrophoresis (results not shown). A second technique used to measure conformational changes was tryptophan fluorescence in response to various treatments of N34–355 or N1–355. As shown in Figures 4(B) and 4(C), treatment with radicicol decreased the amplitude of tryptophan fluorescence and shifted the λmax, indicating a conformational change occurred in both of the truncated versions of GRP94. On the other hand, the brief heat shock used to activate peptide binding did not affect either the amplitude or λmax, but shifted the red region of the spectrum. Treatment of the protein with EGTA also led to a change in tryptophan fluorescence, but the change was distinct from that caused by radicicol treatment: the decrease in fluorescence amplitude was less severe and the λmax was blue-shifted in the presence of EGTA, but red-shifted by 3–4 nm in the presence of radicicol (Figures 4B and 4C). When analysed by CD, the far-UV spectra of Ca2+-loaded or Ca2+-free protein were virtually identical (Figure 4D), indicating no change in the secondary structure. Therefore, Ca2+ binding induces a tertiary structure change in the protein and the conformational change induced by Ca2+ is different from that induced by radicicol, even though the absence of Ca2+ and the presence of radicicol inhibit peptide binding to a similar extent.
Figure 4. Conformational changes upon Ca2+ binding.
(A) Reactivity of the monoclonal antibody 9G10. N34–355 modified with biotin near its C-terminus was immobilized via its N-terminal His tag on Ni-NTA plates under the described conditions. It was then incubated with the antibody 9G10 and developed using an HRP-conjugated secondary antibody and colour development was quantified with a plate reader. (B), (C) Changes in intrinsic tryptophan fluorescence of the truncated chaperone. Tryptophan fluorescence of either N34–355 (2.5 μM) or N1–355 (1 μM), excited selectively at 295 nm, was monitored under different conditions. The emission spectra for the untreated protein in buffer A at room temperature, the protein after a 10 min heat shock at 50°C, radicicol-inhibited protein and EGTA-chelated (1 mM) protein are shown. The data in (B) are representative of two experiments and in (C) of three experiments with different protein preparations. Amino acids 1–33 of GRP94 do not contain tryptophan or tyrosine and are dispensable for peptide binding [10]. (D) CD spectra of Ca2+-bound and Ca2+-free N34–355. The molar ellipticity (theta) is plotted as a function of wavelength in the far-UV region. N34–355 solutions (2.8 μM) in 10 mM Mops, pH 7, were placed in a 0.1-cm-path-length cuvette and spectra were measured at 25°C with a JASCO 810 instrument. The spectra were then buffer-corrected. Ca2+-bound protein was purified and stored in complete buffer A, whereas the Ca2+-free N34–355 was purified and stored in buffers lacking Ca2+.
Given that Ca2+ binding regulates peptide binding at a distance, we asked whether the regulation affects the association of peptide or its stability once bound. Several lines of evidence suggest that Ca2+ binding regulates peptide binding by affecting the on-rate. Firstly, solution binding experiments show that, in the presence of Ca2+, the rate of peptide binding is 20.5 mmol of peptide bound/mol of chaperone per min, whereas in the absence of Ca2+ it is less than 6 mmol/min (Figure 5). Furthermore, in the absence of Ca2+ there is a distinct lag phase, but eventually the level of binding approaches that observed in the presence of Ca2+ (Figure 5A), indicating that the fraction of protein molecules capable of peptide binding is not affected. Similar enhancement of the rate of association by Ca2+ was observed with a solid-phase binding assay using peptide-coated plates (results not shown).
This enhancement by Ca2+ was observed when the chaperone was treated by a brief 50°C heat shock. It has been argued that heat shock denatures GRP94 and therefore the activity observed under such conditions is not representative of the activity of the protein in vivo [26]. Therefore, we examined whether Ca2+ enhances peptide binding when the reaction is conducted entirely at room temperature without the heat-shock treatment. We demonstrated previously [10] that in the absence of the heat-shock treatment the binding kinetics are slow and saturation of peptide binding requires hours instead of minutes. As shown in Figure 5(B), the presence of Ca2+ stimulated peptide binding, even without heat- shocking the protein, demonstrating that peptide binding and its regulation are fundamentally similar with or without heat shock. Under the latter conditions, however, the major effect of Ca2+ was not on the slope of the binding curve (1 mmol compared with 0.6 mmol peptide bound/mol chaperone per min), but rather on the saturation level of binding (Figure 5B). This effect is consistent with the interpretation that Ca2+ acts to increase the fraction of protein in the active conformation and that, without Ca2+, only approximately half of the molecules are competent to bind peptide.
A second reason for focusing on the on-rate of peptide binding is that, although EGTA inhibits when present during peptide binding, addition of EGTA after peptide binding did not dissociate the complex (Figure 5C). Third, thrombin-cleaved N34–355 loses its peptide-binding activity (Figure 5D); the residual activity is proportional to the amount of intact N34–355 left after the enzymatic digestion (for example, Figure 3B), but not to the total protein in the reaction. We therefore infer that the conformational change upon Ca2+ binding promotes the association of peptide with the curved β-sheet in the N-terminal domain, rather than augmenting peptide binding by affecting the dissociation rate. This conclusion is in line with the previous observation [11] that, after the peptide–protein complex was formed, thrombin digestion and EGTA treatment did not dissociate the peptide from the 22.4 kDa fragment.
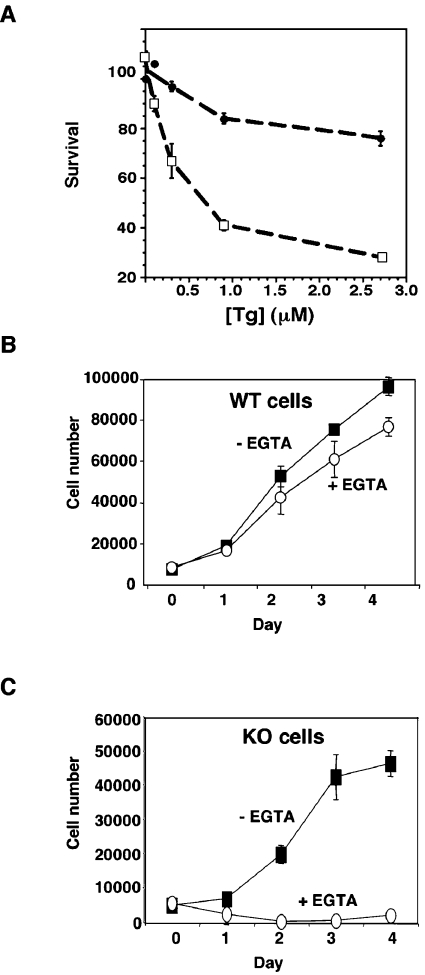
We have demonstrated that GRP94 is required for the cellular stress response to serum deprivation (growth-factor withdrawal) due to its activity as a chaperone and, presumably, via its ability to bind peptides (O. Ostrovsky and Y. Argon, unpublished work). In order to determine whether the Ca2+ binding activity of GRP94 is also important for stress responses, we used a homozygous ES cell line with both GRP94 alleles deleted by homologous recombination in comparison with a wild-type ES cell line. Both lines were derived from blastocysts from an intercross between grp94−/+ mice, as described elsewhere (S. Wanderling, O. Ostrovsky and Y. Argon, unpublished work). The response of the ES cells to Ca2+ perturbation was first measured by the toxicity of Tg (thapsigargin), a specific inhibitor of the ER ATPase-Ca2+ pump. As shown by dose–response experiments (Figure 6A), grp94−/− embryonic fibroblasts are more sensitive to Tg treatment than wild-type cells. Treatment with Tg for 7 h lead to only 20% cell death, even at the higher concentrations, whereas grp94−/− cells showed substantial cell death at 300 nM within this time frame, with 50% of cells dead at 700 nM (Figure 6A). After longer times of treatment, GRP94-sufficient cells are also progressively affected, but at each treatment concentration/time, the grp94−/− cells are affected more severely (results not shown).
Figure 6. Grp94−/− cells are hypersensitive to calcium perturbation.
(A) Hypersensitivity to Tg treatment. Wild-type (●) and grp94−/− (□) ES cells were treated with the indicated concentrations of Tg in triplicate wells and cell viability was assessed after 7 h using an XTT colorimetric assay. (B) Proliferation of wild-type (WT) ES cells when grown in the presence or absence of 1 mM EGTA in the medium. The number of viable cells was counted and cells were evaluated over several doubling times. (C) Proliferation of grp94−/− ES cells when grown in the presence or absence of 1 mM EGTA in the medium. KO, knockout.
Another method to perturb Ca2+ homoeostasis is to culture cells in medium containing EGTA, to remove any extracellular Ca2+ source. As shown in Figure 6(B), wild-type cells continue to proliferate when grown with or without EGTA, but grp94−/− cells stop growing in Ca2+-depleted medium, although they do not die and remained attached to the plates (Figure 6C). Such hypersensitivity to Ca2+ perturbation is specific for GRP94 ablation, because calreticulin-deficient cells are not hypersensitive to Tg and have normal Ca2+ stores [27]. Calreticulin is as abundant as GRP94 in the ER and has a similar capacity for Ca2+, approx. 30 μM. Thus the effect of GRP94 ablation, despite the presence of a number of other ER Ca2+-binding site proteins, suggests that some unique activity of GRP94 is related to Ca2+ homoeostasis and is important for cell viability.
DISCUSSION
The present study establishes a functional role for Ca2+ binding by the chaperone GRP94. GRP94 is not only a passive high-capacity reservoir for Ca2+, but rather its activity is regulated by Ca2+. Furthermore, despite the redundancy of Ca2+-binding proteins in the ER, the ability of GRP94 to bind Ca2+ is uniquely important in the cellular context, since in the absence of GRP94, cells are hypersensitive to perturbations in Ca2+ homoeostasis.
Our results show that the peptide-binding activity of GRP94, which is likely to be related to the chaperone activity of the protein, requires Ca2+. As shown in Figure 3(E), a Ca2+ ion binds to a site within the charged linker domain of GRP94 (amino acids 266–355), inducing a conformational change that promotes the on-rate of peptide binding to the curved β-sheet in the N-terminal domain, without substantially affecting the off-rate. The structural basis for this conformational change remains to be determined. Although the charged linker domain is included in the constructs used by Soldano et al. [24] to solve the structure of the N-terminal portion of GRP94, this linker is not visible in the crystal structures. However, indirect evidence suggests that the charged linker affects the conformation of the peptide-binding site, because the C-terminal 10 amino acids of the linker domain fold back on to the β-sheet in the crystal structure [24]. More evidence for a conformational cross-talk between the two domains is the loss of a monoclonal-antibody-binding site in the charged linker domain when the N-terminal domain is bound by the inhibitors geldanamycin or radicicol [11]. Therefore, it is quite possible that modulation of the conformation of the charged linker affects the ability of the N-terminal domain to bind peptides.
Recent work employing SPR (surface plasmon resonance) challenged the specificity of peptide binding to GRP94, concluding that the binding of the same peptide used here was non-specific and inhibited by Ca2+ [26]. Therefore it was important to establish specificity and regulation of binding. The specificity of peptide binding was demonstrated in the present study by the saturable binding isotherm, by the sensitivity of binding to Ca2+ and the known sensitivity to geldanamycin and radicicol. Importantly, we extended previous work by showing that, regardless of whether the protein has been heat-shocked or not, it is still able to bind the peptide with the same saturation level and a molar ratio close to 1:1, strongly suggesting a single peptide-binding site. None of these observations would be expected if peptide binding is merely adsorptive. The heat-shock treatment does not create new binding sites and only accelerates the association rate; therefore it is a valid experimental method to study the activity of the protein. We ascribed the results in [26] to the use of an inactive protein due to the procedure of coupling it to the Biacore chip (C. A. Makarewich and Y. Argon, unpublished work). As the stoichiometry of peptide binding is the same whether full-length GRP94 or the truncated N34–355 protein is used [10], the peptide-binding characteristics shown in the present study and in previous work [10] also represent the binding activity of the full-length chaperone.
The exact site of Ca2+ binding has not yet been mapped. The charged linker domain does not contain any canonical EF-hand metal-binding sites. However, amino acids 266–290 and 329–334 of GRP94 contain several stretches of three to six consecutive Asp-Glu residues, each of which could potentially co-ordinate Ca2+ atoms. Site-directed mutagenesis would be necessary to precisely map the Ca2+-binding sites. Full-length GRP94 has been reported to possess several Ca2+-binding sites with affinity at the range of 1–5 μM [19]. The site demonstrated in the present study in the charged linker of N34–355 has approx. 10-fold lower affinity, so there must be other, higher-affinity sites elsewhere in full-length GRP94.
As GRP94 resides in the lumen of the ER, where free Ca2+ fluctuates between low micromolar to approximately millimolar concentrations [22], it is important to note that its higher-affinity Ca2+-binding sites are tuned to the range of free luminal Ca2+. The site in the charged linker should be occupied under most luminal Ca2+ concentrations, but would be empty under severe Ca2+ depletion, when free Ca2+ can be as low as 1 μM. Consequently, the peptide-binding activity would be minimal under conditions that deplete the ER Ca2+ stores. It would be important to determine whether the Ca2+ effect on peptide binding is important for the chaperone activity of GRP94 in vivo, as this is one possible connection between the in vitro results of the present study and the hypersensitivity of GRP94-deficient cells to depletion of Ca2+ stores.
There is a precedent for Ca2+-regulated protein binding in the ER, as both of the lectin-type chaperones, calnexin and calreticulin, have a Ca2+-binding site of similar affinity to that of GRP94 [20] and this site maps to a region distinct from, but close to, the carbohydrate-recognition site, and occupancy of this site positively influences the chaperone activity of calnexin and calreticulin [21,28,29]. For example, Di Jeso et al. [30] found that Tg treatment induces the premature exit of thyroglobulin folding intermediates from the calnexin/calreticulin cycle, while stabilizing and prolonging thyroglobulin interactions with BiP (immunoglobulin heavy-chain-binding protein) and GRP94. ER Ca2+ depletion also inhibited the folding of membrane proteins such as LRP (lipoprotein-receptor-related protein) [31] and TCR (T-cell receptor) [32].
Although we have uncovered a mechanism that can regulate GRP94 activity in the cell, it is not immediately obvious why the presence of GRP94 is important for the ER Ca2+ stores. No interaction between GRP94 and proteins involved in Ca2+ homoeostasis has been reported, and therefore the hypersensitivity of grp94−/− cells to Tg may indicate a separate function of GRP94, distinct from its chaperone activity. Nonetheless, this function is a property specific for GRP94, because ablation of calreticulin, whose concentration in the lumen is similar to that of GRP94 and estimated at 30 μM, does not lead to similar hypersensitivity. Similarly, overexpression of the membrane-bound chaperone calnexin also does not affect Ca2+ stores [27,33]. The observed hypersensitivity is not likely to be due to depletion of the storage capacity, because not only calreticulin, but also protein disulfide isomerase, BiP and other ER proteins, bind multiple Ca2+ atoms per molecule of protein [34] and their abundance in the ER is unchanged in grp94−/− cells (O. Ostrovsky and Y. Argon, unpublished work). Therefore it is more likely that there is a functional or physical association between GRP94 and either the Ca2+ pumps, the Ca2+ leakage channels in the ER membrane or a regulatory protein. A specific function for GRP94 in Ca2+ homoeostasis may be inferred from the importance of GRP94 levels in muscle cells. Gorza et al. [35]. found that over-expression of GRP94 protects cardiomyocytes from the toxic effects of high free intracellular Ca2+ and that a reduced level of GRP94 compromises the fusion competence of skeletal myoblasts [36].
A further important consequence of the finding that the peptide-binding ability of GRP94 is stimulated in the presence of Ca2+ is for the design and use of GRP94 as a vaccine. Full-length GRP94, and more recently, the N1–355 portion of the chaperone, have been used to stimulate cytotoxic T-cell production against viruses and tumours, both in animal models and in clinical trials [12,35]. In a typical application, purified chaperone is loaded with peptides that are recognized by T-cells and are injected into the recipient, in order to be taken up by dendritic cells and macrophages. Once in these cells, the bound peptide is delivered to MHC class I molecules, which subsequently traffic to the surface of the dendritic cell or macrophage and present the peptide to T-cells. The efficiency of loading in vitro is affected by Ca2+ levels, as shown in the present study, and it is also possible that peptide unloading in recipient cells is affected. It is, therefore, important to understand how Ca2+ regulates peptide binding and release in order to optimize the design of chaperone-mediated vaccines.
Acknowledgments
We thank Dr Jan Burkhardt for insightful comments and suggestions, Dr S. Lund-Katz, Dr J. Orange and the Children's Hospital of Philadelphia protein core facility for the use of their instrumentation, Dr G. Reddy (Protein-Peptide Core Facility, Biological Sciences Division, University of Chicago) for peptide synthesis and S. Berardi and P. Xu for protein purification. This work was funded by grants from the National Institutes of Health (AI-30178), from the W. W. Smith Foundation (to Y. A.) and, in part, by a grant from the Pennsylvania Department of Health. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations or conclusions. O. O. was supported by a fellowship from the Juvenile Diabetes Research Foundation. T. G. was supported in part by training grant HL-07237 from the National Institutes of Health (NIH).
References
- 1.Melnick J., Dul J. L., Argon Y. Sequential interaction of the chaperones BiP and GRP94 with immunoglobulin chains in the endoplasmic reticulum. Nature. 1994;370:373–375. doi: 10.1038/370373a0. [DOI] [PubMed] [Google Scholar]
- 2.Randow F., Seed B. Endoplasmic reticulum chaperone gp96 is required for innate immunity but not cell viability. Nat. Cell Biol. 2001;3:891–896. doi: 10.1038/ncb1001-891. [DOI] [PubMed] [Google Scholar]
- 3.Argon Y., Simen B. B. GRP94, an ER chaperone with protein and peptide binding properties. Semin. Cell Dev. Biol. 1999;10:495–505. doi: 10.1006/scdb.1999.0320. [DOI] [PubMed] [Google Scholar]
- 4.Schulte T. W., Akinaga S., Murakata T., Agatsuma T., Sugimoto S., Nakano H., Lee Y. S., Simen B. B., Argon Y., Felts S., et al. Interaction of radicicol with members of the heat shock protein 90 family of molecular chaperones. Mol. Endocrinol. 1999;13:1435–1448. doi: 10.1210/mend.13.9.0339. [DOI] [PubMed] [Google Scholar]
- 5.Srivastava P. K., Udono H. Heat shock protein–peptide complexes in cancer immunotherapy. Curr. Opin. Immunol. 1994;6:728–732. doi: 10.1016/0952-7915(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 6.Blachere N. E., Li Z., Chandawarkar R. Y., Suto R., Jaikaria N. S., Basu S., Udono H., Srivastava P. K. Heat shock protein–peptide complexes, reconstituted in vitro, elicit peptide-specific cytotoxic T lymphocyte response and tumor immunity. J. Exp. Med. 1997;186:1315–1322. doi: 10.1084/jem.186.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suto R., Srivastava P. K. A mechanism for the specific immunogenicity of heat shock protein-chaperoned peptides. Science. 1995;269:1585–1588. doi: 10.1126/science.7545313. [DOI] [PubMed] [Google Scholar]
- 8.Berwin B., Rosser M. F., Brinker K. G., Nicchitta C. V. Transfer of GRP94(Gp96)-associated peptides onto endosomal MHC class I molecules. Traffic. 2002;3:358–366. doi: 10.1034/j.1600-0854.2002.30505.x. [DOI] [PubMed] [Google Scholar]
- 9.Li H. T., Yan J. B., Li J., Zhou M. H., Zhu X. D., Zhang Y. X., Tien P. Enhancement of humoral immune responses to HBsAg by heat shock protein gp96 and its N-terminal fragment in mice. World J. Gastroenterol. 2005;11:2858–2863. doi: 10.3748/wjg.v11.i19.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vogen S. M., Gidalevitz T., Biswas C., Simen B. B., Stein E., Gulmen F., Argon Y. Radicicol-sensitive peptide binding to the N-terminal portion of GRP94. J. Biol. Chem. 2002;277:40742–40750. doi: 10.1074/jbc.M205323200. [DOI] [PubMed] [Google Scholar]
- 11.Gidalevitz T., Biswas C., Ding H., Schneidman-Duhovny D., Wolfson H. J., Stevens F., Radford S., Argon Y. Identification of the N-terminal peptide binding site of glucose-regulated protein 94. J. Biol. Chem. 2004;279:16543–16552. doi: 10.1074/jbc.M313060200. [DOI] [PubMed] [Google Scholar]
- 12.Biswas C., Sriram U., Ciric B., Ostrovsky O., Gallucci S., Argon Y. The N-terminal fragment of GRP94 is sufficient for peptide presentation via professional antigen presenting cells. Int. Immunol. 2006;18:1147–1157. doi: 10.1093/intimm/dxl049. [DOI] [PubMed] [Google Scholar]
- 13.Macer D., Koch G. L. R. Identification of a set of calcium-binding protein in reticuloplasm, the luminal content of the endoplasmic reticulum. J. Cell Sci. 1988;91:61–70. doi: 10.1242/jcs.91.1.61. [DOI] [PubMed] [Google Scholar]
- 14.Little E., Ramakrishnan M., Roy B., Gazit G., Lee A. S. The glucose-regulated proteins (GRP78 and GRP94): functions, gene regulation, and applications. Crit. Rev. Eukaryot. Gene Expr. 1994;4:1–18. doi: 10.1615/critreveukargeneexpr.v4.i1.10. [DOI] [PubMed] [Google Scholar]
- 15.Lee A. S. Coordinated regulation of a set of genes by glucose and calcium ionophores in mammalian cells. Trends Biochem. Sci. 1987;12:20–23. [Google Scholar]
- 16.Drummond I. A., Lee A. S., Resendez E., Jr, Steinhardt R. A. Depletion of intracellular calcium stores by calcium ionophore A23187 induces the genes for glucose-regulated proteins in hamster fibroblasts. J. Biol. Chem. 1987;262:12801–12805. [PubMed] [Google Scholar]
- 17.Nigam S. K., Goldberg A. L., Ho S., Rohde M. F., Bush K. T., Sherman M. A set of endoplasmic reticulum proteins possessing properties of molecular chaperones includes Ca2+-binding proteins and members of the thioredoxin superfamily. J. Biol. Chem. 1994;269:1744–1749. [PubMed] [Google Scholar]
- 18.Cala S. E., Jones L. R. GRP94 resides within cardiac sarcoplasmic reticulum vesicles and is phosphorylated by casein kinase II. J. Biol. Chem. 1994;269:5926–5931. [PubMed] [Google Scholar]
- 19.Van P. N., Peter F., Soling H. D. Four intracisternal calcium-binding glycoproteins from rat liver microsomes with high affinity for calcium. No indication for calsequestrin-like proteins in inositol 1,4,5-trisphosphate-sensitive calcium sequestering rat liver vesicles. J. Biol. Chem. 1989;264:17494–17501. [PubMed] [Google Scholar]
- 20.Baksh S., Michalak M. Expression of calreticulin in Escherichia coli and identification of its Ca2+ binding domains. J. Biol. Chem. 1991;266:21458–21465. [PubMed] [Google Scholar]
- 21.Vassilakos A., Michalak M., Lehrman M. A., Williams D. B. Oligosaccharide binding characteristics of the molecular chaperones calnexin and calreticulin. Biochemistry. 1998;37:3480–3490. doi: 10.1021/bi972465g. [DOI] [PubMed] [Google Scholar]
- 22.Meldolesi J., Pozzan T. The endoplasmic reticulum Ca2+ store: a view from the lumen. Trends Biochem. Sci. 1998;23:10–14. doi: 10.1016/s0968-0004(97)01143-2. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura K., Zuppini A., Arnaudeau S., Lynch J., Ahsan I., Krause R., Papp S., De Smedt H., Parys J. B., Muller-Esterl W., et al. Functional specialization of calreticulin domains. J. Cell Biol. 2001;154:961–972. doi: 10.1083/jcb.200102073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soldano K. L., Jivan A., Nicchitta C. V., Gewirth D. T. Structure of the N-terminal domain of GRP94: Basis for ligand specificity and regulation. J. Biol. Chem. 2003;278:48330–48338. doi: 10.1074/jbc.M308661200. [DOI] [PubMed] [Google Scholar]
- 25.Edwards D. P., Weigel N. L., Schrader W. T., O'Malley B. W., McGuire W. L. Structural analysis of chicken oviduct progesterone receptor using monoclonal antibodies to the subunit B protein. Biochemistry. 1984;23:4427–4435. doi: 10.1021/bi00314a029. [DOI] [PubMed] [Google Scholar]
- 26.Ying M., Flatmark T. Binding of the viral immunogenic octapeptide VSV8 to native glucose-regulated protein Grp94 (gp96) and its inhibition by the physiological ligands ATP and Ca2+ FEBS J. 2006;273:513–522. doi: 10.1111/j.1742-4658.2005.05084.x. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura K., Bossy-Wetzel E., Burns K., Fadel M. P., Lozyk M., Goping I. S., Opas M., Bleackley R. C., Green D. R., Michalak M. Changes in endoplasmic reticulum luminal environment affect cell sensitivity to apoptosis. J. Cell Biol. 2000;150:731–740. doi: 10.1083/jcb.150.4.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corbett E. F., Michalak K. M., Oikawa K., Johnson S., Campbell I. D., Eggleton P., Kay C., Michalak M. The conformation of calreticulin is influenced by the endoplasmic reticulum luminal environment. J. Biol. Chem. 2000;275:27177–27185. doi: 10.1074/jbc.M002049200. [DOI] [PubMed] [Google Scholar]
- 29.Corbett E. F., Oikawa K., Francois P., Tessier D. C., Kay C., Bergeron J. J., Thomas D. Y., Krause K. H., Michalak M. Ca2+ regulation of interactions between endoplasmic reticulum chaperones. J. Biol. Chem. 1999;274:6203–6211. doi: 10.1074/jbc.274.10.6203. [DOI] [PubMed] [Google Scholar]
- 30.Di Jeso B., Ulianich L., Pacifico F., Leonardi A., Vito P., Consiglio E., Formisano S., Arvan P. Folding of thyroglobulin in the calnexin/calreticulin pathway and its alteration by loss of Ca2+ from the endoplasmic reticulum. Biochem. J. 2003;370:449–458. doi: 10.1042/BJ20021257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Obermoeller L. M., Chen Z., Schwartz A. L., Bu G. Ca2+ and receptor-associated protein are independently required for proper folding and disulfide bond formation of the low density lipoprotein receptor-related protein. J. Biol. Chem. 1998;273:22374–22381. doi: 10.1074/jbc.273.35.22374. [DOI] [PubMed] [Google Scholar]
- 32.Wileman T., Kane L. P., Carson G. R., Terhorst C. Depletion of cellular calcium accelerates protein degradation in the endoplasmic reticulum. J. Biol. Chem. 1991;266:4500–4507. [PubMed] [Google Scholar]
- 33.Arnaudeau S., Frieden M., Nakamura K., Castelbou C., Michalak M., Demaurex N. Calreticulin differentially modulates calcium uptake and release in the endoplasmic reticulum and mitochondria. J. Biol. Chem. 2002;277:46696–46705. doi: 10.1074/jbc.M202395200. [DOI] [PubMed] [Google Scholar]
- 34.Papp S., Dziak E., Michalak M., Opas M. Is all of the endoplasmic reticulum created equal?. The effects of the heterogeneous distribution of endoplasmic reticulum Ca2+-handling proteins. J. Cell Biol. 2003;160:475–479. doi: 10.1083/jcb.200207136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vitadello M., Penzo D., Petronilli V., Michieli G., Gomirato S., Menabo R., Di Lisa F., Gorza L. Overexpression of the stress protein Grp94 reduces cardiomyocyte necrosis due to calcium overload and simulated ischemia. FASEB J. 2003;17:923–925. doi: 10.1096/fj.02-0644fje. [DOI] [PubMed] [Google Scholar]
- 36.Gorza L., Vitadello M. Reduced amount of the glucose-regulated protein GRP94 in skeletal myoblasts results in loss of fusion competence. FASEB J. 2000;14:461–475. doi: 10.1096/fasebj.14.3.461. [DOI] [PubMed] [Google Scholar]
- 37.Ishii T., Udono H., Yamano T., Ohta H., Uenaka A., Ono T., Hizuta A., Tanaka N., Srivastava P. K., Nakayama E. Isolation of MHC class I-restricted tumor antigen peptide and its precursors associated with heat shock proteins hsp70, hsp90, and gp96. J. Immunol. 1999;162:1303–1309. [PubMed] [Google Scholar]