Abstract
The proteolytic processing of procollagen V is complex and depends on the activity of several enzymes among which the BMP-1 (bone morphogenetic protein-1)/tolloid metalloproteinase and the furin-like proprotein convertases. Few of these processing interactions could have been predicted by analysing the presence of conserved consensus sequences in the proα1(V) chain. In the present study we opted for a cell approach that allows a straightforward identification of processing interactions. A construct encompassing the complete N-terminal end of the proα1(V) chain, referred to as Nα1, was recombinantly expressed to be used for enzymatic assays and for antibody production. Structural analysis showed that Nα1 is a monomer composed of a compact globule and an extended tail, which correspond respectively to the non-collagenous Nα1 subdomains, TSPN-1 (thrombospondin-1 N-terminal domain-like) and the variable region. Nα1 was efficiently cleaved by BMP-1 indicating that the triple helix is not required for enzyme activity. By mutating residues flanking the cleavage site, we showed that the aspartate residue at position P2′ is essential for BMP-1 activity. BMP-1 activity at the C-terminal end of the procollagen V was assessed by generating a furin double mutant (R1584A/R1585A). We showed that, in absence of furin activity, BMP-1 is capable of processing the C-propeptide even though less efficiently than furin. Altogether, our results provide new relevant information on this complex and poorly understood mechanism of enzymatic processing in procollagen V function.
Keywords: extracellular matrix, metalloproteinase, procollagen, proteolytic processing, site-directed mutagenesis, transient cell transfection
Abbreviations: ADAMTS, adisintegrin and metalloproteinase domain with thrombospondin type 1 repeats; BMP-1, bone morphogenetic protein-1; DMEM, Dulbecco's modified Eagle's medium; HEK-293 cell, human embryonic kidney cell; mAb, monoclonal antibody; PCPE, procollagen C-proteinase enhancer; TSPN-1, thrombospondin-1 N-terminal domain-like; α1TH, α1 triple helix domain
INTRODUCTION
Most extracellular matrix proteins are synthesized by cells as precursors that undergo subsequent proteolytic processing into mature and functional molecules. Among these proteins are members of the collagen superfamily. Collagens undergo a great variety of proteolytic modifications involved in biosynthesis, fibrillogenesis, functional activation, shedding of transmembrane collagens and production of functional collagen-derived fragments also called matricryptins. The fate and functions of the released fragments derived from collagens, i.e. propeptides, collagen ectodomains and other cryptic non-collagenous domains, are still under intensive investigation [1]. A large repertoire of proteinases is responsible for these processing interactions. Included among such enzymes are the ADAMTS (a disintegrin and metalloproteinase domain with thrombospondin type 1 repeats) and the BMP-1 (bone morphogenetic protein-1)/tolloid families of metalloproteinases, and more recently the furin-like proprotein convertases [2]. Tolloid metalloproteinases play key roles in the control and orchestration of extracellular matrix deposition, cohesion, mineralization and cross-linking through proteolytic processing of the various extracellular matrix proteins. Their enzymatic activities include processing of fibrillar procollagens [3], laminin 5 and collagen VII [4,5], maturation of small leucine-rich proteoglycans [6,7], proteolytic activation of lysyl oxidase [8] and the release of bioactive domains from SIBLING (small integrin-binding ligand N-linked glycoprotein) proteins (dentin sialophosphoprotein and dentin matrix protein-1) [9] and perlecan [10].
The proteolytic processing of procollagen V is complex and depends on the activity of several enzymes. Collagen V is a minor fibrillar collagen with a broad distribution in tissues such as dermis, tendons, bones, blood vessels and cornea in which it can assemble in three different known stoichiometries. The heterotrimer α1(V)2α2(V) is the most abundant and is widely distributed, whereas the heterotrimer α1(V)α2(V)α3(V) and the homotrimer α1(V)3 show more restricted distributions [11,12]. The physiological relevance of these three molecular forms is unclear. However, a wealth of evidence favours the role of the heterotrimer α1(V)2α2(V) in the control of fibril assembly [13], a function that is not shared by the homotrimer α1(V)3 [14]. A key step in collagen fibril formation is the proteolytic removal of the non-collagenous N- and C-propeptides. BMP-1 cleaves proα1(V) at both N- and C-termini. These processing cleavages release a part of the N-propeptide at the N-terminus, the TSPN-1 (thrombospondin-1 N-terminal domain-like) domain, and the C-propeptide at the C-terminus [15,16]. ADAMTS-2 was recently shown to cleave the heterotrimer proα1(V)2α2(V) extracted from skin and the recombinant homotrimer proα1(V)3 [17]. From the determination of the cleavage site, it was deduced that the released fragment includes the TSPN-1 domain and the variable region [17]. The C-propeptide of the recombinant homotrimer proα1(V)3 was shown to be cleaved rapidly in the conditioned medium of transfected HEK-293 cells (human embryonic kidney cells) [12]. It was further shown that an endogenous furin-like proprotein convertase was responsible for this cleavage [15]. Interestingly, not all of these processing interactions could have been predicted by analysing the presence of conserved consensus sequences in the N- and C-terminal regions of the proα1(V) chain or based on our knowledge of the processing of the fibrillar procollagens.
Here, the proteolytic processing specificity of the proα1(V) chain by BMP-1 and by furin-like proprotein convertases was analysed in detail by site directed mutagenesis. In vitro digestion assays are commonly used to investigate matrix protein processing and give, in many cases, satisfactory results. However, fastidious extraction and purification steps are often necessary to obtain limited amounts of unprocessed proteins and active enzymes since most of them are present in trace amounts in tissues. These problems have been partly solved by expressing recombinant matrix proteins and enzymes, although this alternative method does not avoid purification procedures. Therefore, in the present study, the proteolytic processing of procollagen V was approached by using transient cell transfection for allowing rapid and straightforward analysis of processing interactions. Using this method, we provide new and reliable information on the enzymatic cleavage specificity of procollagen V.
MATERIALS AND METHODS
Plasmids
Plasmids pCEP4-proα1(V) containing the full-length cDNA of the human proα1(V) chain, and pCEP4-BMP-1 were previously described [12,18]. The pCEP4-Nα1(V) construct was obtained from pCEP4-proα1(V), after a KpnI/XhoI digestion (from nt 1 to 1783) and subcloning in pCEP4 previously digested with the same enzymes. Nα1 is a small domain of 64 kDa containing the NC3, COL2 and NC2 domains and 11 triplets from COL1 (Figure 1A).
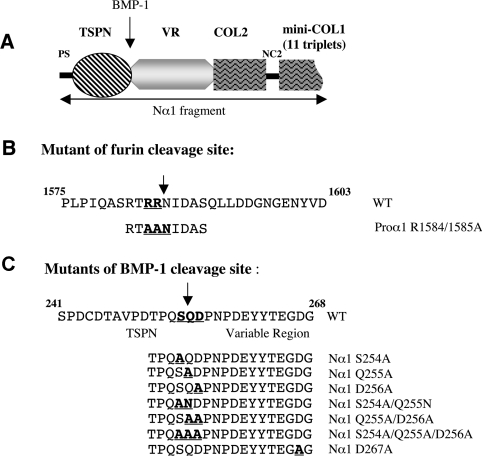
Figure 1. Constructs and mutants of the human proα1(V) chain.
(A) Schematic representation of the N-propeptide construct referred to as Nα1. It consists of NC3, COL2, NC2 and 11 triplets from COL1. (B) Sequences of the C-propeptide encompassing the furin cleavage site and of the R1584A/R1585A mutant. The two arginine residues at positions 1584 and 1585 were mutated to alanine. (C) Sequences of the N-propeptide encompassing the BMP-1 cleavage site and of the BMP-1 cleavage site mutants. COL, collagenous domain; NC, non-collagenous domain. Mutated residues are underlined. Arrows indicate cleavage sites.
Mutagenesis
For mutagenesis, Nα1 and proα1(V) had to be subcloned into the pcDNA3 plasmid (Invitrogen). After KpnI/XhoI digestion of Nα1 from pCEP4-Nα1 and KpnI/KpnI digestion of proα1(V) from pCEP4-proα1(V), fragments were introduced into pcDNA3 linearized with KpnI/XhoI or KpnI/KpnI digestions respectively. Mutation of the putative cleavage site of the α1(V) C-propeptide by furin (Figure 1B) was done on pcDNA3-proα1(V), and different simple, double or triple mutations of the BMP-1 cleavage site of Nα1 (Figure 1B) were done on pcDNA3-Nα1. All mutations were performed using the QuikChange® II XL site-directed mutagenesis kit (Stratagene) according to the manufacturer's instructions, using the following oligonucleotides: R1584A/R1585A: forward 5′-GCATCCAGGACGGCGGCG-AACATCGACGCC-3′ and reverse 5′-GGCGTCGATGTTCGC-CGCCGTCCTGGATGC-3′; S254A forward 5′-CCTGACACCCCACAGGCGCAGGACCCCAATCC-3′ and reverse 5′-GGATTGGGGTCCTGCGCCTGTGGGGTGTCAGG-3′; Q255A forward 5′-GACACCCCACAGTCGGCGGACCCCAATCC-3′ and reverse 5′-GGATTGGGGTCCGCCGACTGTGGGGTGTC-3′; D256A forward 5′-CCACAGTCGCAGGCCCCCAATCCAGATG-3′ and reverse 5′-CATCTGGATTGGGGGCCTGCGACTGTGG-3′; D267A forward 5′-GAATATTACACGGAAGGAGCCGGCGAGGGTGAG-3′ and reverse 5′-CTCACCCTCGCCGGCTCCTTCCGTGTAATATTC-3′; S254A/Q255N forward 5′-GACACCCCACAGGCGAACGACCCCAATCC-3′ and reverse 5′-GGATTGGGGTCGTTCGCCTGTGGGGTGTC-3′; Q255A/D256A forward 5′-GACACCCCACAGTCGGCGGCCCCCAATCC-3′ and reverse 5′-GGATTGGGGGCCGCCGACTGTGGGGTGTC-3′; S254A/Q255A/D256A forward 5′-GACACCCCACAGGCGGCGGCCCCCAATCC-3′ and reverse 5′-GGATTGGGGGCCGCCGCCTGTGGGGTGTC-3′. The cDNA sequences of the mutants were thoroughly checked.
Cell culture
HEK-293 EBNA cells were grown in DMEM (Dulbecco's modified Eagle's medium) medium supplemented with 10% (v/v) fetal calf serum and penicillin–streptomycin cocktail (all from Sigma–Aldrich) at 37°C in a 5% CO2 incubator. Stable cell lines were selected and grown in the same medium supplemented with 200 μg/ml hygromycin B (Calbiochem).
Production and purification of recombinant proteins
pCEP4-BMP-1 and pCEP4-Nα1 were transfected in HEK-293 EBNA cells by electroporation and the transfected cells were selected by adding hygromycin B (200 μg/ml), over 7–10 days, to DMEM culture medium supplemented with 10% fetal calf serum [12]. Resistant HEK-293 EBNA cell media were tested for expression of the recombinant proteins by SDS/6% PAGE followed by Coomassie Blue staining. Large amounts of serum-free medium from transfected HEK-293 EBNA cells were collected every 48 h and stored at −20°C until used. The purified BMP-1 was obtained as previously described [18]. The Nα1 fragment was purified from conditioned media by two steps of ion-exchange chromatography. Conditioned medium (360 ml) was dialysed against 50 mM Tris/HCl (pH 7.6), 100 mM NaCl and 2 M urea. After centrifugation, the supernatant was passed over a DEAE column (DE 52; Whatman) and subsequently eluted with a linear 0–0.6 M NaCl gradient. Pools containing purified recombinant Nα1 fragment were recovered and dialysed against 50 mM Tris/HCl (pH 7.6) and 100 mM NaCl. Samples were then subjected to a HitrapQ column (Amersham Biosciences) and eluted by a NaCl gradient. Recombinant protein-containing fractions were analysed by SDS/PAGE on a 6% gel and dialysed against 50 mM Tris/HCl (pH 7.6) and 100 mM NaCl. Purified Nα1 fragment was stored at −20°C until used.
Polyclonal and mAbs (monoclonal antibodies)
Polyclonal antibodies to BMP-1 were kindly provided by Dr Efrat Kessler (Goldschlegen Eye Research Institute, Tel Aviv University, Tel Aviv, Israel) [19]. For production of mAb against the N-propeptide of the proα1(V), mice were immunized with the purified recombinant Nα1 fragment. Among the tested hybridomas, three clones produced an antibody able to recognize Nα1 both by ELISA and Western blotting. Using the various recombinant N-propeptide domains, it was determined that the clones named 6A7 and 18G5 were specific for the variable region and for the NC2 domain respectively. Neither of them recognized the TSPN-1 domain (Figure 2C).
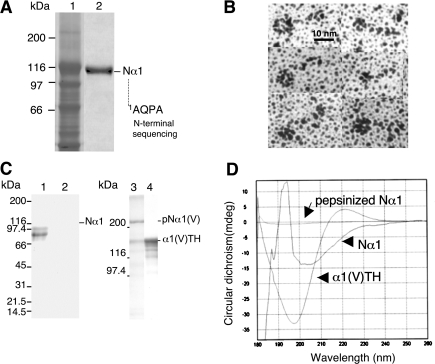
Figure 2. Production and characterization of Nα1 fragment and antibodies.
(A) SDS/6% PAGE analysis of recombinant Nα1 fragment produced in HEK-293 cells. Electrophoretic patterns of serum-free medium from transfected cells (lane 1) and of the purified Nα1 fragment (lane 2). (B) Rotary-shadowing images of recombinant purified Nα1 fragment revealing the presence of a compact globular domain (TSPN-1 domain) and an extended rod-like domain (variable region). (C) SDS/PAGE (5–20% gradient) analysis of Nα1 (lane 1) and Nα1 digested with pepsin (lane 2) and SDS/6% PAGE analysis of α1(V) homotrimeric molecule treated without (lane 3) and with pepsin (lane 4). (D) CD spectra of α1(V)TH, Nα1 and Nα1 after pepsin digestion. Buffer baselines were systematically subtracted.
To obtain antibodies able to recognize specifically the TSPN-1 region, rabbits were immunized with the recombinant TSPN-1 domain produced in Escherichia coli. The TSPN-1 cDNA (nt 258–723) was generated by PCR using the Nα1 clone as template. Two oligonucleotides flanking the desired sequence were designed, forward: 5′-TATGAATTCCCACCAAGCAGCTGTACCCTG-3′ and reverse: 5′-TATCTGCAGGCTGTAGTGCTCACAGTAATC-3′, the underlined sequences introducing an EcoRI site at the 5′-end and a PstI site at the 3′-end. The resulting PCR product was subcloned in the EcoRI and PstI sites of a pT7/7-6His expression vector [20]. The resulting plasmid, named pTSPN1-6His, encoded the TSPN-1 domain of the human proα1(V) chain, under the control of the E. coli phage T7 promoter. E. coli host strain BL21 (DE3) was transformed with pTSPN1-6His and cells were harvested after 2 h of IPTG (isopropyl β-D-thiogalactoside) induction as previously described [21]. Purified TSPN-1 domain was obtained by subjecting cell lysates to a Ni-NTA (Ni2+-nitrilotriacetate)–agarose column (Qiagen). The recombinant protein was analysed by SDS/15% PAGE followed by Coomassie Blue staining. Polyclonal antibodies obtained after immunization with the recombinant protein were purified on a CNBr-activated Sepharose 4B column coupled with the purified recombinant Nα1 fragment as indicated by the manufacturer (Amersham Biosciences). The purified antibodies were able to recognize Nα1 (Figure 2C, lane 1) and the recombinant TSPN-1 domain (results not shown) both by ELISA and Western blotting. Rabbit polyclonal antibodies to human pepsinized collagen V were obtained from Novotec (Lyon, France).
Transient cell transfection
For transient transfection experiments, wild-type HEK-293 EBNA cells or HEK-293 EBNA cells stably transfected with pCEP4-BMP-1 were plated on to 100 mm Petri dishes with 1.5×106 cells and transfected on the following day with the different plasmids using the calcium phosphate transfection kit from Invitrogen. Twenty-four hours after transfection, cells were washed three times with PBS and placed in serum-free medium supplemented with 50 μg/ml sodium ascorbate. Media were collected 48 h after transfection for protein analysis. Efficiency of transfection was analysed by transfecting the cells with a plasmid that expresses GFP (green fluorescent protein). The percentage of transfection was calculated by counting green cells 48 h after transfection with an inversed microscope equipped with fluorescence (Nikon). The values varied from 50 to 70%.
Protein analysis
Proteins were recovered from transfected cell media by addition of 1% Triton X-100 followed by precipitation with 10% (v/v) trichloroacetic acid. After centrifugation, pellets were washed twice with ethanol and acetone, resuspended in Laemmli buffer and analysed by SDS/12% PAGE, SDS/10% PAGE or SDS/6% PAGE as indicated, under reducing conditions. Proteins were electrotransferred on to PVDF membranes (Immobilon-P; Millipore) overnight in 10 mM Caps [3-(cyclohexylamino)propane-1-sulfonic acid; pH 11] and 5% (v/v) methanol. Then, membranes were saturated for 2 h in PBS supplemented with 10% (w/v) non-fat milk. For Nα1 digestion by BMP-1, double immunolabelling using two different colorimetric detection kits was performed to discriminate the BMP-1 band from the Nα1 bands. Membranes were first probed with polyclonal primary antibodies against the TSPN-1 domain followed by incubation with alkaline phosphatase-coupled goat anti-rabbit secondary antibodies (Bio-Rad) and detection using an alkaline phosphatase colorimetric kit (AP Color kit; Bio-Rad). Then membranes were probed with polyclonal primary antibodies to BMP-1 followed by incubation with peroxidase-coupled goat anti-rabbit secondary antibodies (Dako, Copenhagen, Denmark) and the signal was detected using a peroxidase colorimetric kit (Sigma–Aldrich). For the furin site mutants, membranes were probed with the monoclonal 6A7 antibody followed by alkaline phosphatase-coupled goat anti-mouse secondary antibodies incubation (Bio-Rad) and detected using an alkaline phosphatase colorimetric kit. For the BMP-1 mutants, Nα1 and TSPN-1 fragments were detected with polyclonal primary antibodies against the TSPN-1 domain, followed by incubation with horseradish peroxidase-coupled goat anti-rabbit secondary antibodies (Dako) and signals were detected using an ECL® (enhanced chemiluminescence) reagent (Amersham Biosciences) and X-ray film exposure. When indicated, 18G5 antibody was used to detect the Nα1 fragment after BMP-1 cleavage. Membranes were then de-hybridized according to the manufacturer's instructions and probed with antibodies to BMP-1 and treated as described above.
Analytical and electron microscopy methods
Amino acid sequence analysis was performed by automated Edman degradation using an Applied Biosystems 473A protein sequencer. For N-terminal sequencing of the TSPN-1 released fragment, the membrane, after immunotransfer, was treated with pyroglutamate aminopeptidase (Boehringer–Mannheim) to remove the pyroglutamic acid blocking groups before performing automated Edman degradation.
For rotary shadowing, purified Nα1 fragment was diluted to 5–10 μg/ml with 0.1 M ammonium acetate. Samples were mixed with glycerol (1:1), sprayed on to freshly cleaved mica sheets and immediately placed on the holder of a MED 010 evaporator (Balzers). Rotary shadowing was carried out as previously described [22]. Observations of replicas were performed with a Philips CM120 microscope at the ‘Centre Technique des Microstructures’ (Université Lyon 1, Villeurbanne, France).
The possible triple helix folding of Nα1 was ascertained by pepsin digestion experiments and CD. Pepsin digestion experiments were performed on the Nα1 fragment and the recombinant homotrimeric α1(V) as positive control [12]. Samples were treated with pepsin for 2 h at room temperature (22°C) at an enzyme/substrate ratio of 1:10 in acetic acid (0.5 M). Digestion products were analysed by SDS/PAGE [5–20% gradient (for Nα1) or 6% (for the procollagen V homotrimer)], under reducing conditions, followed by Coomassie Blue staining. The Nα1 fragment was analysed by CD before and after pepsin treatment. The pepsinized recombinant homotrimer collagen V, referred to as α1TH (α1 triple helix domain) [12], was analysed as the control. Spectra were recorded at 20°C in 50 mM acetic acid (for α1TH) or 20 mM sodium phosphate (pH 7.0) (for Nα1) on a Chirascan apparatus (Applied Photophysics). Measurements were performed with either 0.5 or 0.2 mm path length cells. Spectra were collected at 0.5 nm intervals over the wavelength range from 260 to 180 nm.
RESULTS
Expression and characterization of recombinant Nα1 fragment
The Nproα1(V) fragment, referred to as Nα1, was designed to encompass the complete N-propeptide of the human α1(V) chain, including the NC3, COL2 and NC2 domains plus 11 triplets of COL1 (Figure 1A). The fragment contains the BMP-1 cleavage site, which is located between the TSPN-1 domain and the variable region (Figure 1A). The theoretical molecular mass of the corresponding fragment is 64 kDa but electrophoretic analysis of serum-free medium from Nproα1(V)-transfected HEK-293 EBNA cells demonstrated a protein band at 116 kDa (Figure 2A, lane 1), which was absent from non-transfected cell medium (results not shown). The identity of the recombinant protein was confirmed by N-terminal sequencing after electrotransfer. The determined sequence, Ala-Gln-Pro-Ala, starts with the first amino acid of the proα1(V) chain after the peptide signal cleavage site [12]. This indicates that the recombinant protein, even though it migrated much slower than expected, corresponds to the Nproα1(V) fragment. This is in agreement with previous results on the recombinant production of the entire proα1(V) chain. Collagenase digestion of the recombinant proα1(V) chains revealed an 86 kDa fragment corresponding to the entire N-propeptide [12]. The difference in migration (116 kDa versus 86 kDa) correlates with the presence in the construct of an additional sequence encoding the complete COL2 and NC2 domains and the 11 triplets of the COL1 domain (Figure 1A). Two steps of anion-exchange chromatography were performed to purify the recombinant fragment (Figure 2A). Rotary shadowing of the purified Nα1 fragment revealed a homogeneous preparation of 50-nm-long molecules with a globular domain at one extremity, the TSPN-1 domain, and an extended tail corresponding to the variable region (Figure 2B). This observation attests to the proper folding of the NC3 domain. The COL2 and COL1 domains were not visible in our preparations indicating that these domains do not fold into stable triple helices. Moreover, formation of a trimer should be seen as a fish-spear shape with three TSPN-1 globular domains at one end of the molecule. We definitively excluded the possibility that Nα1 folds into a triple helix from pepsin digestion experiments and CD analysis. As shown in Figure 2(C), Nα1 was completely digested by pepsin (Figure 2C, lane 2), whereas pepsin digestion of the α1(V) homotrimer under the same conditions showed a pepsin-resistant band (Figure 2C, lane 4) corresponding to the triple helix domain referred to as α1TH [12]. CD spectra were monitored for Nα1 before and after pepsin digestion and compared with the spectrum of α1TH that was typical of a triple helix conformation (a negative minimum peak at 197 nm and a positive peak at approx. 222 nm) (Figure 2D). The spectrum obtained for Nα1 before pepsinization corresponded to signals from the different subdomains of the fragment, particularly the non-collagenous domains, TSPN-1 and the variable region (Figure 2D). After pepsin digestion, no particular signal was monitored for Nα1 (Figure 2D) corroborating the results obtained with SDS/PAGE analysis (Figure 2C).
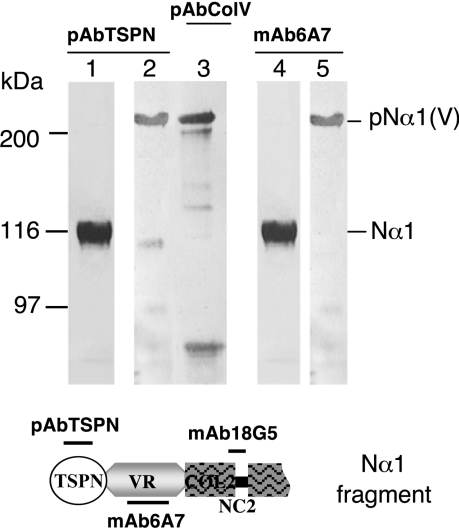
The purified fragment Nα1 was used to obtain mAbs to different regions of the N-propeptide. None of the hybridomas obtained recognized the TSPN-1 domain. The mAb to the N-propeptide, called 6A7, whose epitope was located in the variable region, was selected for the present study. It was shown to recognize the complete proα1(V) chain and the Nα1 fragment (Figure 3, lanes 4 and 5) but not the released BMP-1 fragment, the TSPN-1 domain (results not shown). Polyclonal antibodies to the TSPN-1 domain were obtained by immunizing rabbits with the purified recombinant domain produced in bacteria. These antibodies, pAbTSPN, recognized the complete proα1(V) chain, the Nα1 fragment (Figure 3, lanes 1 and 2) and the recombinant TSPN-1 domain (results not shown).
Figure 3. Western-blot characterization of the polyclonal antibodies to the TSPN-1 domain (pAbTSPN) and the mAb (mAb6A7) directed against the variable region of the Nα1 fragment.
pAbTSPN (lanes 1 and 2) and the mAb 6A7 (lanes 4 and 5) recognized the complete proα1(V) chain (lanes 2 and 5) and the Nα1 fragment (lanes 1 and 4). Lane 3 shows proα1(V) chain recognition by polyclonal antibodies directed against the collagen V triple helix (from Novotec). Epitopes of the different antibodies are indicated on the schematic representation of the Nα1 fragment presented below. Left: running positions of protein standards are indicated in kDa.
Nα1 is processed by BMP-1
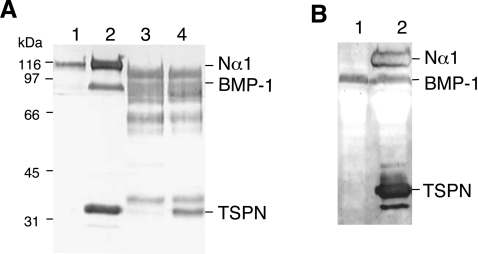
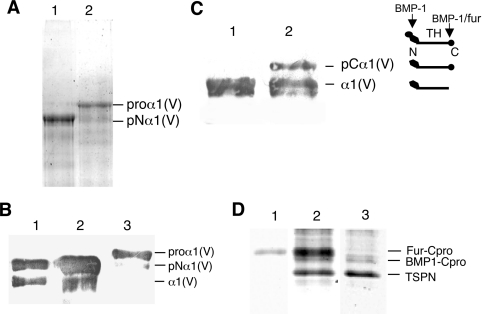
HEK-293 cells were transiently transfected with both Nα1 and BMP-1 constructs, in order to visualize digestion products immediately after SDS/PAGE analysis or immunoblotting. We chose the HEK-293 cell line for several reasons. These cells have been successfully used in the past for production of both matrix proteins and enzymes because correct protein folding and high expression level are achieved. In addition, they express endogenous furin [23], a proprotein convertase enzyme involved in functional processing of metalloproteinases, notably BMP-1 and ADAMTS. BMP-1 processing of the C-propeptide of fibrillar collagens is known to be independent of the triple helical conformation [16]. To test whether the N-propeptide cleavage by BMP-1 is also conformation-independent, we chose to perform experiments using the monomeric N-propeptide recombinant fragment as substrate. Processing experiments using a cell factory showed that a released 34 kDa fragment is detected in conditioned medium from cells co-transfected with Nα1 and BMP-1 constructs using pAbTSPN (Figure 4A, lane 2). This band was not detected when cells were transfected with Nα1 construct alone (Figure 4A, lane 1). The production of active BMP-1 by cells after co-transfection was assessed by probing the same membrane with BMP-1 antibodies as described in the Materials and methods section (Figure 4A, lane 2). In contrast with the control (Figure 4A, lane 1), a clear band migrating at the expected position was detected in cells co-transfected with both constructs (Figure 4A, lane 2). N-terminal sequence of the released fragment confirmed that it corresponds to the N-terminal portion of the Nα1 fragment. The processed Nα1 fragment was identified by probing the membrane with the 18G5 antibody (results not shown) and the N-terminal sequence, Xaa-Asp-Pro-Asn-Pro, was determined after deblocking with pyroglutamate endopeptidase. It corresponds to the sequence previously described by others [15]. In vitro experiments were carried out in parallel with the purified recombinant fragment and enzyme. Western-blot analysis of samples after overnight incubation at 37°C of the purified Nα1 and BMP-1 also showed the appearance of a 34 kDa fragment recognized by pAbTSPN. The same blot was probed with BMP-1 antibodies to reveal the presence of the enzyme band in the assay contrary to the control in which BMP-1 was omitted (Figure 4A, lanes 3 and 4). However, in contrast with the processing assays in cells, considerable protein breakdown was observed after overnight incubation. A similar experiment was performed using cells stably transfected with the BMP-1 construct (Figure 4B, lane 1). Analysis of conditioned media after single transfection with the Nα1 construct revealed the presence of a dense band corresponding to the released TSPN-1 domain (Figure 4B, lane 2). Our results indicate that, as for C-propeptide processing, triple helical conformation is not necessary for BMP-1 cleavage of collagen V N-propeptide.
Figure 4. BMP-1 cleavage of the N-propeptide domain of the proα1(V) chain.
(A) Western-blot analysis of BMP-1 processing of the N-propeptide of the proα1(V) chain in HEK-293 cells (lanes 1 and 2) compared with in vitro experiments with purified proteins (lanes 3 and 4). Electrophoretic patterns of HEK-293 cell media transfected with Nα1 construct alone (lane 1) and co-transfected with BMP-1 and Nα1 constructs (lane 2). Electrophoretic patterns of purified recombinant Nα1 fragment incubated without (lane 3) or with (lane 4) recombinant BMP-1. (B) Western-blot analysis of BMP-1 processing of the N-propeptide of the proα1(V) chain in HEK-293 cells expressing BMP-1. Electrophoretic patterns of HEK-293 BMP-1 cell medium (lane 1) and of medium from HEK-293 BMP-1 cells transfected with Nα1 construct (lane 2). Membranes were probed with pAbTSPN and reprobed with anti-BMP-1 polyclonal antibodies. Left: running positions of protein standards are indicated in kDa.
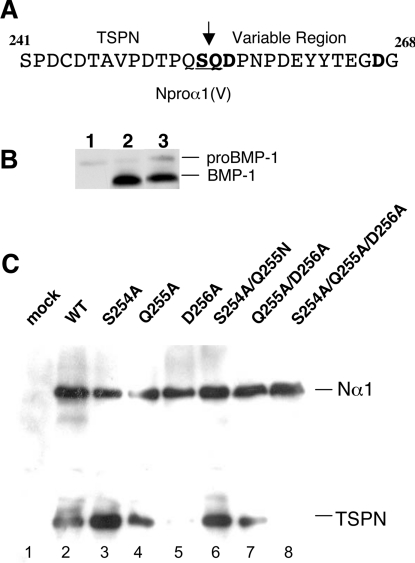
BMP-1 cleavage specificity of Nα1 processing
With the exception of procollagens V and XI N-terminal processing, all the previously identified BMP-1 cleavage sites contain an aspartic acid residue at the P1′ position that appears to be indispensable for BMP-1 activity [16]. The BMP-1 cleavage site in the N-propeptide of the proα1(V) chain is different from the consensus site described for other matrix proteins [24] and corresponds to the Ser254/Gln255 peptide bond between the TSPN-1 domain and the variable region (Figure 5A). The P1′ residue which is indispensable for the cleavage is thus not an aspartic acid residue but a glutamine. To investigate which residue is essential for BMP-1 activity, six different mutants were generated from the Nα1 wild-type construct (Figure 1C): three single mutants S254A, Q255A and D256A, two double mutants S254A/Q255N and Q255A/D256A and the triple mutant S254A/Q255A/D256A. Mutation to Alanine of Gln255 at the P1′ position of the cleavage site might introduce a known cleavage site for BMP-1, Alanine-Aspartic acid [16], with regard to the presence of an aspartic acid residue at the P2′ position (Figure 1B). To address this possibility, Gln255 was mutated to asparagine in the double mutant S254A/Q255N. The D267A mutant located downstream to the cleavage site was also generated as a control (Figure 1C). A screening for BMP-1 cleavage efficiency was assayed by transfection of mutant and wild-type constructs into BMP-1 HEK-293 EBNA cells (Figure 5C) for which BMP-1 expression level was checked using antibodies against BMP-1 (Figure 5B). Alternatively, co-transfection of both Nα1 and BMP-1 constructs in HEK-293 EBNA cells was performed to confirm the results (results not shown). Conditioned media were analysed by Western blotting using anti-TSPN-1 antibodies. The results showed that N-propeptide cleavage was either unaffected or only slightly affected in cells expressing the single mutants S254A and Q255A and the double mutant S254A/Q255N (Figure 5C, lanes 3, 4 and 6) as well as in D267A control (results not shown). In striking contrast, the results showed that the P2′ residue aspartic acid is crucial for BMP-1 activity (Figure 5C, lane 5). In agreement with this result, BMP-1 cleavage of the double mutant Q255A/D256A was greatly affected (Figure 5C, lane 7). Transfection with the triple mutant construct completely abolished the N-propeptide cleavage by BMP-1 (Figure 5C, lane 8). Our results highlight the importance of the aspartic acid residue at the P2′ position in the α1(V) N-propeptide cleavage by BMP-1.
Figure 5. BMP-1 cleavage specificity of Nα1 processing analysed by site-directed mutagenesis.
(A) Sequence for the N-propeptide encompassing the BMP-1 cleavage site. The mutated residues Ser254, Gln255, Asp256 and Asp267 are shown in boldface; the BMP-1 cleavage site is underlined. (B) Western-blot analysis showing the level of BMP-1 expression in HEK-293 cells transfected with BMP-1. Electrophoretic patterns of cell media transfected with a mock plasmid as negative control (lane 1), and with BMP-1 construct (lanes 2 and 3). A low level of proBMP-1 is observed in wild-type HEK-293 cells transfected with a control plasmid, while the high expression level of the mature form of BMP-1 is reproducibly observed in transfected HEK-293 cell media. (C) Electrophoretic patterns of cleavage products obtained by processing wild-type Nα1 and mutant constructs using cell factory assays. Western blots were performed to monitor efficiency of BMP-1 cleavage. Membranes were probed with pAbTSPN. Cells expressing BMP-1 (B; lane 2) were transfected with a mock plasmid as negative control (lane 1), wild-type Nα1 construct (lane 2) or with the mutant constructs: S254A (lane 3); Q255A (lane 4), D256A (lane 5), S254A/Q255N (lane 6), Q255A/D256A (lane 7) and S254A/Q255A/D256A (lane 8). Mutants D256A, S254A/Q255A/D256A and Q255A/D256A showed no or weak BMP-1 activity. Right: running positions of the different processed and unprocessed forms.
Processing of the procollagen V C-propeptide by BMP-1
BMP-1 and furin are both capable of in vitro processing of the proα1(V) C-propeptide chain [16]. Proteolytic C-propeptide removal by furin occurred between Arg1585 and Asn1586 (Figure 1B), a cleavage site located upstream of the previously identified cleavage site of BMP-1 (Asp1594/Asp1595) [15,16]. In vitro, processing of the proα1(V) C-propeptide by furin is more efficient than processing by BMP-1. We thus took advantage of the ‘in cellulo’ method to study α1(V) C-propeptide processing by BMP-1 in the absence of furin cleavage. The use of decanoyl-RVKR-chloromethyl ketone to protect from furin activity in HEK-293 cells only inhibited furin cleavage by approx. 50–70% [16]. We thus sought to abolish the furin cleavage site of the proα1(V) chain by mutating to alanine residues the two arginine residues, Arg1584 and Arg1585, located at positions P2 and P1 of the cleavage site (Figure 1B). The furin mutant and wild-type constructs were transiently transfected into HEK-293 cells and conditioned media were analysed by SDS/PAGE. As can be seen (Figure 6A), the R1584A/R1585A mutations efficiently protected the C-propeptide cleavage from furin activity (Figure 4A, lane 2). Only a very faint band migrating at the pNα1 position was observed in conditioned medium of mutant cells by Western blotting with the 6A7 antibody (Figure 6B, lane 3). The proα1R1584A/R1585A construct was then transfected into BMP-1 HEK-293 cells to analyse C-propeptide cleavage efficiency compared with the wild-type (Figures 6C and 6D). BMP-1 processing of proα1R1584A/R1585A homotrimers yielded two bands recognized by the 6A7 antibody (Figure 6C, lane 2). The largest corresponds to the pCα1(V) form that migrated with a mobility faster than that of pNα1 (Figure 6B, lane 3). Under the same experimental conditions, processing of the wild-type homotrimer by BMP-1 gave a unique band corresponding to the mature α1(V) chain (Figure 6C, lane 1). In agreement with these results, SDS/12% PAGE analysis (Figure 6D) revealed the absence of the band corresponding to the furin cleavage in BMP-1 HEK-293 cells transiently transfected with proα1R1584A/R1585A construct (Figure 6D, lane 3 compared with lanes 1 and 2). The BMP-1 activity on the N-propeptide in the cell factory assay was confirmed by the presence of a faster band of same intensity (Figure 6D, lanes 2 and 3), which corresponds to the N-terminal portion of the proα1(V) homotrimer released by BMP-1. As can be seen (Figure 6D), the C-propeptide cleavage by BMP-1 was not enhanced in the absence of furin cleavage. The corresponding band migrating at approx. 38 kDa was of similar intensity in wild-type and mutant samples (Figure 6D, lanes 2 and 3). These results confirm in vitro experiments [16] and indicate that BMP-1 is capable of processing the C-propeptide even though less efficiently than furin.
Figure 6. Effect of furin site mutagenesis on the α1(V) C-propeptide cleavage by BMP-1.
(A) SDS/6% PAGE analysis of transfected HEK-293 cell medium with the human proα1(V) construct (lane 1) and with the mutant construct proα1R1584A/R1585A (lane 2). (B) Westernblot analysis of (lane 1) purified pNα1(V) homotrimer as running position standard, transfected HEK-293 cell medium with the human proα1(V) construct (lane 2) and with the mutant construct proα1R1584A/R1585A (lane 3). Membranes were probed with mAb6A7. Western blot shows that the C-propeptide cleavage is completely abolished in HEK-293 cells transfected with the furin mutant. (C) Western-blot analysis (mAb6A7) of the cleavage products in HEK-293 cells expressing BMP-1, transfected with the proα1(V) construct (lane 1) and with the mutant construct proα1R1584A/R1585A (lane 2). Right panel: schematic representation of the different proα1(V) processed forms and cleavage sites. N, N-propeptide; C, C-propeptide; TH, triple helix domain. (D) Electrophoretic patterns of cleavage products obtained by processing proα1(V) (lanes 1 and 2) and the furin mutant proα1R1584A/R1585A (lane 3) in HEK-293 cells expressing recombinant BMP-1 and endogenous furin (lanes 2 and 3) and in wild-type HEK-293 cells (lane 1). Samples were run on an SDS/12% PAGE and stained with Coomassie Blue. Right: running positions of the different processed forms.
DISCUSSION
Removal of the N- and C-propeptides of fibrillar collagens is accomplished by procollagen proteinases of the ADAMTS and BMP-1/TLD (tolloid) families respectively. This post-translational event plays a crucial role in fibril assembly [25] and proteolytic products of extracellular matrix proteins may have important biological functions [2]. Collagen V shows a partial retention of N-propeptide that inhibits lateral fibril growth [26]. The excision of the N-propeptide can occur at different sites in a tissue-specific manner and be ensured by BMP-1 and/or ADAMTS-2 [15,17]. Another feature of the minor fibrillar collagen V is that enzyme activities responsible for C-propeptide cleavage (BMP-1 and/or furin) are chain-dependent [16,27].
In order to analyse processing of the procollagen V at the molecular level, we took the advantages of a cell approach based on the transient transfection of cells with plasmids that each encode one of the partners (enzyme and substrate) followed by direct analysis of digestion products. The major advantages of this procedure are the rapid screening of processing interactions, the lack of protein breakdown during purification and enzyme digestion steps and the analysis of enzyme activity in a cellular environment.
Procollagen V can be processed at the C-terminal end by two enzymes, furin and BMP-1. The consensus recognition sequence for furin cleavage is Arg-Xaa-Arg/Lys-Arg (Xaa for any other residue). Excision of the C-propeptide from the proα1(V) chain by BMP-1 activity occurs at a site (Asp1594/Asp1595) next to the furin cleavage site (Arg1585/Asn1586) [15]. Kessler et al. [16] showed that the C-propeptide processing by BMP-1 was much less efficient than furin cleavage and 4-fold less efficient than the cleavage of the N-propeptide by BMP-1. Partial inhibition of furin activity, using enzyme inhibitors, showed that BMP-1 is capable of cleaving the C-propeptide from the intact molecule [16]. To accurately analyse the physiological relevance of BMP-1 cleavage, it was important to carry out experiments in a cellular environment and in the complete absence of furin activity. The double mutation (R1584A/R1585A) completely abolished furin activity and showed that the C-propeptide fragment released by BMP-1 activity did not result exclusively from further cleavage of the furin-derived fragment. However, a consistent finding over the course of several experiments was the lower efficiency of the C-propeptide cleavage by BMP-1 compared with furin and no enhancement of BMP-1 activity was obtained after mutation of the furin cleavage site. Given the strong conservation of this domain in all the fibrillar procollagens [28], a common processing mechanism was expected. However, this proved not to be the case. It has been shown that the C-propeptides of the α1 and α3 chains of collagen V, a member of the fibrillar collagen family, are efficiently cleaved by furin activity [15,27]. The PCPE (procollagen C-proteinase enhancer) is a potent stimulator of the BMP-1 processing of the major fibrillar collagens [24]. But the possible role of PCPE as an enhancer of BMP-1 cleavage of the fibrillar collagen V C-propeptide remains an open question. Low amounts of PCPE were detected in the HEK-293 cell media [18]. The results presented here indicate either that PCPE enhancement of BMP-1 processing is not targeted to all members of the collagen fibrillar family or that the cells produce insufficient amount of PCPE to efficiently increase BMP-1 activity. The later hypothesis is more likely since maximum enhancement activity appears to occur at a PCPE/procollagen molar ratio of 1:1 [29].
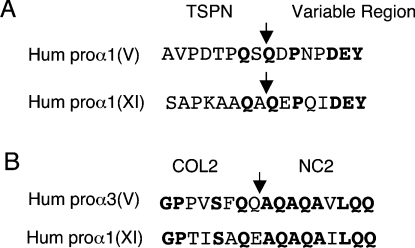
The proα1(V) N-propeptide BMP-1 cleavage site, which occurs at the peptide bond Ser254/Gln255, differs from those previously described [16], most remarkably in the absence of an invariant aspartate residue at the P1′ position. Proteolytic processing of proα1(XI) by BMP-1 occurs at an analogous cleavage site (Figure 7) [30]. The proα3(V) chain was shown to be cleaved at a previously unreported site [31]. Although the primary sequence of the proα3(V) chain is highly homologous with proα1(V), BMP-1 cleavage occurs at a different peptide bond located between Gln463 and Ala464 (Figure 7). In that case, the complete N-propeptide is released. Again, the cleavage sequence lacks an aspartate residue at the P1′ position and residues adjacent to the site showed no homology with the residues flanking any previously characterized cleavage sites of BMP-1 (Figure 7). Along this line, Colige et al. [17] showed that ADAMTS-2 processes the aminopropeptide of proα1(V) homotrimer at the end of the variable domain at a cleavage site (Pro-Ala) different from the previously described sites (Pro/Ala-Glu) for ADAMTS-2. In generating mutants to the previously identified cleavage site, we demonstrate that the residue crucial for BMP-1 cleavage is the aspartic acid at the P2′ position of the cleavage site, whereas the P1′ glutamine residue does not influence BMP-1 activity. This result was unexpected. First, the P1′ glutamine residue is conserved in the proα1(V) and proα1(XI) chains of various species [15] but the mutation Q255A and the double mutation S254A/Q255N BMP-1 only slightly affect the activity of BMP-1. Secondly, the presence of a conserved aspartic acid residue is known to be important for BMP-1 cleavage but at the P1′ position. The presence of a conserved P3′ proline residue adjacent to the aspartic acid residue can play an important role in BMP-1 activity although proline residues are not found at similar positions in all previously identified BMP-1 cleavage sites. The fact that mutation of the P1 serine has no effect on BMP-1 activity is less surprising since this residue is not conserved in the proα1(XI) chains [15].
Figure 7. Alignment of sequences corresponding to the BMP-1 cleavage site of the N-propeptide of the human proα1(V) (A) and proα3(V) (B) chains with human proα1(XI).
COL, collagenous domain; NC, non-collagenous domain. Conserved residues are shown in boldface; arrows indicate known BMP-1 cleavage sites in human (A) proα1(V) and proα1(XI) and (B) proα3(V) (accession numbers are NM000093, NM001854 and NM015719 respectively).
Fibrillar collagens are the most abundant structural proteins in the extracellular matrix. They undergo N- and C-propeptide processing which implicates specific proteinases. Collagen V is a minor fibrillar collagen that can be distinguished from the others by its capacity to control fibrillogenesis. In addition, this molecule is submitted to a particular processing and is involved in fundamental processes such as development and human connective tissues disorders. Here, in exploring the N- and C-terminal proteolytic processing of proα1(V) chain using several mutants, our results provide new relevant information on this complex and poorly understood mechanism essential to the function of the extracellular matrix proteins.
Acknowledgments
We thank Dr Efrat Kessler for the gift of BMP-1 antibodies and for helpful discussions on BMP-1 specificity, Jean-Claude Cortay (Laboratoire de Virologie et Pathogénèse Virale, UMR5537, Université Lyon 1, Lyon, France) for providing the pT7/7-6His expression vector plasmid and Yvette Descollonges (Institut de Biologie et Chimie des Proteines, UMR5086, Université Lyon 1, Lyon, France) for the production of monoclonal antibody to the N-propeptide of the human α1(V) chain. CD experiments were performed with the help of Rolland Montserret at the platform ‘Production and Purification of Proteins’ from the IFR 128 BioSciences Gerland (Université Lyon 1). This work was supported by grants from the ‘Association pour la Recherche contre le Cancer’ (no. 3652), the Emergence Research Program (Région Rhône-Alpes), GIS Maladies Rares Inserm (A04115SP) and the European Commission (Contract NMP2-CT-2003-504017). M. B. is the recipient of an Emergence Research Program bursary (Région Rhône-Alpes).
References
- 1.Ricard-Blum S., Ruggiero F. The collagen superfamily: from the extracellular matrix to the cell membrane. Pathol. Biol. 2005;53:30–42. doi: 10.1016/j.patbio.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 2.Trackman P. C. Diverse biological functions of extracellular collagen processing enzymes. J. Cell. Biochem. 2005;96:927–937. doi: 10.1002/jcb.20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prockop D. J., Sieron A. L., Li S. W. Procollagen N-proteinase and procollagen C-proteinase. Two unusual metalloproteinases that are essential for procollagen processing probably have important roles in development and cell signalling. Matrix Biol. 1998;16:399–408. doi: 10.1016/s0945-053x(98)90013-0. [DOI] [PubMed] [Google Scholar]
- 4.Sasaki T., Gohring W., Mann K., Brakebusch C., Yamada Y., Fassler R., Timpl R. Short arm region of laminin-5 gamma2 chain: structure, mechanism of processing and binding to heparin and proteins. J. Mol. Biol. 2001;314:751–763. doi: 10.1006/jmbi.2001.5176. [DOI] [PubMed] [Google Scholar]
- 5.Colombo M., Brittingham R. J., Klement J. F., Majsterek I., Birk D. E., Uitto J., Fertala A. Procollagen VII self-assembly depends on site-specific interactions and is promoted by cleavage of the NC2 domain with procollagen C-proteinase. Biochemistry. 2003;42:11434–11442. doi: 10.1021/bi034925d. [DOI] [PubMed] [Google Scholar]
- 6.Scott I. C., Imamura Y., Pappano W. N., Troedel J. M., Recklies A. D., Roughley P. J., Greenspan D. S. Bone morphogenetic protein-1 processes probiglycan. J. Biol. Chem. 2000;275:30504–30511. doi: 10.1074/jbc.M004846200. [DOI] [PubMed] [Google Scholar]
- 7.Ge G., Seo N. S., Liang X., Hopkins D. R., Hook M., Greenspan D. S. Bone morphogenetic protein-1/tolloid-related metalloproteinases process osteoglycin and enhance its ability to regulate collagen fibrillogenesis. J. Biol. Chem. 2004;279:41626–41633. doi: 10.1074/jbc.M406630200. [DOI] [PubMed] [Google Scholar]
- 8.Panchenko M. V., Stetler-Stevenson W. G., Trubetskoy O. V., Gacheru S. N., Kagan H. M. Metalloproteinase activity secreted by fibrogenic cells in the processing of prolysyl oxidase. Potential role of procollagen C-proteinase. J. Biol. Chem. 1996;271:7113–7119. doi: 10.1074/jbc.271.12.7113. [DOI] [PubMed] [Google Scholar]
- 9.Steiglitz B. M., Ayala M., Narayanan K., George A., Greenspan D. S. Bone morphogenetic protein-1/Tolloid-like proteinases process dentin matrix protein-1. J. Biol. Chem. 2004;279:980–986. doi: 10.1074/jbc.M310179200. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez E. M., Reed C. C., Bix G., Fu J., Zhang Y., Gopalakrishnan B., Greenspan D. S., Iozzo R. V. BMP-1/Tolloid-like metalloproteases process endorepellin, the angiostatic C-terminal fragment of perlecan. J. Biol. Chem. 2005;280:7080–7087. doi: 10.1074/jbc.M409841200. [DOI] [PubMed] [Google Scholar]
- 11.Fichard A., Kleman J. P., Ruggiero F. Another look at collagen V and XI molecules. Matrix Biol. 1995;14:515–531. doi: 10.1016/s0945-053x(05)80001-0. [DOI] [PubMed] [Google Scholar]
- 12.Fichard A., Tillet E., Delacoux F., Garrone R., Ruggiero F. Human recombinant α1(V) collagen chain. Homotrimeric assembly and subsequent processing. J. Biol. Chem. 1997;272:30083–30087. doi: 10.1074/jbc.272.48.30083. [DOI] [PubMed] [Google Scholar]
- 13.Birk D. E. Type V collagen: heterotypic type I/V collagen interactions in the regulation of fibril assembly. Micron. 2001;32:223–237. doi: 10.1016/s0968-4328(00)00043-3. [DOI] [PubMed] [Google Scholar]
- 14.Chanut-Delalande H., Fichard A., Bernocco S., Garrone R., Hulmes D. J. S., Ruggiero F. Control of heterotypic fibril formation by collagen V is determined by chain stoichiometry. J. Biol. Chem. 2001;276:24352–24359. doi: 10.1074/jbc.m101182200. [DOI] [PubMed] [Google Scholar]
- 15.Imamura Y., Steiglitz B. M., Greenspan D. S. Bone morphogenetic protein-1 processes the NH2-terminal propeptide and furin-like proprotein convertase processes the COOH-terminal propeptide of pro-α1(V) collagen. J. Biol. Chem. 1998;273:27511–27517. doi: 10.1074/jbc.273.42.27511. [DOI] [PubMed] [Google Scholar]
- 16.Kessler E., Fichard A., Chanut-Delalande H., Brusel M., Ruggiero F. Bone morphogenetic protein-1 (BMP-1) mediates C-terminal processing of procollagen V homotrimer. J. Biol. Chem. 2001;276:27051–27057. doi: 10.1074/jbc.M102921200. [DOI] [PubMed] [Google Scholar]
- 17.Colige A., Ruggiero F., Vandenberghe I., Dubail J., Kesteloot F., Van beeumen J., Beschin A., Brys L., Lapière C. M., Nusgens B. Domains and maturation processes that regulate the activity of ADAMTS-2, a metalloproteinase cleaving the aminopropeptide of fibrillar procollagens types I–III and V. J. Biol. Chem. 2005;280:34397–34408. doi: 10.1074/jbc.M506458200. [DOI] [PubMed] [Google Scholar]
- 18.Moali C., Font B., Ruggiero F., Eichenberger D., Rousselle P., François V., Oldberg A., Bruckner-Tuderman L., Hulmes D. J. S. Substrate-specific modulation of a multisubstrate proteinase. C-terminal processing of fibrillar procollagens is the only BMP-1-dependent activity to be enhanced by PCPE-1. J. Biol. Chem. 2005;280:24188–24194. doi: 10.1074/jbc.M501486200. [DOI] [PubMed] [Google Scholar]
- 19.Parsons M., Kessler E., Laurent G. J., Brown R. A., Bishop J. E. Mechanical load enhances procollagen processing in dermal fibroblasts by regulating levels of bone morphogenetic protein-1 (BMP-1)/procollagen C-proteinase. Exp. Cell Res. 1999;252:319–331. doi: 10.1006/excr.1999.4618. [DOI] [PubMed] [Google Scholar]
- 20.Nègre D., Bonod-Bidaud C., Geourjon C., Deléage G., Cozzone A. J., Cortay J. C. Definition of a consensus DNA-binding site for the Escherichia coli pleiotropic regulatory protein, FruR. Mol. Microbiol. 1996;21:257–266. doi: 10.1046/j.1365-2958.1996.6341350.x. [DOI] [PubMed] [Google Scholar]
- 21.Delacoux F., Fichard A., Geourjon C., Garrone R., Ruggiero F. Molecular features of the collagen V heparin binding site. J. Biol. Chem. 1998;273:15069–15076. [PubMed] [Google Scholar]
- 22.Ruggiero F., Burillon C., Garrone R. Human corneal fibrillogenesis. Collagen V structural analysis and fibrillar assembly by stromal fibroblasts in culture. Invest. Ophthalmol. Vis. Sci. 1996;37:1749–1760. [PubMed] [Google Scholar]
- 23.Tillet E., Mann K., Nischt R., Pan T. C., Chu M. L., Timpl R. Recombinant analysis of human α1 (XVI) collagen. Evidence for processing of the N-terminal globular domain. Eur. J. Biochem. 1995;228:160–168. doi: 10.1111/j.1432-1033.1995.tb20245.x. [DOI] [PubMed] [Google Scholar]
- 24.Ge G., Greenspan D. S. Developmental roles of the BMP1/TLD metalloproteinases. Birth Defects Res. C Embryo Today. 2006;78:47–68. doi: 10.1002/bdrc.20060. [DOI] [PubMed] [Google Scholar]
- 25.Kadler K. E., Hojima Y., Prockop D. J. Assembly of collagen fibrils de novo by cleavage of the type I pC-collagen with procollagen C-proteinase. Assay of critical concentration demonstrates that collagen self-assembly is a classical example of an entropy-driven process. J. Biol. Chem. 1987;262:15696–15701. [PubMed] [Google Scholar]
- 26.Linsenmeyer T. F., Gibney E., Igoe F., Gordon M., Fitch J. M., Fessler L. I., Birk D. E. Type V collagen: molecular structure and fibrillar organization of the chicken α1(V) NH2-terminal domain, a putative regulator of corneal fibrillogenesis. J. Cell Biol. 1993;121:1181–1189. doi: 10.1083/jcb.121.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Unsöld C., Pappano W. N., Imamura Y., Steiglitz B. M., Greenspan D. S. Biosynthetic processing of the pro-a1(V)pro-a2(V) collagen heterotrimer by bone morphogenetic protein-1 and furin-like proprotein convertases. J. Biol. Chem. 2002;277:5596–5602. doi: 10.1074/jbc.M110003200. [DOI] [PubMed] [Google Scholar]
- 28.Boot-Handford R. P., Tuckwell D. S., Plumb D. A., Farrington R. C., Poulsom R. A novel and highly conserved collagen (proα1(XXVII)) with a unique expression pattern and unusual molecular characteristics establishes a new clade within the vertebrate fibrillar collagen family. J. Biol. Chem. 2003;278:31067–31077. doi: 10.1074/jbc.M212889200. [DOI] [PubMed] [Google Scholar]
- 29.Moschcovich L., Bernocco S., Font B., Rivkin H., Eichenberger D., Chejanovsky N., Hulmes D. J. S., Kessler E. Folding and activity of recombinant human procollagen C-proteinase enhancer. Eur. J. Biochem. 2001;268:2991–2996. doi: 10.1046/j.1432-1327.2001.02189.x. [DOI] [PubMed] [Google Scholar]
- 30.Medeck R. J., Sosa S., Morris N., Oxford J. T. BMP-1-mediated proteolytic processing of alternatively spliced isoforms of collagen type XI. Biochem. J. 2003;376:361–368. doi: 10.1042/BJ20030894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gopalakrishnan B., Wang W. M., Greenspan D. S. Biosynthetic processing of the pro-α1(V)proα2(V)proα3(V) procollagen heterotrimer. J. Biol. Chem. 2004;279:30904–30912. doi: 10.1074/jbc.M402252200. [DOI] [PubMed] [Google Scholar]