Abstract
The TGF-β (transforming growth factor-β) induces survival signals in foetal rat hepatocytes through transactivation of EGFR (epidermal growth factor receptor). The molecular mechanism is not completely understood, but both activation of the TACE (tumour necrosis factor α-converting enzyme)/ADAM17 (a disintegrin and metalloproteinase 17; one of the metalloproteases involved in shedding of the EGFR ligands) and up-regulation of TGF-α and HB-EGF (heparin-binding epidermal growth factor-like growth factor) appear to be involved. In the present study, we have analysed the molecular mechanisms that mediate up-regulation of the EGFR ligands by TGF-β in foetal rat hepatocytes. The potential involvement of ROS (reactive oxygen species), an early signal induced by TGF-β, and the existence of an amplification loop triggered by initial activation of the EGFR, have been studied. Results indicate that DPI (diphenyleneiodonium) and apocynin, two NOX (NADPH oxidase) inhibitors, and SB431542, an inhibitor of the TβR-I (TGF-β receptor I), block up-regulation of EGFR ligands and Akt activation. Different members of the NOX family of genes are expressed in hepatocytes, included nox1, nox2 and nox4. TGF-β up-regulates nox4 and increases the levels of Rac1 protein, a known regulator of both Nox1 and Nox2, in a TβR-I-dependent manner. TGF-β mediates activation of the nuclear factor-κB pathway, which is inhibited by DPI and is required for up-regulation of TGF-α and HB-EGF. In contrast, EGFR activation is not required for TGF-β-induced up-regulation of those ligands. Considering previous work that has established the role of ROS in apoptosis induced by TGF-β in hepatocytes, the results of the present study indicate that ROS might mediate both pro- and anti-apoptotic signals in TGF-β-treated cells.
Keywords: heparin-binding epidermal growth factor-like growth factor (HB-EGF), hepatocyte, NADPH oxidase (NOX), nuclear factor-κB (NF-κB), reactive oxygen species (ROS), transforming growth factor (TGF)
Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; DHE, dihydroethidine; DPI, diphenyleneiodonium; EGF, epidermal growth factor; EGFR, EGF receptor; EMSA, electrophoretic mobility-shift assay; ERK, extracellular-signal-regulated kinase; HB-EGF, heparin-binding EGF-like growth factor; H2DCFDA, 2′,7′-dichlorodihydrofluorescein diacetate; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor-κB; NOX, NADPH oxidase; PI3K, phosphoinositide 3-kinase; ROS, reactive oxygen species; RT, reverse transcriptase; SBE, Smad-binding element; SOD, superoxide dismutase; TGF, transforming growth factor; TβR-I, TGF-β receptor type I; TNFα, tumour necrosis factor α; XIAP, X-linked inhibitor of apoptosis
INTRODUCTION
TGF-β (transforming growth factor-β) belongs to a family of structurally related polypeptide growth factors that play important roles in growth control, development and differentiation [1]. TGF-β inhibits growth and induces apoptosis in epithelial cells, contributing to the suppression of tumorigenesis. However, recent works have indicated that the cellular response to TGF-β is very complex, in particular in pre-neoplastic or tumour cells, where TGF-β might mediate both pro- and anti-apoptotic signals, and induce epithelial–mesenchymal transition processes, contributing to tumour progression and dissemination [2].
One example of this complex response is observed in liver cells. TGF-β induces apoptosis in foetal rat hepatocytes, by a mechanism dependent on oxidative stress, mitochondrial release of cytochrome c and caspase activation [3]. However, TGF-β also induces survival signals in those cells [4], through transactivation of EGFR (epidermal growth factor receptor), which is required for Akt phosphorylation and cell death rescue [5]. The balance between death and survival signals decides cell fate. Thus, a great proportion (around 60–70%) survive the apoptotic effects of TGF-β and respond to this cytokine by undergoing an epithelial–mesenchymal transition process, which increases their migratory capacity. Thus, understanding the molecular pathways that mediate TGF-β-induced hepatocyte survival might help in the design of new drugs that potentiate its suppressor effects. The molecular mechanisms that mediate EGFR transactivation by TGF-β are not completely understood. Activation of the TACE [TNFα (tumour necrosis factor α)-converting enzyme]/ADAM17 (a disintegrin and metalloproteinase 17; one of the metalloproteases involved in shedding of the EGFR ligands) produces the proteolysis and activation of the EGFR ligands that are anchored in the cell membrane [5]. Furthermore, TGF-β induces up-regulation of TGF-α and HB-EGF (heparin binding epidermal growth factor-like growth factor) [6]. Regulation of TGF-α expression by TGF-β had been proposed previously to occur in human colon carcinoma [7,8] and endothelial [9] cells; however, up until now, nothing was known about the intracellular signal(s) initiated by TGF-β that triggers this effect.
One of the early events taking place in response to TGF-β in foetal rat hepatocytes is generation of ROS (reactive oxygen species), through activation of a NOX (NADPH oxidase)-like system and down-regulation of anti-oxidant genes [10]. With the increased understanding of the proliferation, survival and death signalling pathways, the exact role of ROS in regulating cellular functions is controversial. Growing evidence suggests that ROS within cells act as second messengers in intracellular signalling cascades, which induce and maintain the oncogenic phenotype of cancer cells. However, it is also known that ROS can induce cellular senescence and cell death and can therefore act as anti-tumorigenic agents [11]. In this line of evidence, some important discoveries have underscored the critical role of ROS in the survival signals triggered by TNF-α [notably in the TNF-α-mediated activation of NF-κB (nuclear factor-κB) and JNK (c-Jun N-terminal kinase)], in addition to their known role in cell death (apoptotic and necrotic) pathways [12]. Interestingly, transactivation of EGFR by TNF [13] or endothelin-1 [14] would require ROS generation, and different reports propose that ROS and oxidative stress may regulate the transcription of HB-EGF [15–17].
In the rat gastric epithelial cell line (RGM1 cells), in the first minutes following oxidative and osmotic stress, tyrosine phosphorylation of EGFR is induced, followed by a marked increase in HB-EGF and amphiregulin transcripts [18], which suggests that EGFR might play a crucial role in the stress-induced expression of EGF-like growth factors in gastrointestinal cells. In addition, it is known that TGF-α is able to induce transcription of its own mRNA via a signal due to activation of the EGFR, which is acting predominantly at the post-transcriptional level [19].
According to the above findings, the aim of this work was to analyse the molecular mechanisms that mediate up-regulation of EGFR ligands, HB-EGF and TGF-α, by TGF-β in foetal rat hepatocytes. The possible involvement of ROS, induced by a NOX system, and/or the existence of an amplification loop triggered by the initial activation of the EGFR have been studied. The results indicate that TβR-I (TGF-β receptor I/Alk5)-dependent activation of NOX mediates up-regulation of EGFR ligands by a mechanism that does not require EGFR activity, but depends on a functional NF-κB pathway.
EXPERIMENTAL
Materials
Human recombinant TGF-β1, AG1478, apocynin, PD169316, PD98059 and SN-50 were from Calbiochem (La Jolla, CA, U.S.A.). Culture medium was from Invitrogen (Carlsbad, CA, U.S.A.). Foetal bovine serum was from Sera Laboratories International (Cinder Hill, U.K.). EGF was a gift from the Serono Laboratory (Madrid, Spain). Monoclonal anti-β-actin antibody, DPI (diphenyleneiodonium chloride), glutathione (ethyl ester form), SOD (superoxide dismutase), catalase, rapamycin and SB431542 were from Sigma (Madrid, Spain). LY294002 and SP600125 were from Alexis Biochemicals (Lausen, Switzerland). The fluorescent probes H2DCFH-DA (2′,7′-dichlorodihydrofluorescein diacetate) and DHE (dihydroethidine) were from Molecular Probes (Eugene, OR, U.S.A.). Anti-Akt (CS-9272), anti-(phospho-Akt) (Ser473; CS-9271), and anti-(phospho Smad-2) (Ser465/467; CS-3101) antibodies were from Cell Signaling Technology (Beverly, MA, U.S.A). NF-κB/p65 antibodies, used in Western blot (SC-109) or EMSA (electrophoretic mobility-shift assay) experiments (SC-109X) were from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). Anti-Rac1 (Upstate 05-389) was from Upstate (Lake Placid, NY, U.S.A.). The secondary antibody, Alexa Fluor 488-conjugated anti-rabbit IgG (A11001), was from Molecular Probes.
Cell isolation and culture
Foetal hepatocytes were obtained by collagenase disruption of 20-day-old foetal Wistar rat liver and cultured in non-coated plastic dishes with arginine-free, ornithine-supplemented, M-199 medium as described previously [4]. After attachment and serum starvation for 12–24 h, growth factors were added. Inhibitors were added 30 min before TGF-β treatment.
Western blot analysis
Total protein extracts were obtained as described previously [5], separated by SDS/PAGE (12% polyacrylamide gels) and transferred on to PVDF membranes. After blocking with 5% (w/v) non-fat milk TBST [Tris-buffered saline solution containing 0.05% (v/v) Tween 20] the membranes were incubated overnight with the corresponding antibody in a 0.5% non-fat milk TBST (diluted 1:5000 for β-actin and 1:1000 for all others). After washing and incubating the membrane with an appropriate peroxidase-conjugated antibody (diluted 1:5000) for 1 h at 21°C, antibody binding was revealed using ECL® (GE-Healthcare). β-actin was used as a loading control.
RT (reverse transcriptase)-PCR
Total RNA was obtained using the RNeasy Kit (Qiagen). Complementary DNA was generated by the SuperScript First-Strand Synthesis System for RT-PCR using oligo(dT) as primer.
Rat specific primers sequences for PCR reactions were used: snail: 5′-GCAGCTGGCCAGGCTCTCGGTGGC-3′ (forward) and 5′-GTAGCTGGGTCAGCGAGGGCCTCC-3′ (reverse); tgf-α: 5′-TGGTGCAGGAAGAGAAGC-3′ (forward) and 5′-TGACAGCAGTGGATCAGC-3′ (reverse); hb-egf: 5′-CGGTGGTGCTGAAGCTCTTTC-3′ (forward) and 5′-TGGTAACCAGGGAGGCAGTG-3′ (reverse); nox1: 5′-GGATCACAACCTCACCTTCC-3′ (forward) and 5′-GCTGCATACATCACTGTCACG-3′ (reverse); nox2: 5′-TCAAGTGTCCCCAGGTATCC-3′ (forward) and 5′-CTTCACTGGCTGTACCAAAGG-3′ (reverse); nox4: 5′-TTACTACTGCCTCCATCAAGC-3′ (forward) and 5′-GGAATGATTGGATGTCTCTGC-3′ (reverse); rac1: 5′-TGCGTTCCCTGGAGAGTACATCC-3′ (forward) and 5′-TTGAGTCCTCGCTGTGTGAGTGC-3′ (reverse), and; albumin: 5′-CTGCCGATCTGCCCTCAATAGC (forward) and 5′-GTGCCCACTCTTCCCAGGTTTCT-3′ (reverse). Products were obtained after 30–35 cycles of amplification at annealing temperatures between 60 and 65°C, and were electrophoresed in 0.8–1.2% (w/v) agarose gels. Expression of albumin was analysed as a loading control. The −RT lanes, which lack any kind of RNA, are shown as specificity controls.
NOX activity assay
Cells were detached by trypsinization, pelleted by centrifugation at 2500 g for 5 min at 4°C and resuspended in PBS. NOX activity was analysed as described previously [10]. Briefly, cells were incubated with 250μM NADPH. NADPH consumption was monitored by the decrease in absorbance at λ=340 nm for 5 min. Specific oxidase activity was measured by monitoring the rate of consumption of NADPH inhibited by the addition of 10μM DPI 30 min before the assay. An aliquot of cells was lysed with SDS and used to determine the protein concentration. The results are expressed as pmol of oxidized NADPH/min per mg of protein.
Measurement of intracellular ROS
The oxidation-sensitive fluorescent probes H2DCFDA and DHE were used to analyse the intracellular content of peroxides and superoxide anions respectively. Cells were detached by trypsinization, resuspended in PBS and incubated for 30 min with 5 μM H2DCFDA or 20 μM DHE. Fluorescence intensity was measured by using a FACScan flow cytometer (Becton Dickinson, San Jose, CA, U.S.A.). For each analysis 10000 events were recorded.
EMSA
Specific probes for DNA–protein interaction analyses were used. NF-κB-binding element [corresponding to the NF-κB motif in the iNOS (inducible nitric oxide synthase) promoter]: 5′-tcgaCCAACTGGGACTCTCCCTTTGGGAACA-3′ (forward) and 5′-tcgaTGTTCCCAAAGGGAGAGTCCCAGTTGG-3′ (reverse); Smad-binding element (corresponding to the consensus sequence): 5′-agctGGAGTATGTCTAGACTGACAAGTTAC-3′ (forward) and 5′-agctGTACATTGTCAGTCTAGACATACTCC-3′ (reverse); and AP-1-binding element (corresponding to the collagenase PMA-responsive element): 5′-agctTGATGAGTCAGCCG-3′ (forward) 5′-gatcCGGCTGACTCATCA-3′ (reverse). After annealing of the forward and reverse oligonucleotides, 0.5 μg were radiolabelled using the Klenow enzyme (Roche) and [α32P]dCTP (GE-Healthcare). Radiolabelled oligonucleotides were purified using MicroSpin SC-200 HR columns (GE-Healthcare) and used as probes. Radiolabelling was measured by liquid scintillation counting.
Nuclear protein extracts were obtained as described previously [4]. For specificity controls, 10 μg of protein extract were incubated for 15 min at 4°C with anti-p65 antibody (2 μg) or with unlabelled oligonucleotide (100 ng). After this, 10 μg of all nuclear protein extracts (specificity controls included) were incubated with 150000 counts per million of the appropriate probe and 0.5 μg/μl of polydeoxyinosinic deoxycytidylic acid in a binding solution [40 mM Hepes, pH 8.0, 0.2 mM EDTA, 0.1 M NaCl, 80 mM KCl, 10 mM Mg Cl2, 10% (v/v) glycerol, 2 mM dithiothreitol] for 30 min at 4°C. DNA–protein complexes were separated in 6% (w/v) non-denaturing polyacrylamide gels in 0.5× TBE (1× TBE=45 mM Tris/borate and 1 mM EDTA) at room temperature. Gels were dried and complexes were visualized by autoradiography.
Immunocytochemistry
Cells were cultured on non-coated, glass coverslips. After washing with PBS, cells were fixed with 4% (w/v) paraformaldehyde in PBS for 12 min at room temperature, permeabilized PBS containing 0.1% Triton X-100 and 0.1% BSA for 3 min, and then blocked with PBS containing 1% (w/v) BSA and 10% (v/v) goat serum for 1 h. After blocking, the cells were incubated with the anti-p65 antibody [diluted 1:400 in PBS containing 1% (w/v) BSA], overnight at 4°C. The cells were washed and then incubated with Alexa Fluor 488-conjugated anti-rabbit [diluted 1:200 in PBS containing 1% (w/v) BSA] for 1 h at room temperature. Coverslips were embedded in Vectashield (Vector Laboratories) with DAPI (4′,6-diamidino-2-phenylindole). Cells were visualized using an Olympus BX-60 microscope with the appropriate filters. Representative images were taken with a Spot 4.3 digital camera and software, and edited using Adobe Photoshop.
RESULTS
Activation of the TβR-I and a NOX system is required for the increase in tgf-α and hb-egf transcript levels induced by TGF-β in foetal rat hepatocytes
EGFR activation is crucial for the survival of foetal hepatocytes against the pro-apoptotic signals induced by TGF-β because it mediates the activation of the PI3K (phosphoinositide 3-kinase)/Akt signalling pathway in response to TGF-β treatment [4]. As we have shown previously, inhibition of the EGFR activity with the specific inhibitor AG1478 completely blocked the activation of the PI3K/Akt survival pathway (Figure 1A), leading to large-scale apoptosis of foetal hepatocytes [5]. Analysis of the mRNA levels of the EGFR ligands, TGF-α and HB-EGF, by RT-PCR after TGF-β treatment revealed that TGF-β promoted an increase in the expression of these genes (Figure 1B), which might account for activation of the EGFR pathway. We first wanted to know if this event was being triggered by the canonical TGF-β signalling pathway through TβR-I (ALK 5), therefore we used a specific TβR-I inhibitor, SB431542. This compound completely blocked the up-regulation of both TGF-α and HB-EGF. Next, we analysed the possible implication of ROS production in the up-regulation of EGFR ligands. For this, we cultured the cells in the presence of the flavoprotein inhibitor DPI, which was shown to block completely TGF-β-induced ROS production in a previous studies by our group [10]. As shown in Figure 1(C), DPI blocked the up-regulation of both TGF-α and HB-EGF. In contrast, the expression of a well-known target of TGF-β, Snail, was blocked by the TβRI inhibitor, but not by DPI, indicating that its effect was not unspecific. At the protein level, DPI also blocked the activation of Akt without affecting Smad-2 phosphorylation, demonstrating again that DPI is not impairing classical TGF-β signalling. TβR-I inhibitor completely abolished both Akt activation and Smad-2 phosphorylation (Figure 1D). These data suggest that both up-regulation of EGFR ligands and activation of the PI3K/Akt pathway need the activation of the TβR-I and ROS production.
Figure 1. ROS production and TβR-I are required for up-regulation of EGFR ligands by TGF-β.
Hepatocytes were incubated in the absence or presence of TGF-β (1 ng/ml), the EGFR inhibitor AG1478 (10 μM), the TβR-I inhibitor SB431542 (10 μM) and/or the ROS scavenger, DPI (20 μM), at the indicated times [only a 3h time point was used in (C)]. Protein extracts were obtained to study the activation state of Akt by Western blot (A and D). Smad-2 activation was also studied in (D). Total RNA was obtained to study the expression of tgf-α and hb-egf by RT-PCR (B and C). Expression levels of snail were also analysed in (C). In all cases, a representative experiment of at least four is shown.
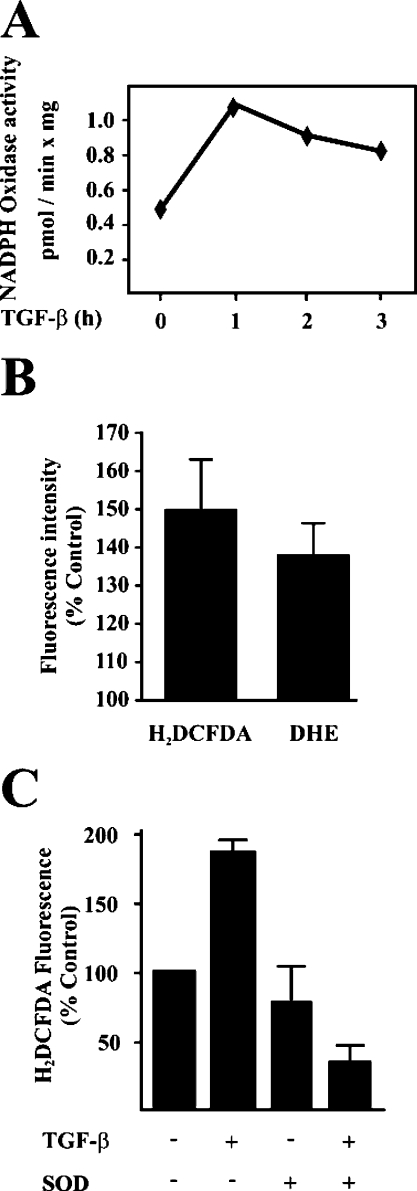
A detailed timing analysis showed that TGF-β induces an early activation of a NOX system in foetal rat hepatocytes (Figure 2A), coincident with an increase in the intracellular content of superoxide anion and peroxide analysed by flow cytometry using DHE and H2DCFDA respectively (Figure 2B). Adding SOD to the culture medium blocked completely the increase in peroxide (Figure 2C), indicating that superoxide anion is the first ROS produced, as expected if a NOX system is involved. Different members of the NOX family of genes are expressed in these cells, included nox1, nox2 and nox4 (Figure 3A), in agreement with previous results obtained in adult hepatocytes [20]. TGF-β1 up-regulated nox4 and increased the protein levels of Rac1 (Figure 3B), a known regulator of Nox2 [21] and some other members of the family. The increase in Rac1 protein level does not correlate with an increase in its transcript level (Figure 3C), which would suggest post-transcriptional regulation is occurring. Both the up-regulation of nox4 and the increase in Rac1 protein level, were blocked in the presence of the TβR-I inhibitor (Figure 3D). Since DPI is not a specific NOX inhibitor, because it inhibits flavoproteins, we next tested a more selective NOX inhibitor, apocynin. Apocynin blocked completely the early NOX activation induced by TGF-β (Figure 4A) and was able to abolish the up-regulation in the expression of tgf-α and hb-egf genes (Figure 4B), thus reaffirming the role of NOX in the control of EGFR ligand expression. A complete inhibition in TGF-β-mediated Akt phosphorylation was observed when foetal hepatocytes were cultured in the presence of apocynin (Figure 4C). Furthermore, adding catalase or SOD to the culture medium also impaired TGF-β-mediated Akt activation. The addition of a permeable form of glutathione also blocked this activation, reinforcing the essential role of ROS-mediated signalling in the TGF-β-regulated survival signals in foetal rat hepatocytes.
Figure 2. TGF-β activates a NOX system in hepatocytes, which produces an increase in the intracellular content of superoxide anion and peroxide.
(A) Cells were incubated with TGF-β (1 ng/ml) at different times. NOX activity assay was performed as described in the Experimental section. A representative experiment of three is shown. (B) Intracellular content of ROS was analysed, as described in the Experimental section, in hepatocytes treated with TGF-β (1 ng/ml) for 3 h. The results are shown as means±S.E.M. for five experiments. (C) The intracellular content of peroxide was analysed in cells incubated for 3 h in the absence or presence of TGF-β (1 ng/ml) and/or 300 units/ml of SOD. The results are shown as means±S.E.M. for three experiments.
Figure 3. TGF-β up-regulates nox4 mRNA transcription and Rac1 protein level.
Hepatocytes were treated with TGF-β (1 ng/ml) at the indicated times. (A) RT-PCR analysis was performed to analyse the expression levels of nox1, nox2 and nox4. (B) Rac1 protein levels were analysed by Western blotting. (C) RT-PCR was performed to analyse rac1 transcript levels. (D) The effect of the TβR-I inhibitor SB431542 (10 μM) on TGF-β-mediated increase in nox4 transcription and Rac1 protein level (3 h treatment with 1 ng/ml TGF-β). In all cases, a representative experiment of at least three is shown.
Figure 4. Apocynin, a selective NOX inhibitor, impairs TGF-β-induced tgf-α and hb-egf up-regulation, and apocynin and antioxidants block Akt activation.
(A) The effect of apocynin (300 μM) over NOX activity was analysed in the absence (C) or presence (T) of TGF-β (1 ng/ml) after 3 h of treatment. (B) Expression levels of tgf-α and hb-egf were analysed by RT-PCR after cell treatment with or without 1 ng/ml TGF-β for 3 h in the presence or absence of apocynin (300 μM). (C) Effect of apocynin on Akt activation examined by Western blotting in cells treated with TGF-β (1 ng/ml) and/or apocynin (300 μM) for 3 h. (D) Effect of the antioxidants catalase (300 units/ml), SOD (300 units/ml) and GSH (0.5 mM) on TGF-β-induced Akt activation in cells treated with 1 ng/ml TGF-β for 3h and examined by Western blotting. A representative experiment of at least three is shown in all cases.
The NF-κB pathway, downstream of ROS, is required for TGF-α and HB-EGF up-regulation
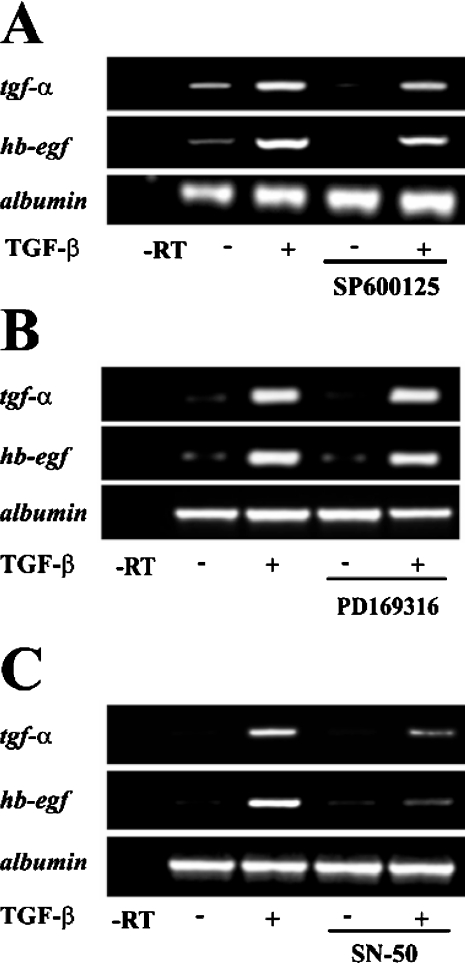
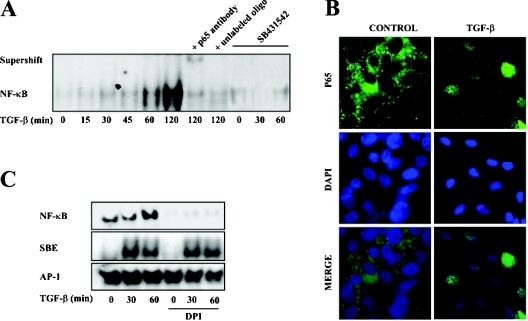
Next, we asked whether the increased expression of the EGFR ligands was being triggered by redox-sensitive signalling pathways. Semiquantitative RT-PCR assays with RNA from cells treated with specific inhibitors of JNK (SP600125) and p38MAPK (mitogen-activated protein kinase; PD169316) showed that these proteins were not involved (Figures 5A and 5B). However, an NF-κB specific inhibitor (SN-50) that selectively impairs the translocation of NF-κB active complexes into the nucleus was able to almost completely block the increased expression of TGF-α and HB-EGF (Figure 5C), thus indicating the possible involvement of this pathway. TGF-β activated NF-κB rapidly, which is shown in the EMSA time course analysis in Figure 6(A). This effect was blocked by the TβR-I inhibitor, implicating the classical TGF-β signalling pathway in the processes studied. Nuclear translocation of the p65 subunit after TGF-β treatment is shown in an immunocytochemistry assay (Figure 6B). Although p65, in the control cells, is located in the cytoplasm, in the TGF-β-treated cells it was observed to co-localize completely with the nuclear stain DAPI. As ROS are well-known activators of NF-κB, we analysed the possibility that ROS were doing so in the present system. For that purpose we used the flavoprotein inhibitor DPI observing that it was able to impair completely the TGF-β-mediated NF-κB activation (Figure 6C). To further assess the lack of interaction between DPI and the TGF-β signalling pathway, transcriptional binding of Smads to their SBE (Smad-binding element) was also analysed. DPI did not affect the TGF-β-stimulated DNA binding activity of Smads (Figure 6C) or the translocation of Smad2 and Smad3 to the nucleus, as analysed by Western blot (results not shown). AP-1 is shown as a transcription factor neither modulated by TGF-β nor by ROS at short times. These results demonstrate that NF-κB activation mediates the increased expression of TGF-α and HB-EGF induced by TGF-β, and that ROS are responsible for this activation.
Figure 5. NF-κB activation is necessary for TGF-β-induced up-regulation of EGFR ligands.
Expression levels of tgf-α and hb-egf were analysed by RT-PCR in cells cultured in the presence or absence of 1 ng/ml TGF-β for 3h, with or without the JNK inhibitor SP600125 (40 μM) (A), the p38MAPK inhibitor PD169316 (800 nM) (B) or the inhibitor of the nuclear translocation of NF-κB, SN-50 (50 μM) (C). In all cases, a representative experiment of at least three is shown.
Figure 6. ROS mediate TGF-β-induced NF-κB activation.
(A) Following the incubation of cells with TGF-β (1 ng/ml) at different times in the presence or the absence of the TβR-I inhibitor SB431542 (10 μM), nuclear protein extracts were obtained. Binding activity of NF-κB was analysed by an EMSA as described in the Experimental section. Specificity controls were performed by incubating nuclear extracts with an anti-p65 antibody and with unlabelled oligonucleotide. A representative experiment of three is shown. (B) Cellular localization of the p65 subunit of NF-κB before (control) and after treatment of hepatocytes with TGF-β (1 ng/ml) was analysed by fluorescent microscopy immunocytochemistry, using DAPI for nuclear staining. A representative experiment of five is shown. (C) The effect of DPI on the binding activity of NF-κB, SBE and AP-1 was performed by EMSA. For this, cells were incubated with TGF-β (1 ng/ml) in the presence or the absence of DPI (20 μM) and nuclear protein extracts were collected. A representative experiment of two is shown.
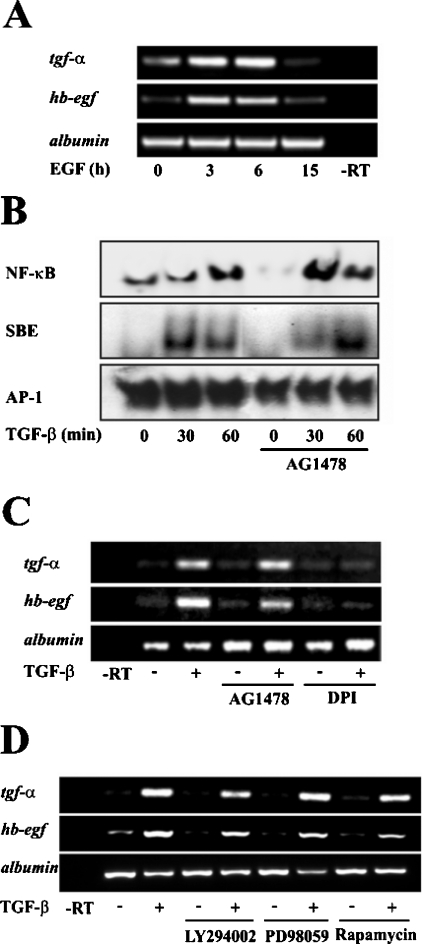
Activation of the EGFR pathway, PI3K or ERKs (extracellularsignal-regulated kinases) is not required for up-regulation of TGF-α or HB-EGF by TGF-β in foetal rat hepatocytes
Results from our and other groups [5] had established that EGF could impair TGF-β-induced apoptosis in different ways, including activation of the PI3K pathway. Moreover, EGF is also capable of increasing the expression of EGFR ligands (Figure 7A). For these reasons, we wondered whether EGFR could be collaborating with ROS to activate NF-κB in the modulation of the expression of its own ligands. Contrary to our expectations, EGFR seemed not to be involved. Using a selective EGFR inhibitor, AG1478, we could not observe either a blocking effect on NF-κB activity, as analysed by EMSA (Figure 7B), or on the expression levels of TGF-α and HB-EGF (Figure 7C), indicating that EGFR does not trigger the early up-regulation of its own ligands. As expected, EGFR does not affect TGF-β signalling through Smad proteins, as the specific EGFR inhibitor does not affect Smad binding to the SBE (Figure 7B). To better substantiate this result, we analysed the implication of possible effectors downstream EGFR activation, such as PI3K, ERKs or mTOR (mammalian target of rapamycin). Using specific inhibitors for these proteins (LY294002 for PI3K, PD 98059 for ERKs and rapamycin for mTOR) we analysed the expression levels of the EGFR ligands TGF-α and HB-EGF, finding that none of these proteins seemed to be involved (Figure 7D). All these data support the idea that EGFR is not necessary for TGF-β-mediated up-regulation of TGF-α and HB-EGF, and that other signalling pathways linked to EGFR activation, such as PI3K, ERKs or mTOR are also not required.
Figure 7. EGFR is not required for TGF-β-induced up-regulation of its own ligands.
(A) Time course analysis of the effect of EGF (20 ng/ml) on EGFR ligand expression by RT-PCR. (B) After treatment of cells with TGF-β (1 ng/ml) at different times in the presence or absence of AG1478 (10 μM), nuclear extracts were collected and the DNA-binding activity of NF-κB, SBE and AP-1 was analysed by EMSA. A representative experiment of two is shown. (C) and (D) The effect of the EGFR inhibitor AG1478 (10 ng/ml), DPI (20 μM), the PI3K inhibitor LY294002 (10 μM), the ERK inhibitor PD98059 (50 μM) and the mTOR inhibitor rapamycin (10 μM) on the expression of EGFR ligands in cells cultured in the presence or absence of TGF-β (1 ng/ml, 3h) was analysed by RT-PCR. A representative experiment of at least three is shown.
DISCUSSION
TGF-β is a potent regulator of epithelial cell proliferation, through growth inhibition and the induction of apoptosis [1]. Indeed, loss of TGF-β-mediated growth restraint has been shown to be associated with an increased risk of transformation [1]. However, there is also evidence that high production and/or activation of TGF-β in tumours can enhance cancer progression by autocrine and/or paracrine mechanisms [2]. It is worthy to note that TGF-β was originally isolated as an agent that was able to induce anchorage-independent growth of mouse fibroblasts [22]. The results of the present study indicate that, in addition to its role in inducing apoptosis in hepatocytes [3], TGF-β controls the expression of EGFR ligands, such as TGF-α or HB-EGF, which contributes to the transactivation of the EGFR pathway, regulating anti-apoptotic signals, such as activation of the Akt pathway (as shown in Figures 1A and 1B). Thus, TGF-β controls both pro- and anti-apoptotic signals, as previously suggested [4], in foetal rat hepatocytes. The balance among these signals controls cell fate, and a great percentage of cells (approx. 70%) survive ([4] and results not shown).
Although previous reports have proposed that TGF-β might regulate the expression of EGFR ligands [6–9], nothing was known about the intracellular signal(s) initiated by TGF-β that triggers this effect. Here we propose that the TβR-I-dependent activation of NOX is necessary for this effect. Different results support this hypothesis: 1) TβR-I-dependent up-regulation of TGF-α and HB-EGF is inhibited by DPI (Figure 1) and apocynin (Figure 4), which are two known inhibitors of the NOX system; 2) TGF-β activates an early NOX pathway, which is coincident with up-regulation of the gene nox4, and the increase in Rac1 protein level, a well-known activator of Nox1 and Nox2 [21] (Figures 2 and 3); and 3) activation of the TβR-I is required for both the increase in nox4 mRNA expression and the enhancement of Rac1 protein level (Figure 3).
Different intracellular signals are controlled by oxidative stress, with later effects in gene transcription. Among them, p38MAPK has been proposed to be activated by ROS in TGF-β-treated foetal rat hepatocytes [23]. p38MAPK is particularly interesting, because a previous report [24] indicated that activation of p38MAPK might regulate the expression of an autocrine survival factor by TGF-β in mesenchymal cells. JNK is another signal under redox regulation, which might contribute to regulate gene transcription through the AP-1 transcription factor [25]. However, the results presented in Figure 5 exclude the involvement of p38MAPK or JNK in the up-regulation of EGFR ligands by TGF-β in hepatocytes. Furthermore, AP-1 DNA-binding activity is not modulated by TGF-β at short times (Figures 6 and 7).
NF-κB has, for some time, been known to be redox regulated and is a direct target for oxidation, which can affect its ability to bind DNA [26]. Many kinases involved in direct or indirect activation of NF-κB are affected by oxidants and, therefore, have the potential to alter NF-κB function [27,28]. Furthermore, previous results have shown that NF-κB might be activated by NOXs in different cell types [29–31]. NF-κB plays an important role during liver neoplastic development through transcriptional regulation of pro-survival genes, such as Bcl-xL or XIAP (X-linked inhibitor of apoptosis) [32]. Interestingly, the NF-κB/Bcl-xL/XIAP axis potently counteracts the TGF-β-induced apoptosis [33] and exerts a general cytoprotective role on pre-neoplastic hepatocytes [34]. Here we propose that a NOX-dependent activation of NF-κB might mediate regulation of the expression of EGFR ligands by TGF-β, which might act by increasing proliferation and preventing apoptosis of liver cells. Results that support this hypothesis are: 1) SN-50, a known inhibitor of the nuclear translocation of NF-κB, blocks the up-regulation of TGF-α and HB-EGF (Figure 5C); 2) TGF-β induces an early activation of the NF-κB DNA-binding, which is dependent on TβR-I activation, and correlates with nuclear translocation of the RelA/p65 subunit (Figures 6A and 6B); 3) DPI impairs NF-κB activation (Figure 6C). Previous reports have proposed a crucial role for NF-κB in the control of transcription of EGFR ligands. Thus, NF-κB activity is required for platelet-activating-factor-mediated up-regulation of HB-EGF in monocytes [35]. NF-κB is also involved in HB-EGF expression in non-parenchymal liver cells after partial hepatectomy [36]. Interestingly, the hepatitis C virus core protein promotes proliferation of human hepatoma cells through up-regulation of TGF-α transcription via activation of NF-κB [37]. Thus, NF-κB might control transcription of EGFR ligands either directly or indirectly by acting on NF-κB target genes whose products are required for TGF-α or HB-EGF transcriptional induction. It has been suggested recently that Smad4 plays a role in the transcriptional activation of NF-κB by TGF-β in colon cancer cells [38]. As shown in Figures 1, 4, 6 and 7, NOX inhibitors do not affect either Smad2 phosphorylation or Smad DNA-binding activity. Smads might contribute to induce expression of nox4, and/or activate NF-κB. Further work is necessary to understand completely whether both ROS elevation and Smad involvement are critical for the transcriptional induction of the EGFR ligands by TGF-β in hepatocytes.
It is worthy to note that, although different data in the literature point to the existence of an autocrine loop for EGFR ligands that is up-regulated as soon as the EGFR is activated [18,19], this pathway does not appear to be involved in the early up-regulation of TGF-α or HB-EGF addressed by TGF-β in hepatocytes. Thus, in spite of the strong effect of extracellular EGF on the expression of TGF-α and HB-EGF (Figure 7A), the EGFR inhibitor AG1478 does not impair either the activation of NF-κB by TGF-β, or the up-regulation of the EGFR ligands (Figure 7). Of course, we cannot exclude the possibility that the EGFR pathway might contribute to the amplification or maintenance of EGFR ligands expression at later times.
In summary, the results of the present study indicate that activation of NOX by TGF-β, which correlates with up-regulation of nox4 and increase in Rac1 protein level, mediates anti-apoptotic signals in foetal rat hepatocytes through up-regulation of EGFR ligands and transactivation of the EGFR pathway. Since previous work has clearly established the role of ROS in the apoptosis induced by TGF-β in different cell types, including hepatocytes [39], the results presented here indicate that ROS might mediate both pro- and anti-apoptotic signals in TGF-β-treated hepatocytes. Our results also support the important role played by NF-κB, downstream of ROS, to control EGFR ligand up-regulation. It is worthy to note that IKKβ-deficient mice die at mid-gestation from uncontrolled liver apoptosis, a phenotype that is remarkably similar to that of mice deficient in both the RelA (p65) and NF-κB1 (p50/p105) subunits of NF-κB [40]. Mice lacking IKKβ (inhibitor of κB kinase β) specifically in both hepatocytes and haematopoietic-derived Kupffer cells exhibit reduced hepatocyte regeneration and diminished induction of hepatomitogens [41]. Taking these results together, it is possible to hypothesize that NF-κB might play an essential role in regulating EGFR ligands under different physiological and pathological situations of the liver.
Acknowledgments
This work was supported by Grants from the Ministerio de Educación y Ciencia (BMC03-524), Comunidad Autónoma de Madrid-Universidad Complutense (CAM/UCM 2005-920359) and IDIBELL-Institut de Recerca Oncològica, Spain. M. M. M. and G. del C. are recipients of fellowships from the Ministerio de Educación y Ciencia, Spain. I. C.-C. has a Comunidad de Madrid, Spain fellowship and C. O. has an IDIBELL-Institut de Recerca Oncològica, Catalunya, Spain fellowship. A. S. is recipient of a research contract (Ramón y Cajal Program) from the Ministerio de Educación y Ciencia/Universidad Complutense de Madrid, Spain.
References
- 1.Siegel P. M., Massague J. Cytostatic and apoptotic actions of TGF-β in homeostasis and cancer. Nat. Rev. Cancer. 2003;3:807–821. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
- 2.Muraoka-Cook R. S., Dumont N., Arteaga C. L. Dual role of transforming growth factor β in mammary tumorigenesis and metastatic progression. Clin. Cancer Res. 2005;11:937–943. [PubMed] [Google Scholar]
- 3.Sanchez A., Alvarez A. M., Benito M., Fabregat I. Apoptosis induced by transforming growth factor-β in foetal hepatocyte primary cultures: involvement of reactive oxygen intermediates. J. Biol. Chem. 1996;271:7416–7422. doi: 10.1074/jbc.271.13.7416. [DOI] [PubMed] [Google Scholar]
- 4.Valdes F., Murillo M. M., Valverde A. M., Herrera B., Sánchez A., Benito M., Fernández M., Fabregat I. Transforming growth factor-β activates both pro-apoptotic and survival signals in foetal rat hepatocytes. Exp. Cell Res. 2004;292:209–218. doi: 10.1016/j.yexcr.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 5.Murillo M. M., Del Castillo G., Sanchez A., Fernandez M., Fabregat I. Involvement of EGF receptor and c-Src in the survival signals induced by TGF-β1 in hepatocytes. Oncogene. 2005;24:4580–4587. doi: 10.1038/sj.onc.1208664. [DOI] [PubMed] [Google Scholar]
- 6.Del Castillo G., Murillo M. M., Alvarez-Barrientos A., Bertran E., Fernández M., Sánchez A., Fabregat I. Autocrine production of TGF-β confers resistance to apoptosis after an epithelial-mesenchymal transition process in hepatocytes: role of EGF receptor ligands. Exp. Cell Res. 2006;312:2860–2871. doi: 10.1016/j.yexcr.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 7.Zipfel P. A., Ziober B. L., Morris S. L., Mulder K. M. Up-regulation of transforming growth factor α expression by transforming growth factor β1, epidermal growth factor, and N, N-dimethylformamide in human colon carcinoma cells. Cell Growth Differ. 1993;4:637–645. [PubMed] [Google Scholar]
- 8.Lynch M. J., Pelosi L., Carboni J. M., Merwin J., Coleman K., Wang R. C., Lin P. F., Henry D. L., Brattain M. G. Transforming growth factor-β1 induces transforming growth factor-α promoter activity and transforming growth factor-α secretion in the human colon adenocarcinoma cell line FET. Cancer Res. 1993;53:4041–4047. [PubMed] [Google Scholar]
- 9.Vinals F., Pouyssegur J. Transforming growth factor β1 (TGF-β1) promotes endothelial cell survival during in vitro angiogenesis via an autocrine mechanism implicating TGF-α signalling. Mol. Cell Biol. 2001;21:7218–7230. doi: 10.1128/MCB.21.21.7218-7230.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrera B., Murillo M. M., Alvarez-Barrientos A., Beltran J., Fernández M., Fabregat I. Source of early reactive oxygen species in the apoptosis induced by transforming growth factor-β in foetal rat hepatocytes. Free Radical Biol. Med. 2004;36:16–26. doi: 10.1016/j.freeradbiomed.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 11.Engel R. H., Evens A. M. Oxidative stress and apoptosis: a new treatment paradigm in cancer. Front Biosci. 2006;11:300–312. doi: 10.2741/1798. [DOI] [PubMed] [Google Scholar]
- 12.Shen H. M., Pervaiz S. TNF receptor superfamily-induced cell death: redox-dependent execution. FASEB J. 2006;20:1589–1598. doi: 10.1096/fj.05-5603rev. [DOI] [PubMed] [Google Scholar]
- 13.Hirota K., Murata M., Itoh T., Yodoi J., Fukuda K. Redox-sensitive transactivation of epidermal growth factor receptor by tumor necrosis factor confers the NF-κB activation. J. Biol. Chem. 2001;276:25953–25958. doi: 10.1074/jbc.M011021200. [DOI] [PubMed] [Google Scholar]
- 14.Chen C. H., Cheng T. H., Lin H., Shih N. L., Chen Y. L., Chen Y. S., Cheng C. F., Lian W. S., Meng T. C., Chiu W. T., Chen J. J. Reactive oxygen species generation is involved in epidermal growth factor receptor transactivation through the transient oxidization of Src homology 2-containing tyrosine phosphatase in endothelin-1 signalling pathway in rat cardiac fibroblasts. Mol. Pharmacol. 2006;69:1347–1355. doi: 10.1124/mol.105.017558. [DOI] [PubMed] [Google Scholar]
- 15.Kayanoki Y., Higashiyama S., Suzuki K., Asahi M., Kawata S., Matsuzawa Y., Taniguchi N. The requirement of both intracellular reactive oxygen species and intracellular calcium elevation for the induction of heparin-binding EGF-like growth factor in vascular endothelial cells and smooth muscle cells. Biochem. Biophys. Res. Commun. 1999;259:50–55. doi: 10.1006/bbrc.1999.0723. [DOI] [PubMed] [Google Scholar]
- 16.Koh Y. H., Suzuki K., Che W., Park Y. S., Miyamoto Y., Higashiyama S., Taniguchi N. Inactivation of glutathione peroxidase by NO leads to the accumulation of H2O2 and the induction of HB-EGF via c-Jun NH2-terminal kinase in rat aortic smooth muscle cells. FASEB J. 2001;15:1472–1474. doi: 10.1096/fj.00-0572fje. [DOI] [PubMed] [Google Scholar]
- 17.Nishimura M., Ookawara T., Eguchi H., Fujiwara N., Yoshihara D., Yasuda J., Mimura O., Suzuki K. Inhibition of gene expression of heparin-binding epidermal growth factor-like growth factor by extracellular superoxide dismutase in rat aortic smooth muscle cells. Free Radical Res. 2006;40:589–595. doi: 10.1080/10715760600615094. [DOI] [PubMed] [Google Scholar]
- 18.Miyazaki Y., Hiraoka S., Tsutsui S., Kitamura S., Shinomura Y., Matsuzawa Y. Epidermal growth factor receptor mediates stress-induced expression of its ligands in rat gastric epithelial cells. Gastroenterology. 2001;120:108–116. doi: 10.1053/gast.2001.20950. [DOI] [PubMed] [Google Scholar]
- 19.Nicolini G., Miloso M., Moroni M. C., Beguinot L., Scotto L. Post-transcriptional control regulates transforming growth factor α in the human carcinoma KB cell line. J. Biol. Chem. 1996;271:30290–30296. doi: 10.1074/jbc.271.47.30290. [DOI] [PubMed] [Google Scholar]
- 20.Reinehr R., Becker S., Eberle A., Grether-Beck S., Haussinger D. Involvement of NADPH oxidase isoforms and Src family kinases in CD95-dependent hepatocyte apoptosis. J. Biol. Chem. 2005;280:27179–27194. doi: 10.1074/jbc.M414361200. [DOI] [PubMed] [Google Scholar]
- 21.Groemping Y., Rittinger K. Activation and assembly of the NADPH oxidase: a structural perspective. Biochem. J. 2005;386:401–416. doi: 10.1042/BJ20041835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moses H. L., Branum E. L., Proper J. A., Robinson R. A. Transforming growth factor production by chemically transformed cells. Cancer Res. 1981;41:2842–2848. [PubMed] [Google Scholar]
- 23.Herrera B., Fernández M., Benito M., Fabregat I. cIAP-1, but not XIAP, is cleaved by caspases during the apoptosis induced by TGF-β in foetal rat hepatocytes. FEBS Lett. 2002;520:93–96. doi: 10.1016/s0014-5793(02)02774-6. [DOI] [PubMed] [Google Scholar]
- 24.Horowitz J. C., Lee D. Y., Waghray M., Keshamouni V. G., Thomas P. E., Zhang H., Cui Z., Thannickal V. J. Activation of the pro-survival phosphatidylinositol 3-kinase/AKT pathway by transforming growth factor-β1 in mesenchymal cells is mediated by p38 MAPK-dependent induction of an autocrine growth factor. J. Biol. Chem. 2004;279:1359–1367. doi: 10.1074/jbc.M306248200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pani G., Colavitti R., Borrello S., Galeotii T. Endogenous oxygen radicals modulate protein tyrosine phosphorylation and JNK-1 activation in lectin-stimulated thymocytes. Biochem. J. 2000;347:173–181. [PMC free article] [PubMed] [Google Scholar]
- 26.Gloire G., Legrand-Poels S., Piette J. NF-κB activation by reactive oxygen species: fifteen years later. Biochem. Pharmacol. 2006;72:1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 27.Pantano C., Reynaert N. L., van der Vliet V., Janssen-Heininger Y. M. Redox-sensitive kinases of the nuclear factor-κB signalling pathway. Antioxid. Redox Signal. 2006;8:1791–1806. doi: 10.1089/ars.2006.8.1791. [DOI] [PubMed] [Google Scholar]
- 28.Storz P., Doppler H., Toker A. Protein kinase Cδ selectively regulates protein kinase D-dependent activation of NF-κB in oxidative stress signalling. Mol. Cell Biol. 2004;24:2614–2626. doi: 10.1128/MCB.24.7.2614-2626.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark R. A., Valente A. J. Nuclear factor-κB activation by NADPH oxidases. Mech. Ageing Dev. 2004;125:799–810. doi: 10.1016/j.mad.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Park H. S., Jung H. Y., Park E. Y., Kim J., Lee W. J., Bae Y. S. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-κB. J. Immunol. 2004;173:3589–3593. doi: 10.4049/jimmunol.173.6.3589. [DOI] [PubMed] [Google Scholar]
- 31.Kustermans G., El Benna J., Piette J., Legrand-Poels S. Perturbation of actin dynamics induces activation in myelomonocytic cells through an NADPH oxidase-dependent pathway. Biochem. J. 2005;387:531–540. doi: 10.1042/BJ20041318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calvin L. G., Wang F., Factor V. M., Kaur S., Venkatraman M., Thorgeirsson S. S., Arsura M. Transforming growth factor-α inhibits the intrinsic pathway of c-Myc-induced apoptosis through activation of nuclear factor-κB in murine hepatocellular carcinomas. Mol. Cancer Res. 2005;3:403–412. doi: 10.1158/1541-7786.MCR-04-0186. [DOI] [PubMed] [Google Scholar]
- 33.Kaur S., Wang F., Venkatraman M., Arsura M. X-linked inhibitor of apoptosis (XIAP) inhibits c-Jun N-terminal kinase 1 (JNK1) activation by transforming growth factor β1 (TGF-β1) through ubiquitin-mediated proteosomal degradation of the TGF-β1-activated kinase 1 (TAK1) J. Biol. Chem. 2005;280:38599–38608. doi: 10.1074/jbc.M505671200. [DOI] [PubMed] [Google Scholar]
- 34.Qiao L., Zhang H., Yu J., Francisco R., Dent P., Ebert M. P., Rocken C., Farrell G. Constitutive activation of NF-κB in human hepatocellular carcinoma: evidence of a cytoprotective role. Hum. Gene Ther. 2006;17:280–290. doi: 10.1089/hum.2006.17.280. [DOI] [PubMed] [Google Scholar]
- 35.Pan Z., Kravchenko V. V., Ye R. D. Platelet-activating factor stimulates transcription of the heparin-binding epidermal growth factor-like growth factor in monocytes: correlation with an increased κB binding activity. J. Biol. Chem. 1995;270:7787–7790. doi: 10.1074/jbc.270.14.7787. [DOI] [PubMed] [Google Scholar]
- 36.Sakuda S., Tamura S., Yamada A., Miyagawa J., Yamamoto K., Kiso S., Ito N., Imanaka K., Wada A., Naka T., et al. Activation of signal transducer and activator transcription 3 and expression of suppressor of cytokine signal 1 during liver regeneration in rats. J. Hepatol. 2002;36:378–384. doi: 10.1016/s0168-8278(01)00282-3. [DOI] [PubMed] [Google Scholar]
- 37.Sato Y., Kato J., Takimoto R., Takada K., Kawano Y., Miyanishi K., Kobune M., Sato Y., Takayama T., Matunaga T., Niitsu Y. Hepatitis C virus core protein promotes proliferation of human hepatoma cells through enhancement of transforming growth factor α expression via activation of nuclear factor-κB. Gut. 2006;55:1801–1808. doi: 10.1136/gut.2005.070417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grau A. M., Datta P. K., Zi J., Halder S. K., Beauchamp R. D. Role of Smad proteins in the regulation of NF-κB by TGF-β in colon cancer cells. Cell. Signaling. 2006;18:1041–1050. doi: 10.1016/j.cellsig.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 39.Herrera B., Alvarez A. M., Sanchez A., Fernandez M., Roncero C., Benito M., Fabregat I. Reactive oxygen species (ROS) mediates the mitochondrial-dependent apoptosis induced by transforming growth factor β in foetal hepatocytes. FASEB J. 2001;15:741–751. doi: 10.1096/fj.00-0267com. [DOI] [PubMed] [Google Scholar]
- 40.Li Z. W., Chu W., Hu Y., Delhase M., Deerinck T., Ellisman M., Johnson R., Karin M. The IKKβ subunit of IκB kinase (IKK) is essential for nuclear factor κB activation and prevention of apoptosis. J. Exp. Med. 1999;189:1839–1845. doi: 10.1084/jem.189.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maeda S., Kamata H., Luo J. L., Leffert H., Karin M. IKKβ couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]