Abstract
A recently cloned isoform of cGMP-dependent protein kinase (cGK), designated type II, was implicated as the mediator of cGMP-provoked intestinal Cl− secretion based on its localization in the apical membrane of enterocytes and on its capacity to activate cystic fibrosis transmembrane conductance regulator (CFTR) Cl− channels. In contrast, the soluble type I cGK was unable to activate CFTR in intact cells, although both cGK I and cGK II could phosphorylate CFTR in vitro. To investigate the molecular basis for the cGK II isotype specificity of CFTR channel gating, we expressed cGK II or cGK I mutants possessing different membrane binding properties by using adenoviral vectors in a CFTR-transfected intestinal cell line, and we examined the ability of cGMP to phosphorylate and activate the Cl− channel. Mutation of the cGK II N-terminal myristoylation site (Gly2 → Ala) reduced cGK II membrane binding and severely impaired cGK II activation of CFTR. Conversely, a chimeric protein, in which the N-terminal membrane-anchoring domain of cGK II was fused to the N terminus of cGK Iβ, acquired the ability to associate with the membrane and activate the CFTR Cl− channel. The potency order of cGK constructs for activation of CFTR (cGK II > membrane-bound cGK I chimer ≫ nonmyristoylated cGK II > cGK Iβ) correlated with the extent of 32P incorporation into CFTR observed in parallel measurements. These results strongly support the concept that membrane targeting of cGK is a major determinant of CFTR Cl− channel activation in intact cells.
The cystic fibrosis transmembrane conductance regulator (CFTR) Cl− channel plays an important role as a mediator of salt and fluid transport across numerous epithelial tissues (1). The absence of functional CFTR Cl− channels in the plasma membrane of epithelial cells either from patients with the genetic disease cystic fibrosis or from CFTR knockout mice causes severe disturbances in the salt and water content of luminal fluids, leading to potentially lethal lung infections and/or pancreas and intestinal obstructions (meconium ileus) (1, 2). In contrast, the hyperactivation of CFTR Cl− channels in intestinal epithelium by several enterotoxins causes a massive secretory diarrhea that is responsible for a large part of the infant death in developing countries (3). Therefore, the activity of CFTR must be regulated strictly under physiological conditions (1, 3). In most epithelia, phosphorylation of the regulatory domain of CFTR by cAMP-dependent protein kinase (cAK), possibly in combination with protein kinase C (4), is believed to be the primary activating step, followed by ATP-binding/hydrolysis at the nucleotide binding domains (1). However, in intestinal epithelium, a recently cloned isotype of cGMP-dependent protein kinase, cGK type II has been implicated as an additional regulator of CFTR (3, 5–8). This pathway for CFTR activation functions independently from that of cAK (8) and is triggered by membrane-permeant cGMP analogs and presumably by guanylin, an endogenous cGMP-elevating peptide hormone (3, 6, 7). Furthermore, pharmacological (9) and gene disruption techniques (10) recently have demonstrated that cGK II is involved critically in the secretory diarrhea caused by heat-stable enterotoxins, which, like guanylin, are known activators of guanylyl cyclase C in intestinal epithelial cells (3, 11).
Type II cGK is a membrane-bound enzyme that is related closely to the soluble type I cGK (consisting of α and β isoforms) (5, 7). Both isotypes have been shown to phosphorylate a number of substrates in vitro with similar kinetics (3, 7, 12, 13), including CFTR and a peptide comprising the regulatory domain of CFTR (CF-2; ref. 8). However, only cGK II, not cGK I, was able to phosphorylate and activate CFTR in membrane patches or whole cells (8, 14), suggesting that the subcellular localization of cGKs, clearly different for types II and I (7, 14), might determine the efficacy of cGK interaction with CFTR under physiological conditions. To investigate this possibility, we expressed cGK II or cGK I mutants possessing different membrane-binding properties in a CFTR-transfected intestinal cell line (IEC-CF7) and examined the ability of cGMP to phosphorylate and activate the CFTR Cl− channel. Here, we report that cGK membrane association is a strong determinant of the potency of cGK forms for activating CFTR Cl− channels in intact cells.
EXPERIMENTAL PROCEDURES
Materials.
Protein A Sepharose was obtained from Pharmacia; 3-isobutyl-1-methyl-xanthine and rat atrial natriuretic peptide (ANP) were obtained from Sigma; cGMP analogs were obtained from BioLog Life Science Institute (Bremen, Germany), and [32P]orthophosphate, [γ-32P]ATP, 125I−, and the enhanced chemiluminescence system were obtained from Amersham. Polyclonal cGK II or cGK I antibodies, raised against recombinant cGK II or cGK Iβ expressed in Escherichia coli, were prepared as described (6). The polyclonal antibody C449 against CFTR (15) and CF-2, a cloned regulatory domain of CFTR (15), were gifts from A. C. Nairn (Rockefeller University, New York). The cGK substrate peptide 2A3 (RRKVSKQE) and the Walsh inhibitor peptide [PKI (5–24) amide] were synthesized by D. Palm (University of Würzburg, Würzburg, Germany).
Construction of cGK Mutants and Adenovirus (Ad) Vectors.
A nonmyristoylated mutant of cGK II (G2A) was constructed by mutation of Gly2 to Ala as described (16). A cGK chimer containing the N-terminal sequence of cGK II fused to the N terminus from cGK Iβ was made subsequent to using the Transformer site-directed mutagenesis kit (CLONTECH) to introduce a unique NheI restriction site in the N terminus of human cGK Iβ (17) just upstream of Leu7, changing Arg5–Asp6 to Leu–Ala. The sequence coding for the first 29 N-terminal amino acids of cGK II was obtained by PCR amplification of a 0.8-kb fragment (beginning at a MluI site in pRc/CMV–cGK II (5) and ending at a new NheI site attached downstream of the codon for Lys29] and was ligated into the mutated cGK Iβ in a pRc/CMV vector. Recombinant replication-deficient (E1 deletion) Ad type 5 containing the coding sequences of rat cGK II (5) or human cGK Iβ (17) were prepared as described (14). The coding sequence of the cGK II-G2A mutant, or of the cGK II-cGK Iβ chimer (cGK chimer), was introduced into the adenoviral vectors similarly as described for cGK II (14). The titer of the adenoviral preparations was ≈1 plaque forming unit/500 particles.
Culture and Infection of IEC-CF7 Cells.
Rat intestine-derived IEC-6 cells stably expressing CFTR (IEC-CF7; ref. 18) were cultured in DMEM supplemented with 5% fetal calf serum, 0.1 unit/ml insulin, 0.2 mg/ml G418, 0.1 mg/ml streptomycin, and 0.04 mg/ml penicillin. Two days after plating, confluent monolayers of cells were infected by adding medium containing the adenoviral vectors (usually 5 × 109 particles/ml).
Determination of Subcellular Localization of cGK Constructs by Immunofluorescence Confocal Microscopy.
IEC-CF7 cell were grown on glass coverslips in 12-well plates. Two days after infection, cells were fixed with 3% paraformaldehyde and subsequently with 100% methanol. After incubation with cGK I- or II-specific antisera (1:100) and an fluorescein isothiocyanate-coupled secondary antibody, cells were embedded in antifading medium consisting of 0.9 M 1,4-diazabicyclo[2.2.2] octane in 60% glycerol, and immunofluorescence was detected by using a Zeiss confocal laser microscope LSM 410. A 488-nm argon laser was used for excitation, and emission was detected by using a 515- to 540-bandpass filter.
Protein Kinase Assays and Immunoblotting.
Two days after infection, cells were suspended in buffer A (150 mM NaCl/10 mM sodium phosphate, pH 7.4/1 mM EDTA) and homogenized by brief sonication. Cytosol and membranes were separated by centrifugation at 150,000 × g for 60 min at 4°C in a Beckman airfuge. Protein kinase activity was determined in 40 μl of 20 mM Tris⋅HCl (pH 7.4), 10 mm MgCl2, 10 mM β-mercaptoethanol, 200 nM of the cAK inhibitor peptide [PKI (5–24) amide], 0.1 mM 3-isobutyl-1-methyl-xanthine, 25 mM β-glycerophosphate, 0.1 mg/ml cGK substrate peptide 2A3, 1 μCi of γ [32P]ATP, 300 μM nonlabeled ATP, and various concentrations of cGMP as described (16). For the measurements of phosphorylation of the cloned regulatory domain of CFTR (CF-2; ref. 15), cGK was extracted from the adenoviral vector-infected IEC-CF7 cells with 0.5 M NaCl and 1% Triton X-100 and purified by affinity chromatography on 8-(2-aminoethyl) amino-cAMP Sepharose (8). CF-2 (0.1 μM) was phosphorylated by incubation with purified cGK bound to the affinity gel in the buffer used for 2A3 phosphorylation, except for the presence of 50 μM ATP and 15 μM cGMP. Subsequently, CF-2 was separated on 12.5% SDS/PAGE, and the 32P incorporated was quantitated by the Bio-Rad Molecular Imaging System GS-363. Expression of cGK constructs was determined by immunoblotting and was quantitated by a PhosphoImager (Molecular Dynamics) as described (14). Specific activities of the cGK constructs were calculated by dividing the cGMP-stimulated phosphotransferase activities by the amount of cGK protein in the corresponding homogenates.
125I− Efflux Studies.
Confluent IEC-CF7 cells grown in 12-well plates and infected 2 days earlier were loaded with tracer 125I− (5 μCi/ml) for 1.5 h (19). After removal of the extracellular isotope, fractional 125I− efflux was determined at 37°C by the addition and consecutive replacement of the extracellular medium at 1- to 3-min intervals as described (14, 19).
Phosphate Labeling and Immunoprecipitation of CFTR.
Two days after infection, confluent cells grown in 6-well plates (Costar) were incubated at 37°C for 1 h in phosphate-free, modified Meyler solution supplemented with 0.5 mCi/ml [32P]orthophosphate (14). Labeled cells were incubated for an additional 20 min with membrane-permeable cGMP analogs in the same medium. 32P-labeled CFTR was immunoprecipitated with affinity, purified anti-CFTR antibody C449 as described (14, 15), separated by 6% SDS/PAGE, visualized by autoradiography, and quantitated with the Molecular Imaging System GS-363 (Bio-Rad).
RESULTS
Expression of cGK Mutants with Different Membrane Binding Properties.
The membrane binding of cGK II was shown to be critically dependent on the presence of a myristoyl group at the cGK II N terminus and could be disrupted by mutating the Gly at position 2 to an Ala (G2A; ref. 16). Conversely, we postulated that it could be possible to target the soluble cGK Iβ to the membrane by attaching the first 29 amino acids of cGK II, containing the myristoylation signal plus a polybasic domain (16), to the cGK Iβ N terminus. This cGK chimer as well as the G2A mutant were cloned into adenoviral (Ad) vectors like those used previously for wild-type cGK constructs for efficient expression in the CFTR-transfected intestinal cell line IEC-CF7, which contains very little endogenous soluble cGK and no detectable cGK II (14). Infection of IEC-CF7 cells with the recombinant viral vectors (5 × 109 particles/ml) resulted in a relatively high level of expression of G2A, cGK chimer, and cGK Iβ (1.7 ± 0.3, 2.3 ± 0.5, and 2.4 ± 0.6 μg/mg protein, respectively, as assessed by immunoblotting; n = 3; data not shown), whereas cGK II was expressed at a 3- to 4-fold lower level (0.6 ± 0.2 μg/mg protein; n = 3). The various cGK constructs exhibited: (i) similar Kas for cGMP (0.6–0.8 μM; data not shown) and (ii) similar specific activities for phosphorylating the cGK-specific peptide 2A3 (0.5–1 μmol of phosphate incorporated per minute per milligram enzyme protein; data not shown. cf. Fig. 1 and ref. 14 for cGK Iβ). Furthermore, the cGK constructs, purified from infected IEC-CF7 cells (see Materials and Methods), phosphorylated the recombinant regulatory domain of CFTR (CF-2) with similar specific activities in vitro (1.0–1.8 arbitrary units/min/mg protein; data not shown; cf. ref. 8).
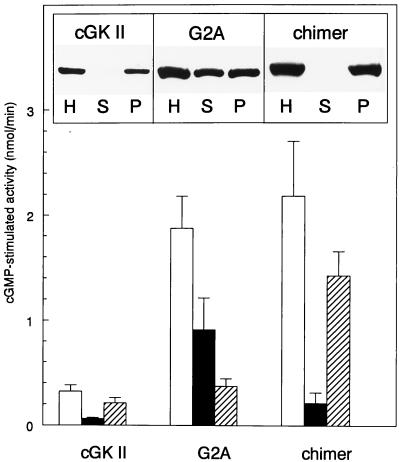
Figure 1.
Adenoviral vector expression of cGK constructs in IEC-CF7 cells. Rat intestinal IEC-CF7 cells were infected with 5 × 109 particles/ml (≈107 plaque forming units/ml) of replication-deficient adenoviral vectors containing the cDNA of either cGK II, a myristoylation-deficient cGK II mutant (G2A), or the N terminus of cGK II fused to the N terminus of cGK Iβ (chimer). Two days after infection, cells were harvested, homogenized (open bar), and separated into cytosolic (filled bar) and membrane (150,000 × g, hatched bar) fractions. Phosphotransferase activity (nmol/min) was determined in the presence or absence of 10 μM cGMP and was expressed per milligram of homogenate protein after correction for basal activity in the absence of cGMP. Shown are means ± SD of three experiments. (Inset) Immunoblots of the homogenate (H; 10 μg protein), cytosol (S), and membrane (P) fractions from cells infected with adenoviral vectors containing the cGK constructs described above and labeled with cGK I- or cGK II-specific antibody.
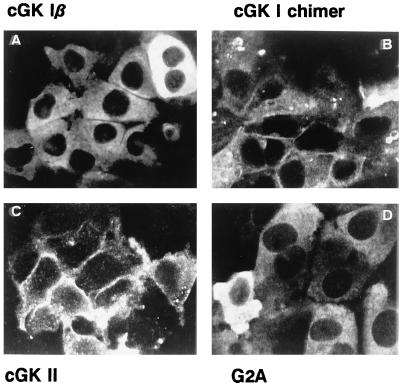
The cGK mutants expressed differed in their subcellular localizations. Whereas cGK II was recovered mainly in the particulate fraction, a major part of the kinase activity associated with the myristoylation-deficient cGK II mutant (G2A) was recovered in the cytosolic fraction (Fig. 1), although a portion of the G2A mutant did remain associated with the particulate fraction. Expressed cGK Iβ was almost completely cytosolic (14). In contrast, the cGK chimer, containing the cGK II myristoylation sequence, was targeted predominantly to the membrane, similar to cGK II. Immunofluorescence confocal microscopy confirmed the cytosolic localization of cGK Iβ and G2A and the presence of cGK II and the cGK chimer in the plasma membrane (Fig. 2). Additional localization of the cGK chimer in intracellular compartments was also evident. Furthermore, highly efficient expression of the various cGK constructs was detected in most IEC-CF7 cells, although a small percentage (5–10%) of cells contained considerably more cGK immunoreactivity than the average (Fig. 2). The efficient expression of active cGK mutants in IEC-CF7 cells displaying different membrane binding properties provided a basis for the meaningful comparison of the effects of these mutants on CFTR Cl− channel activity and CFTR phosphorylation.
Figure 2.
Subcellular localization of cGK proteins expressed in IEC-CF7 cells. (A) Rat intestinal IEC-CF7 cells were infected with replication-deficient Ad (5 × 109 particles/ml) containing the cDNA of either cGK Iβ. (B) The N terminus of cGK II fused to the N terminus of cGK Iβ. (C) cGK II. (D) G2A, a myristoylation-deficient cGK II mutant. Two days after infection, localization of the expressed cGK proteins was determined by using immunofluorescence confocal microscopy. Specificity of the cGK staining was confirmed by the absence of signal in mock-infected IEC-CF7 cells (not shown).
Activation of CFTR by cGK Mutants.
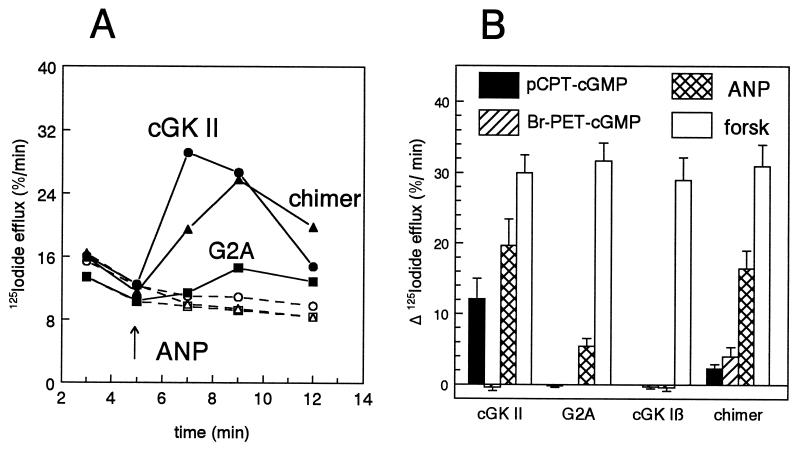
Activation of CFTR in IEC-CF7 cells can be measured accurately and sensitively by monitoring the 125I− efflux from these cells (14). We have shown previously that membrane-permeable cGMP analogs, or the cGMP-elevating hormone ANP, failed to provoke CFTR-channel opening in mock-infected IEC-CF7 cells (14). In agreement with this previous study, cGK II but not cGK Iβ expression rendered CFTR susceptible to activation by cGMP (Fig. 3B). However, abolishment of the N-terminal myristoylation of cGK II by the G2A mutation strongly impaired the ability of cGK II to stimulate CFTR-mediated 125I− efflux, although some small residual activity remained (Figs. 3 and 4). Conversely, the fusion of the N-terminal myristoylation sequence of cGK II to cGK Iβ produced a cGK chimer capable of stimulating 125I− efflux in response to cGMP or ANP. The activation of CFTR by this cGK I chimer permitted the comparison of the cGMP analog sensitivity of cGK II and Iβ within the same cell type. β-Phenyl-1,N2-etheno-8-bromo-cGMP (8-Br-PET-cGMP), defined as a cGK I selective agonist, (20 μM; refs. 20 and 21), provoked a clear increase in 125I− efflux in cGK chimer expressing IEC-CF7 cells but not in cGK II containing cells (Fig. 3B). Conversely, the cGK II-selective agonist 8-(4-chlorophenylthio)-cGMP (50 μM; ref. 21) was a potent activator of CFTR in cGK II-expressing cells but provoked only one-half of the 8-Br-PET-cGMP-induced increase in 125I− efflux in cGK chimer expressing cells (Fig. 3B).
Figure 3.
Iodide efflux in IEC-CF7 cells expressing cGK constructs with different membrane binding properties. Rat intestinal IEC-CF7 cells stably transfected with CFTR Cl− channels were infected with replication-deficient Ad (5 × 109 particles/ml) containing the cDNA of either cGK II, a myristoylation-deficient cGK II mutant (G2A), the N terminus of cGK II fused to the N terminus of cGK Iβ (chimer), or wild-type cGK Iβ. Two days after infection, CFTR activity was monitored by using measurements of fractional 125I− efflux. (A) Time course of 125I− efflux. At 5 min (arrow), either 0.1 μM ANP (closed symbols) or vehicle (open symbols) was added to cells expressing cGK II (•, ○), cGK chimer (▴, ▵), or G2A (▪, □). (B) Maximal increment in 125I− efflux determined at 2 min after addition of forskolin and ANP in the case of cGK II, at 4 min in the case of other constructs, and at 7 min after addition of cGMP analogs to IEC-CF7 cells infected as indicated. 125I− efflux was corrected for basal efflux in the absence of the agonists at the same time points. Concentration of agonists used: 8-(4-chlorophenylthio)-cGMP, 50 μM (filled bars); 8-Br-PET-cGMP, 20 μM (hatched bars); ANP, 0.1 μM (crosshatched bars); and forskolin, 10 μM (forsk; open bars). Data are means ± SD of three to four experiments.
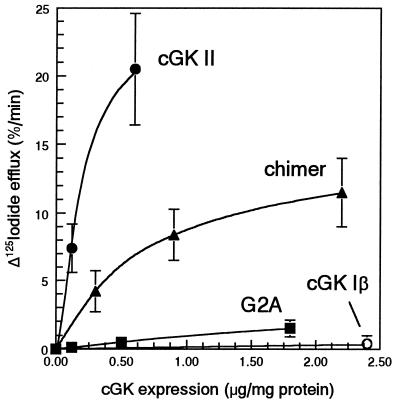
Figure 4.
Iodide efflux in IEC-CF7 cells expressing various levels of cGK constructs. Rat intestinal IEC-CF7 cells stably transfected with CFTR Cl− channels were infected with different doses of replication-deficient Ad containing the cDNA of either cGK II (•), a myristoylation-deficient cGK II mutant (G2A, ▪), the N terminus of cGK II fused to the N terminus of cGK Iβ (chimer, ▴), or cGK Iβ (○). The doses of Ad used were: 1 and 5 × 109 particles/ml in the case of cGK II; 0.25, 1, and 4 × 109 particles/ml in the case of G2A and chimer; and 5 × 109 particles/ml in the case of cGK Iβ. Two days after infection, the initial cGK-mediated increase in CFTR activity was monitored by measurement of the increment in fractional 125I− efflux 2 min after addition of 0.1 μM ANP. The average level of cGK expression obtained for each dose of virus was determined by immunoblotting and was plotted against the mean ± SD of the corresponding increase in 125I− efflux (n = 3).
To determine the relative potencies of the various cGK mutants in activating CFTR, the effect of different cGK II expression levels was measured on the initial ANP-induced increase in 125I− efflux. At a physiologically relevant expression level of ≈0.6 μg of cGK II per milligram protein (for comparison rat enterocytes contain 0.2–0.4 μg of cGK II per milligram protein; ref. 14), we estimated that cGK II is ≈3-fold more potent than the cGK chimer, and 30-fold more potent than G2A (Fig. 4). cGK Iβ was clearly the least potent activator of CFTR because its expression at an even fourfold higher level (2.4 μg/mg protein) did not lead to any measurable stimulation of 125I− efflux in response to ANP (Figs. 3B and 4).
Phosphorylation of CFTR by cGK Mutants.
To investigate the molecular mechanism of CFTR activation by the cGK mutants, we determined their effect on the in situ phosphorylation of CFTR. As shown in Fig. 5, membrane-permeable cGMP analogs increased 32P incorporation into CFTR in cells expressing cGK II, cGK chimer, or G2A. However, the level of cGMP-stimulated CFTR phosphorylation was higher in cGK II-expressing cells than in cells expressing cGK chimer and much higher than in cells expressing the myristoylation mutant G2A. Furthermore, the basal level of CFTR phosphorylation was higher in cells expressing cGK II than in cells expressing the other cGK constructs (Fig. 5).
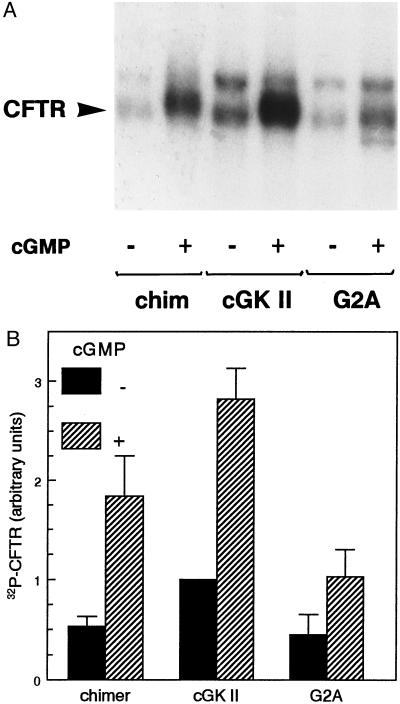
Figure 5.
Phosphorylation of CFTR in IEC-CF7 cells expressing cGK constructs. Rat intestinal IEC-CF7 cells, stably transfected with CFTR Cl− channels, were infected with replication-deficient Ad (5 × 109 particles/ml) containing the cDNA of either cGK II, a myristoylation-deficient cGK II mutant (G2A), or the N terminus of cGK II fused to the N terminus of cGK Iβ (chimer). Two days after infection, cells were labeled metabolically with inorganic 32P for 1 h and subsequently incubated for 20 min with vehicle [−cGMP (A); filled bars (B)] or 50 μM 8-(4-chlorophenylthio)-cGMP in the case of cGK II and G2A or 20 μM 8-Br-PET-cGMP in the case of cGK Iβ chimer [+cGMP (A); hatched bars (B)]. Subsequently, CFTR was immunoprecipitated and separated by 6% SDS/PAGE. (A) Autoradiograph showing 32P-phosphorylated CFTR (20-day exposure). The position of CFTR (180–190 kDa), determined by using in vitro 32P-phosphorylated CFTR (8, 14), is indicated with an arrowhead. The 220-kDa protein running above CFTR is nonspecific (14). (B) The amount of 32P incorporated into CFTR was quantitated by using a PhosphoImager and was expressed relative to the basal 32P incorporation into CFTR in Ad–cGK II-infected IEC-CF7 cells. Data are means ± SD of three experiments.
DISCUSSION
Our results indicated that the membrane localization of cGK II was an important determinant of the capacity of cGK II to activate CFTR. Mutation (G2A) of the cGK II myristoylation site impaired the membrane-anchoring of cGK II and reduced cGK II activation of CFTR by 30-fold, although cGMP activation of the mutated cGK II remained unchanged. A relatively small residual activation of CFTR by ANP, observed in G2A expressing IEC-CF7 cells, may derive from the G2A (30–40% of the G2A kinase activity) still associated with the particulate fraction of these cells. This fraction of particulate G2A was considerably greater than that observed previously in experiments with the same construct in COS-1 cells (10–20%, ref. 16). There appears to be some difference in the membrane association of cGK II in these two cell types, which may depend on the extent of cytoskeletal participation in cGK II anchoring. The solubilization of wild-type cGK II from IEC-CF7 cells with Triton X-100 required addition of 0.5 M NaCl in IEC-CF7 cells but not in COS-1 cells (cf. ref. 16; A.B.V., unpublished observations). A cytoskeletal association of the residual particulate pool of G2A in IEC-CF7 cells in the present study also may explain why particulate G2A was not detected in the plasma membrane by confocal microscopy.
Furthermore, this study also indicates that the failure of soluble cGK Iβ to activate CFTR in whole cells predominantly results from the lack of cGK Iβ membrane/substrate targeting. Notably, this study and previous studies (8) demonstrated no lack of intrinsic recognition of CFTR as a substrate by cGK I because CFTR or its regulatory domain, CF-2, was a good substrate for cGK I in vitro. Now, fusion of amino acids 1–29 of cGK II to the N terminus of cGK I produced a chimer capable of opening CFTR Cl− channels, showing that the N-terminal membrane-anchoring piece of cGK II dramatically improved CFTR activation by the type I isozyme. Although the gain of function observed with the cGK I/II chimer is most likely due to the acquisition of a myristoyl anchor, additional cGK II-specific targeting motifs may be present in the N terminus or in other regions of cGK II. The latter possibility may explain why the chimer was 60–70% less effective than cGK II as an activator of CFTR in the IEC-CF7 cell model. Although the chimer was found rather exclusively in the pellet fraction (after 150,000 × g centrifugation), this fraction contains a heterogeneous mixture of different membranes, and without possible additional regions of cGK II for appropriate membrane attachment, some chimer may be located in intracellular membranes not containing CFTR (cf. Fig. 2). Membrane attachment also may cause certain conformational changes in cGK II that cannot be mimicked completely by cGK I. In any case, there was a clear correlation between myristoylation and activation of CFTR, i.e., cGK II > chimer > G2A > cGK Iβ. The same rank order of these constructs was observed for phosphorylation of CFTR, indicating that CFTR phosphorylation also was dependent on the membrane association of cGK and that phosphorylation was the key step in CFTR activation by cGK.
The dependence of CFTR activation in intact cells on cGK subcellular localization supports the emerging concept that compartmentalization of protein kinases and their substrates is a major determinant of their interaction under physiological conditions (22). Targeting of a kinase to its substrate may involve kinase lipidation, as exemplified by cGK II and the tyrosine kinases of the Src family (23), or may require specific anchoring to scaffolding proteins, as shown for some cAKs (24). Modulation of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/kainate channel and the L-type Ca2+ channel by cAK was shown to require cAK binding to specific anchoring proteins (25, 26). Of interest, preliminary studies suggest a similar requirement for membrane anchoring of cAK for CFTR activation. Type II cAK, which associates with membrane anchor proteins (26), and not the unanchored type I cAK, was identified as the cAK isozyme responsible for cAMP-provoked CFTR activation in whole cells (27). The importance of the subcellular localization of cGK I for interaction with certain substrates like vimentin (28, 29) and the importance of nuclear translocation of cGK I for gene regulation (30) also has been described.
One potential problem that could complicate approaches using expression of recombinant enzymes to study physiological effects of cGK (or other kinases) is that certain cells may lack the appropriate substrates or anchor proteins. For example, the regulation of the L-type Ca2+ channel by the catalytic subunit of cAK shown in cardiac myocytes could not be demonstrated always in transfection systems (cf. refs. 26 and 31). So far, the cytoskeletal protein vimentin is the only specific anchoring protein that has been described for cGK I (29). Design of cGK I constructs that would be targeted to membrane-bound substrates such as phospholamban and the IP3-receptor (28) may prove useful for further investigating the relationship between subcellular localization and function in intact cells.
The observed advantage of cGK and CFTR colocalization for their interaction raises the consideration that cGK II may interact less efficiently with cytosolic substrates. It is therefore tempting to speculate that other cGK II substrates yet to be defined in brain (32, 33), kidney (34), and bone (10) may turn out to be membrane proteins. Identification of physiological cGK II substrates in addition to CFTR constitutes a major challenge for the future.
Acknowledgments
We acknowledge A. C. Nairn for donating the CFTR-antibody and the CFTR regulatory domain protein CF-2. Our study was supported by the Netherlands Organization for Scientific Research, the Deutsche Forschungsgemeinschaft, and an European Community Human Capital and Mobility grant. A.S. is a fellow of the German Cardiology Society.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: ANP, atrial natriuretic peptide; cAK, cAMP-dependent protein kinase; CFTR, cystic fibrosis transmembrane conductance regulator; cGK, cGMP-dependent protein kinase; 8-Br-PET-cGMP, β-phenyl-1,N2-etheno-8-bromo-cGMP; Ad, adenovirus; G2A, Gly at position 2 to an Ala.
References
- 1.Welsh M J, Tsui L-C, Boat T F, Beaudet A L. In: The Metabolic & Molecular Basis of Inherited Disease. Scriver C R, Beaudet A L, Sly W S, Valle D, editors. III. New York: McGraw–Hill; 1995. pp. 3799–3876. [Google Scholar]
- 2.Smith J J, Travis S M, Greenberg E P, Welsh M J. Cell. 1996;85:229–236. doi: 10.1016/s0092-8674(00)81099-5. [DOI] [PubMed] [Google Scholar]
- 3.Vaandrager A B, De Jonge H R. Adv Pharmacol. 1994;26:253–283. doi: 10.1016/s1054-3589(08)60057-5. [DOI] [PubMed] [Google Scholar]
- 4.Jia Y, Mathews C J, Hanrahan J W. J Biol Chem. 1997;272:4978–4984. doi: 10.1074/jbc.272.8.4978. [DOI] [PubMed] [Google Scholar]
- 5.Jarchau T, Häusler C, Markert T, Pöhler D, Vandekerckhove J, De Jonge H R, Lohmann S M, Walter U. Proc Natl Acad Sci USA. 1994;91:9426–9430. doi: 10.1073/pnas.91.20.9426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Markert T, Vaandrager A B, Gambaryan S, Pöhler D, Häusler C, Walter U, De Jonge H R, Jarchau T, Lohmann S M. J Clin Invest. 1995;96:822–830. doi: 10.1172/JCI118128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lohmann S M, Vaandrager A B, Smolenski A, Walter U, De Jonge H R. Trends Biochem Sci. 1997;22:307–312. doi: 10.1016/s0968-0004(97)01086-4. [DOI] [PubMed] [Google Scholar]
- 8.French P J, Bijman J, Edixhoven M, Vaandrager A B, Scholte B J, Lohmann S M, Nairn A C, De Jonge H R. J Biol Chem. 1995;270:26626–26631. doi: 10.1074/jbc.270.44.26626. [DOI] [PubMed] [Google Scholar]
- 9.Vaandrager A B, Bot A G M, De Jonge H R. Gastroenterology. 1997;112:437–443. doi: 10.1053/gast.1997.v112.pm9024297. [DOI] [PubMed] [Google Scholar]
- 10.Pfeifer A, Aszódi A, Seidler U, Ruth P, Hofmann F, Fässler R. Science. 1996;274:2082–2086. doi: 10.1126/science.274.5295.2082. [DOI] [PubMed] [Google Scholar]
- 11.Wedel B J, Garbers D L. FEBS Lett. 1997;410:29–33. doi: 10.1016/s0014-5793(97)00358-x. [DOI] [PubMed] [Google Scholar]
- 12.Pöhler D, Butt E, Meissner J, Müller S, Lohse M, Walter U, Lohmann S M, Jarchau T. FEBS Lett. 1995;374:419–425. doi: 10.1016/0014-5793(95)01168-e. [DOI] [PubMed] [Google Scholar]
- 13.Gamm D M, Francis S M, Angelotti T P, Corbin J D, Uhler M D. J Biol Chem. 1995;270:27380–27388. doi: 10.1074/jbc.270.45.27380. [DOI] [PubMed] [Google Scholar]
- 14.Vaandrager A B, Tilly B C, Smolenski A, Schneider-Rasp, Bot A G M, Edixhoven M, Scholte B J, Jarchau T, Walter U, Lohmann S M, et al. J Biol Chem. 1997;272:4195–4200. doi: 10.1074/jbc.272.7.4195. [DOI] [PubMed] [Google Scholar]
- 15.Picciotto M R, Cohn J A, Bertuzzi G, Greengard P, Nairn A C. J Biol Chem. 1992;267:12742–12752. [PubMed] [Google Scholar]
- 16.Vaandrager A B, Ehlert E M E, Jarchau T, Lohmann S M, De Jonge H R. J Biol Chem. 1996;271:7025–7029. doi: 10.1074/jbc.271.12.7025. [DOI] [PubMed] [Google Scholar]
- 17.Sandberg M, Natarajan V, Ronander I, Kalderon D, Walter U, Lohmann S M, Jahnsen T. FEBS Lett. 1989;255:321–329. doi: 10.1016/0014-5793(89)81114-7. [DOI] [PubMed] [Google Scholar]
- 18.Bijman J, Dalemans W, Kansen M, Keulemans J, Verbeek E, Hoogeveen A, De Jonge H R, Wilke M, Dreyer D, Lecocq J-P, et al. Am J Physiol. 1993;264:L229–L235. doi: 10.1152/ajplung.1993.264.3.L229. [DOI] [PubMed] [Google Scholar]
- 19.Vaandrager A B, Bajnath R, Groot J A, Bot A G M, De Jonge H R. Am J Physiol. 1991;261:G958–G965. doi: 10.1152/ajpgi.1991.261.6.G958. [DOI] [PubMed] [Google Scholar]
- 20.Sekhar K R, Hatchett R J, Shabb J B, Wolfe L, Francis S H, Wells J N, Jastorff B, Butt E, Chakinala M M, Corbin J D. Mol Pharmacol. 1992;42:103–108. [PubMed] [Google Scholar]
- 21.Vaandrager A B, Edixhoven M, Bot A G M, Kroos M A, Jarchau T, Lohmann S M, Genieser H-G, De Jonge H R. J Biol Chem. 1997;272:11816–11823. doi: 10.1074/jbc.272.18.11816. [DOI] [PubMed] [Google Scholar]
- 22.Monchly-Rosen D. Science. 1995;268:247–251. doi: 10.1126/science.7716516. [DOI] [PubMed] [Google Scholar]
- 23.Casey P J. Science. 1995;268:221–225. doi: 10.1126/science.7716512. [DOI] [PubMed] [Google Scholar]
- 24.Scott J D, McCarty S. Mol Endocrinol. 1994;8:5–11. doi: 10.1210/mend.8.1.8152430. [DOI] [PubMed] [Google Scholar]
- 25.Rosenmund C, Carr D W, Bergeson S E, Nilaver G, Scott J D, Westbrook G L. Nature (London) 1994;368:853–856. doi: 10.1038/368853a0. [DOI] [PubMed] [Google Scholar]
- 26.Johnson B D, Scheuer T, Catterall W A. Proc Natl Acad Sci USA. 1994;91:11492–11496. doi: 10.1073/pnas.91.24.11492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steagall W K, Kelley T J, Drumm M L. Pediatr Pulmonol Suppl. 1996;13:211. (abstr.). [Google Scholar]
- 28.Lincoln T M, Cornwell T L. FASEB J. 1993;7:328–338. doi: 10.1096/fasebj.7.2.7680013. [DOI] [PubMed] [Google Scholar]
- 29.MacMillan-Crow L A, Lincoln T M. Biochemistry. 1994;33:8035–8043. doi: 10.1021/bi00192a007. [DOI] [PubMed] [Google Scholar]
- 30.Gudi T, Lohmann S M, Pilz R. Mol Cell Biol. 1997;17:5244–5254. doi: 10.1128/mcb.17.9.5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zong X, Schreieck J, Mehrke G, Welling A, Schuster A, Bosse E, Flockerzi V, Hofmann F. Pflügers Arch. 1995;430:340–347. doi: 10.1007/BF00373908. [DOI] [PubMed] [Google Scholar]
- 32.Uhler M D. J Biol Chem. 1993;268:13586–13591. [PubMed] [Google Scholar]
- 33.El-Husseini A, Bladen C, Vincent S R. J Neurochem. 1995;64:2814–2817. doi: 10.1046/j.1471-4159.1995.64062814.x. [DOI] [PubMed] [Google Scholar]
- 34.Gambaryan S, Häusler C, Markert T, Pöhler D, Jarchau T, Walter U, Haase W, Kurtz A, Lohmann S M. J Clin Invest. 1996;98:662–670. doi: 10.1172/JCI118837. [DOI] [PMC free article] [PubMed] [Google Scholar]