Abstract
We have proposed that the stability of bimanual coordination is influenced by the complexity of the representation of the task goals. Here we present two experiments to explore this hypothesis. First, we examined whether a temporal event structure is present in continuous movements by having participants vocalize while producing bimanual circling movements. Participants tended to vocalize once per movement cycle when moving in-phase. In contrast, vocalizations were not synchronized with anti-phase movements. While the in-phase result is unexpected, the latter would suggest anti-phase continuous movements lack an event structure. Second, we examined the event structure of movements marked by salient turn-around points. Participants made bimanual wrist flexion movements and were instructed to move ‘in synchrony’ with a metronome, without specifying how they should couple the movements to the metronome. During in-phase movements, participants synchronized one hand cycle with every metronome beat; during anti-phase movements, participants synchronized flexion of one hand with one metronome beat and extension of the other hand with the next beat. The results are consistent with the hypothesis that the instability of anti-phase movements is related to their more complex (or absent) event representation relative to that associated with in-phase movements.
Keywords: coordination, constraints, in-phase, anti-phase, stability
1.0 Introduction
Constraints on bimanual coordination have been extensively examined in the motor control literature leading to a number of accounts regarding the relative stability of in-phase movements compared to anti-phase movements. It has become generally accepted that coordination is influenced by a ‘coalition of constraints’ (for reviews see Carson & Kelso, 2004; Swinnen & Wenderoth, 2004). For instance, the stability of in-phase movements has been associated with a preference for homologous muscle activations (Cattaert, Semjen, & Summers, 1999; Cohen, 1971). However, a preference for in-phase movements is observed even when the movements involve non-homologous muscles (Buchanan & Kelso, 1993; Jeka & Kelso, 1995; Kelso, Buchanan, & Wallace, 1991; Kelso & Jeka, 1992) as well as when one movement is performed by one person and the other by a different person (e.g., interactions between two people, Schmidt, Carello, & Turvey, 1990). Thus, constraints are not limited biomechanics.
Many observations have been made of cognitive constraints on bimanual actions (see Carson & Kelso, 2004; Swinnen & Wenderoth, 2004). The coupled oscillator model (Haken, Kelso, & Bunz, 1985; Yamanishi, Kawato, & Suzuki, 1980) cannot account for these observations. However, recently we presented a theoretical account of cognitive constraints. According to the event representation theory, differences in the representation of the task goal underlie the relative stability of in-phase rhythmic movements over anti-phase movements (Spencer, Semjen, Yang, & Ivry, in press). Specifically, in-phase movements entail a simpler representation of the task goal than the representation associated with anti-phase movements. We refer to this goal representation as the temporal event structure (e.g., Ivry, Spencer, Zelaznik, & Diedrichsen, 2002; Spencer et al., in press). The event structure is the abstract representation of the goals or features that demarcate a movement. In our experiment, participants were required to perform rhythmic in-phase movements (synchronized movements using homologous muscles of the limbs) or anti-phase movements (alternating movements, 180° out of phase). The participants were asked to say “Ba” repeatedly as they moved once the hand movement patterns were established. No instructions were provided regarding the manner in which the manual and vocal actions were to be coordinated. As expected, participants synchronized the vocalizations with their hand movements. However, we observed a qualitative difference in the form of this synchronization between the two tasks. When moving the hands in-phase, half of the participants vocalized once per hand cycle and the other half vocalized twice per cycle. Moreover, some participants switched to the one vocalization mode at faster rates. In contrast, for anti-phase movements, participants vocalized twice per hand cycle across all rates.
We assume that these vocalizations reveal how the task goal is represented. As such, the results indicate a difference in the event structure for in-phase and anti-phase movements (Spencer et al., in press). The preference to vocalize twice per cycle in the latter condition suggests that the anti-phase pattern entails a more complex event structure and as such, offers a process-based account for the greater instability of this pattern.
A second experiment provided direct evidence that pattern stability is related to the complexity of the event structure (Spencer et al., in press, Experiment 2). Participants made anti-phase movements and, over the course of a trial, increased movement frequency. In different conditions, the participants were required to vocalize either once or twice per hand cycle. As indicated by the rate at which transitions to the in-phase pattern became evident, participants were most stable when vocalizing once per cycle. Moreover, imposing the simpler event structure led to more stable performance compared to a third condition in which the movements were performed without any vocalizations.
Here we further examine the relationship of event structure and coordination stability during bimanual movements. In Experiment 1, we use the concurrent vocalization procedure during bimanual circle drawing. We have proposed that the continuous nature of this task does not entail an event structure (e.g., Ivry et al., 2002; Spencer, Zelaznik, Diedrichsen, & Ivry, 2003). As such, we expected to see a reduced tendency to synchronize the manual and vocal actions. In Experiment 2, we replace the vocalizations with an external metronome to evaluate if the distinct synchronization patterns for in- and anti-phase movements are specific to the dual-task demands imposed by the vocalization task. Together, these results extend the event-based account of coordination constraints illustrating that it is not influenced by dual-task demands and is limited to tasks which have an event-structure.
2.0 Experiment 1
In our analysis of pattern stability, we have focused on movements that would seem amenable to an event-based representation (Spencer, Ivry, Hazeltine, & Semjen, 2004; Spencer et al., in press). For example, in movements involving wrist flexion and extension, the turn-around points provide salient events during each movement cycle. However, such singularities are absent in some types of movements such as continuous circle drawing. Our studies with normal participants (Zelaznik, Spencer, & Ivry, 2002) and neurological populations (Spencer et al., 2003) indicate that the temporal control and coordination of continuous movements may involve distinct neural processes and systems than those associated with movements in which the cycles are demarcated by some salient feature. In terms of representation, we have posited that continuous movements lack an event structure (Ivry et al., 2002; Spencer et al., 2003).
To further explore this hypothesis, we had participants perform the concurrent vocalization task during bimanual circle drawing. We tested the strong prediction that the participants will not synchronize the vocal and manual actions during these continuous movements.
2.1. Methods
2.1.1. Participants
Twelve college-aged participants (ten female; two male) participated in exchange for class credit. Procedures were approved by the Institutional Review Board at UC Berkeley. Informed consent was provided prior to the experiment.
2.1.2. Task and Procedure
A template with two 10-cm diameter circles was positioned on the tabletop in front of the participant. The center-to-center distance of the circles was 20 cm. The participants traced the templates, bimanually, with the tip of the index finger. Participants were assigned to either the In-Phase or Anti-Phase groups. All participants began a trial with the finger tips positioned at the top of the circle and were instructed to make smooth repetitive circles during the trial. Participants in the In-Phase group were told to start each trial by moving “both fingers to the outside (away from the midline) simultaneously”. Participants in the Anti-Phase group were instructed to start each trial by moving the fingers “to the right together.”
We did not use a metronome to establish the movement rate for each trial since the metronome would likely impose an event structure. Rather, we began each block with a set of five short trials with the aim of training the participants to move at one of the target rates: 1200 ms, 1000 ms, 750 ms, or 500 ms. For these trials, participants completed seven cycles per trial. The target-rate goal was maintained for the entire block. After each trial, the experimenter provided feedback regarding the produced cycle duration and instructed the participant to either move faster or slower, and indicated whether the change in movement speed should be small or large. In addition, participants also completed a block in which they moved at a self-selected rate (“spontaneous”).
The last five trials of each block contained 20 movement cycles and feedback was no longer provided. During these trials, participants were instructed to “Say ‘Ba’ repeatedly as you move.” We provided no additional instructions. In particular, we did not instruct the participants to synchronize the vocal and manual actions.
Half of the participants in each group performed the blocks in descending rate order with the spontaneous rate condition performed first; the remaining participants performed the blocks in ascending rate order with the spontaneous rate block last.
Between blocks, participants were provided with one of three tongue twisters to repeat as fast as possible for 30 s (see Spencer et al., in press). The tongue twisters were included to reduce carry-over effects from one block to the next that might arise in terms of how the participants synchronized the vocal and manual tasks.
2.1.3. Data acquisition and analysis
Kinematic markers (Ascension miniBIRD) were taped to the right and left index fingers. The x and y position of these markers were sampled at a rate of 138-Hz. Cycle durations were computed as the time between local maxima in the x-dimension (the point furthest to the right for the right hand; the point furthest to the left for the left hand).
2.2. Results and discussion
Participants approximated the goal cycle duration for each block in both the In- and Anti-Phase conditions (Fig. 1). The spontaneous rate (SPO) chosen by the In-Phase group (1475 ms) was faster than that of the Anti-Phase group (1878 ms), and both means were slower than any of the instructed rates.
Fig. 1.
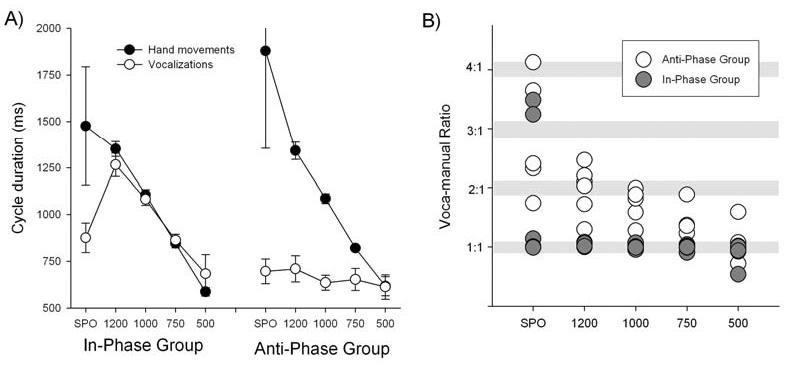
A) Mean hand cycle duration (filled circles) and vocalization rates (open circles) for the target durations in Experiment 1. Error bars represent the standard error (the variation of the adopted cycle durations across subjects). B) Vocal-manual ratios for individual subjects across rates.
The vocal-manual ratio (see Spencer et al., in press) was also computed. This is the ratio of the vocalization cycle duration: hand cycle duration calculated on a trial-by-trial basis. As the ratios remained consistent within a block, these values were then averaged. Given that there is some variability in both cycle duration measures, ratios were classified as synchronized 1:1 if they fell between .9:1 and 1.1:1. Likewise, ratios between 1.9:1 and 2.1:1 were classified as synchronized at 2:1. The ratios produced are presented in Fig. 1B.
Contrary to our prediction, participants in the In-Phase group synchronized their vocalizations with the hand movements, producing one “Ba” per hand cycle. This is evident in the mean vocalization rates which essentially match the circling rates (see Fig. 1A) as well as in the ratios (Fig. 1B) which were consistently classified as 1:1. One exception is the spontaneous rate condition. Here, three subjects synchronized their vocalizations with the hand movements (classified as 1:1); the remaining subjects did not show synchrony. In the 500 ms condition, one subject failed to synchronize vocalizations and hand movements.
In contrast, the participants in the Anti-Phase group tended to not synchronize their vocal and manual actions at any of the circling rates. Rather, these participants tended to adopt a single vocalization rate (around 660 ms) across all hand movement rates as depicted in Fig. 1A. Although this plot suggests that the two actions were synchronized at the fastest rate, this is likely due to the fact that the instructed rate was close to the constant vocalization rate. In fact, only three participants made productions which were classified as synchronized at a 1:1 ratio (Fig. 1B).
In sum, we obtained mixed support for the prediction that participants would fail to spontaneously synchronize vocal and manual responses during circle drawing. This lack of coupling was clear during anti-phase movements, a result that is interesting given the powerful tendency people manifest to synchronize rhythmic actions of varied effectors (Buchanan & Kelso, 1993; Jeka & Kelso, 1995; Kelso et al., 1991; Kelso & Jeka, 1992). We suggest that the uncoupling arises because circle drawing does not entail an event structure and thus, there are no salient points within each movement cycle for aligning the vocalizations. On the other hand, we did observe strong coupling during in-phase movements, suggesting that there was a salient event for this pattern1. While admittedly post-hoc, this coupling may be related to visual or somatosensory events being more salient in this condition compared to the anti-phase movements (Mechsner et al., 2001). In-phase movements are, by definition, symmetrical throughout the cycle and include a point at which the two hands come close together.
3.0 Experiment 2
One concern with the vocalization task in Experiment 1 and our previous studies (Spencer et al., 2004; Spencer et al., in press) is that it introduces a dual-task situation (or triple-task if you consider bimanual movements a dual-task situation). We employed it as a probe on how participants represent the event structure of the task goals, yet recognize that the task itself may alter that event structure. For example, it could impose an event structure on the movement patterns. This concern would not, of course, account for why we obtain different vocalization patterns for in-phase and anti-phase movements (see Experiment 1 and Spencer et al., in press)
An alternative way to compare the representation of in- and anti-phase movements is by replacing the vocalization task with an auditory metronome. While the utilization of a metronome is one of the most common methods to establish rhythmic movements, we are unaware of any studies that have examined whether there are differences in how people spontaneously choose to synchronize in- and anti-phase movements with the tones. Given our assumption that that these two patterns entail different event structures, we expect that this will be reflected in the spontaneous organization during paced movements. In particular, we predict that participants will spontaneously produce a 1:1 ratio of metronome cycles to movement cycles for the in-phase pattern and a 2:1 ratio for the anti-phase pattern.
3.1 Method
3.1.1. Participants
Twenty-two participants (19 female; 3 male) volunteered for this experiment. As this experiment was extremely brief (about 7 mins), participants were tested in a motor learning experiment following this task. Participants received course credit for their participation in these experiments.
3.1.2. Task and procedure
Participants were seated comfortably and positioned their elbows on the table in front of them with their arm flexed such that the forearms were held perpendicular to the table surface. This configuration was adopted for two reasons. Primarily, it was chosen to prevent the participant from making any contact with the table with the hands as this haptic feedback would influence the event structure associated with the task (Spencer et al., in press). Moreover, this is the configuration used in the previous study and we want to remain consistent across experiments. The task was to produce flexion and extension movements at the wrist. Participants were assigned to either the In-Phase group or the Anti-phase group. Participants in the In-Phase group were instructed to move the wrists up together and down together and those in the Anti-Phase group were instructed to move one hand up while moving the other hand down. In each trial, participants were presented with a metronome (20-ms tone length) with a rate of 725 ms. Participants were instructed to synchronize the wrist movements with the metronome. Importantly, no information was provided as to how the movements should be aligned with the metronome. Each participant completed five trials of 20 cycles.
3.1.3. Data acquisition and analysis
As in Experiment 1, kinematic markers were attached to each index finger. Cycle durations were computed as the time between local maxima in the z-dimension (perpendicular to the table surface, i.e., up-down).
3.2. Results and discussion
Participants synchronized their movements with the metronome as instructed and similar movement amplitudes were adopted for the In- and Anti-Phase groups. To capture the participants' initial conceptualization of the task goal, we examined the mean cycle duration of the first trial (Fig. 2A). For the In-Phase group, eight of the eleven participants (73%) immediately adopted a movement rate close to that of the metronome (mean = 728 ms). The other three participants in this group adopted a hand movement rate that was twice that of the metronome rate (mean = 1450 ms). This pattern was reversed for the Anti-Phase group. Seven of these participants (64%) adopted a cycle duration that was twice that of the metronome rate (mean = 1445 ms) while the other four participants matched their movement rate to the metronome rate (mean = 728 ms).
Fig. 2.
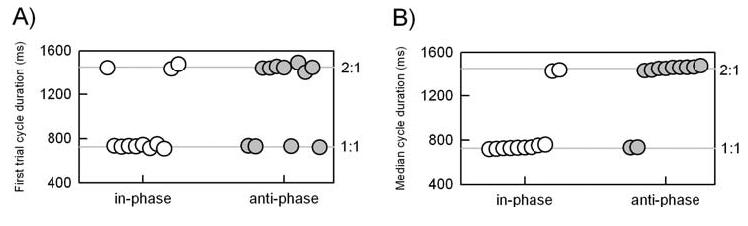
Hand cycle duration adopted A) on the first trial and B) the median across all trials. Each circle represents an individual participant's performance. Lateral spread of the symbols within each group is merely to prevent overlap of the data points; performance is ordered by the median cycle duration. White circles are for participants in the In-Phase group; gray circles are for participants in the Anti-Phase group.
For the most part, participants maintained their self-selected initial synchronization profile for the other four trials (Fig. 2B). There were three exceptions. One participant in the In-Phase group switched from a 2:1 metronome:manual ratio2 to a 1:1 ratio. Two participants in the Anti-Phase group switched from a 1:1 ratio to a 2:1 ratio. Thus, the separation of the two groups became more pronounced over time and were significantly different (χ2(1, N = 22) > 8.9, p < .01).
Experiment 2 provides a simple demonstration of how the task goals differ for in-phase and anti-phase movements. For in-phase movements, the participants generally adopted a movement rate such that one cycle was produced with each metronome beat. We assume that they conceptualize the task as consisting of one salient event per cycle. For anti-phase movements, the participants generally adopted a movement rate such that one cycle was completed with every two beats of the metronome. This result is consistent with the hypothesis that the task representation for these participants consists of two events per cycle. A parsimonious view of both groups is that one phase of the movement is aligned with the metronome; for example, the participants align wrist flexion with the metronome (Carson, 1996). For in-phase movements, the event is simultaneous for the two hands; for anti-phase movements, the event is 180° out of phase for the two hands.
4.0 Discussion
We have proposed an account of pattern stability during rhythmic bimanual movements that focuses on differences in the temporal representation of in- and anti-phase movements (Ivry, Diedrichsen, Spencer, Hazeltine, & Semjen, 2004). The basic premise is that anti-phase movements entail a more complex event-based representation than in-phase movements. A loss of stability occurs when movement rate increases because the person is unable to sustain this more complex representation. From this perspective phase transitions indicate a shift from a complex representation to a simpler representation (Spencer et al., in press).
The present experiments extend this work in two novel ways. We used a concurrent vocalization task in Experiment 1 to probe the event structure of a continuous movement pattern. Based on previous work, we hypothesized that movements such as circle drawing do not entail an event structure (Ivry et al., 2002; Spencer et al., 2003). As such, we predicted that participants would not synchronize their vocalizations with the hand movements. The results for participants performing anti-phase movements were consistent with this prediction. However, in-phase movements were tightly synchronized with the vocalizations. At present, we can only speculate on this difference, offering the idea that certain salient perceptual features in the in-phase condition were readily incorporated with the vocalizations to produce an integrated representation (Hommel, Musseler, Aschersleben, & Prinz, 2001).
We eliminated the vocalization task in Experiment 2 to investigate whether the vocalization task itself may be causing, or at least contributing to the different results for in- and anti-phase movements. Here we simply looked at how participants spontaneously synchronized their movements with an external metronome. Again, we observed a striking qualitative difference between in- and anti-phase movements, with participants adopting a 1:1 metronome:manual ratio for the former and a 2:1 ratio for the latter. This difference is essentially the same as what we observed with the vocalization task (Spencer et al., in press), indicating that the vocalization task does provide a useful probe for exploring the underlying temporal representation. Taken together, these results indicate that anti-phase movements entail a more complex representation consisting of two events per movement cycle.
It is important to emphasize that the event representation hypothesis provides a theoretical account of the cognitive constraints on bimanual actions. Undoubtedly, biomechanics provide additional constraints on coordinated movements. It has been proposed that cognitive constraints and biomechanical constraints interact as a coalition of constraints on movement (Carson & Kelso, 2004; Swinnen & Wenderoth, 2004). It remains to be determined how the relative roles of such constraints vary across task domains.
Acknowledgments
This work was supported by NIH grants NS048012, NS30256, NS17778, and NS40813. The authors would like to thank Stephanie Yang and Jackson Liang for their help in data collection.
Footnotes
It is difficult to infer the exact location in the manual cycle when the vocalization occurred. For example, when tapping with a metronome, the finger tap leads the metronome although the phenomenal experience is that the events are coincident. Similarly, in in-phase circling it seems that the vocalization is coincident with the point at which the two hands are closest to each other. However, defining the perceived vocalization onset to measure this relationship is arbitrary.
We have chosen to represent this as a metronome:manual ratio (rather than manual:metronome) to be consistent with Experiment 1 and previous studies (Spencer et al., 2004; Spencer et al., in press) in which used a vocalization:manual ratio.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Buchanan JJ, Kelso JAS. Posturally induced transitions in rhythmic multijoint limb movements. Experimental Brain Research. 1993;94:131–142. doi: 10.1007/BF00230476. [DOI] [PubMed] [Google Scholar]
- Carson RG. Neuromuscular-skeletal constraints upon the dynamics of perception-action coupling. Experimental Brain Research. 1996;110:99–110. doi: 10.1007/BF00241379. [DOI] [PubMed] [Google Scholar]
- Carson RG, Kelso JAS. Governing coordination: Behavioral principles and neural correlates. Experimental Brain Research. 2004;154:267–274. doi: 10.1007/s00221-003-1726-8. [DOI] [PubMed] [Google Scholar]
- Cattaert D, Semjen A, Summers JJ. Simulating a neural cross-talk model for between-hand interference during bimanual circle drawing. Biological Cybernetics. 1999;81:343–358. doi: 10.1007/s004220050567. [DOI] [PubMed] [Google Scholar]
- Cohen L. Synchronous bimanual movements performed b homologous and nonhomologous muscles. Perceptual and Motor Skills. 1971;32:639–644. doi: 10.2466/pms.1971.32.2.639. [DOI] [PubMed] [Google Scholar]
- Fink PW, Foo P, Jirsa VK, Kelso JAS. Local and global stabilization of coordination by sensory information. Experimental Brain Research. 2000;134:9–20. doi: 10.1007/s002210000439. [DOI] [PubMed] [Google Scholar]
- Haken H, Kelso JAS, Bunz H. A theoretical model of phase transition in human hand movements. Biological Cybernetics. 1985;51:347–356. doi: 10.1007/BF00336922. [DOI] [PubMed] [Google Scholar]
- Hommel B, Musseler J, Aschersleben G, Prinz W. The theory of event coding (TEC): A framework for perception and action planning. Behavioral and Brain Sciences. 2001;24:849–878. doi: 10.1017/s0140525x01000103. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Diedrichsen J, Spencer RMC, Hazeltine E, Semjen A. A cognitive neuroscience perspective on bimanual coordination and interference. In: Swinnen SP, Duyens J, editors. Interlimb Coordination. Kluwer Academic; Norwell, MA: 2004. pp. 259–295. [Google Scholar]
- Ivry RB, Spencer RMC, Zelaznik HN, Diedrichsen J. The cerebellum and event timing. Annals of the New York Academy of Sciences. 2002;978:302–317. doi: 10.1111/j.1749-6632.2002.tb07576.x. [DOI] [PubMed] [Google Scholar]
- Jeka JJ, Kelso JAS. Manipulating symmetry in human two-limb coordination dynamics. Journal of Experimental Psychology: Human Perception and Performance. 1995;21:360–374. doi: 10.1037//0096-1523.21.2.360. [DOI] [PubMed] [Google Scholar]
- Kelso JAS, Buchanan JJ, Wallace SA. Order parameters for the neural organization of single, multijoint limb movement patterns. Experimental Brain Research. 1991;85:432–444. doi: 10.1007/BF00229420. [DOI] [PubMed] [Google Scholar]
- Kelso JAS, Delcolle JD, Schöner GS. Action-perception as a pattern formation process. In: Jeannerod M, editor. Attention and performance. XIII. Erlbaum; Hillsdale, NJ: 1990. pp. 139–169. [Google Scholar]
- Kelso JAS, Jeka JJ. Symmetry breaking dynamics of human multilimb coordination. Journal of Experimental Psychology: Human Perception and Performance. 1992;18:645–668. doi: 10.1037//0096-1523.18.3.645. [DOI] [PubMed] [Google Scholar]
- Mechsner F, Kerzel D, Knoblich G, Prinz W. Perceptual basis of bimanual coordination. Nature. 2001;414:69–73. doi: 10.1038/35102060. [DOI] [PubMed] [Google Scholar]
- Schmidt RC, Carello C, Turvey MT. Phase transitions and critical fluctuations in the visual coordination of rhythmic movements between people. Journal of Experimental Psychology: Human Perception and Performance. 1990;16:227–247. doi: 10.1037//0096-1523.16.2.227. [DOI] [PubMed] [Google Scholar]
- Spencer RMC, Ivry RB, Hazeltine E, Semjen A. Goal-based representation in repetitive bimanual movements. International Journal of Sport and Exercise Psychology. 2004;2:239–254. [Google Scholar]
- Spencer RMC, Semjen A, Yang S, Ivry RB. An event-based account of coordination stability. Psychological Bulletin & Review. doi: 10.3758/bf03193984. in press. [DOI] [PubMed] [Google Scholar]
- Spencer RMC, Zelaznik HN, Diedrichsen J, Ivry RB. Disrupted timing of discontinuous movements by cerebellar lesions. Science. 2003;300:1437–1439. doi: 10.1126/science.1083661. [DOI] [PubMed] [Google Scholar]
- Swinnen SP, Wenderoth N. Two hands, one brain: The cognitive neuroscience of bimanual coordination. Trends in cognitive sciences. 2004;8:18–25. doi: 10.1016/j.tics.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Yamanishi J, Kawato M, Suzuki R. Two coupled oscillators as a model for the coordinated finger tapping by both hands. Biological Cybernetics. 1980;37:219–225. doi: 10.1007/BF00337040. [DOI] [PubMed] [Google Scholar]
- Zelaznik HN, Spencer RMC, Ivry RB. Dissociation of explicit and implicit timing in repetitive tapping and drawing movements. Journal of Experimental Psychology: Human Perception and Performance. 2002;28:575–588. doi: 10.1037//0096-1523.28.3.575. [DOI] [PubMed] [Google Scholar]


