Abstract
IL-15, produced by monocytes and epithelial cells, is a novel cytokine with actions similar to IL-2. IL-15 induces T cell proliferation, B cell maturation and natural killer (NK) cell cytotoxicity, and is a chemoattractant for T cells. We investigated the expression of IL-15 mRNA in blood and cerebrospinal fluid (CSF) mononuclear cells (MNC) in MS, an inflammatory disease of the central nervous system where cytokines are involved. MS patients had higher numbers of IL-15 mRNA-expressing blood MNC than patients with aseptic meningo-encephalitis (AM) and healthy controls. In CSF, MS patients had even higher numbers of IL-15 mRNA-expressing cells than in blood. This discrepancy between IL-15 mRNA expression between blood and CSF MNC was not seen in AM patients. Patients examined during the secondary chronic-progressive phase of MS had higher numbers of IL-15 mRNA-expressing blood MNC compared with patients examined during the relapsing-remitting phase. Levels of IL-15 mRNA-positive blood MNC were similar in patients with AM, myasthenia gravis, non-inflammatory neurological diseases and healthy controls. Taken together these data indicate that IL-15 mRNA expression is up-regulated in MS, further suggesting a role for proinflammatory cytokines in the pathogenesis of MS.
Keywords: multiple sclerosis, cytokines, IL-15
INTRODUCTION
Currently, MS is considered to be an inflammatory immune-mediated disease of the central nervous system (CNS), the aetiology of which remains enigmatic. Perivascular mononuclear cell infiltration and activation of the immune system are considered to lead ultimately to demyelination and astrogliosis. Cytokines produced by infiltrating inflammatory cells and resident cells in the brain are proposed to play a major role in directing and regulating the immune response as well as mediating tissue damage [1]. There exists ample evidence showing dysregulation of proinflammatory cytokines, with an up-regulation of both T cell and macrophage-derived cytokines, not only systemically but also locally in the cerebrospinal fluid (CSF) and in MS brain lesions [1–5].
IL-15 is a recently characterized cytokine with biological functions resembling those of IL-2, of which induction of T cell proliferation might be the most important [6]. Although IL-15 shares no significant homology with IL-2, the three-dimensional structures of the two cytokines are similar. The effects of IL-15 are mediated through the β- and γ-chains of the IL-2 receptor and a unique IL-15 receptor α-chain [7]. IL-15 mRNA is expressed in a wide range of human tissues, including placenta, skeletal muscle, kidney and liver. Activated T cells express no detectable levels of IL-15 mRNA, while peripheral blood monocytes and epithelial cells express this cytokine at high levels [6]. Besides acting as a T cell growth factor, IL-15 can promote the induction of cytolytic effector cells, including natural killer (NK) cells and cytotoxic T cells, up-regulate the production of proinflammatory cytokines by NK cells and T cells, and induce B cell maturation and isotype switching [6, 8–10]. IL-15 is also a potent chemoattractant for T cells, and may protect these cells from apoptosis [11, 12].
To our knowledge, no information about the involvement of IL-15 in MS is available. We adopted the highly sensitive method of in situ hybridization with synthetic oligonucleotide probes to measure IL-15 mRNA expression in mononuclear cells (MNC) separated from paired samples of blood and CSF from patients with MS and control subjects.
PATIENTS AND METHODS
Patients
Blood and, in suitable patients, CSF were collected from four different patient categories and one group of healthy volunteers. Thirty-two patients, aged 25–71 years (mean 45 years) had clinically definite or laboratory supported MS [13]. Ten patients were examined during exacerbation, which was defined as a sudden appearance of new, or the abrupt reappearance or worsening of previously present symptoms and signs, lasting > 24 h and occurring within 1 month before examination. Eight patients were examined during remission and 14 patients were examined during the secondary chronic-progressive phase of MS. The duration of MS varied between 1 and 31 years (mean 9 years). Fifteen patients had no or slight disability defined as < 3 on the expanded disability status scale (EDSS) [14], while 17 patients had moderate or severe disability defined as an EDSS ≥ 3. CSF was obtained from 18 of the patients. None of the patients was treated with any immunomodulatory drugs or interferon-beta (IFN-β) during the last year previous to examination.
Twenty-two patients, aged 21–83 years (mean 47 years) had acute aseptic meningo-encephalitis (AM). The cause of AM was neuroborreliosis in six patients and unknown in the remaining patients. The duration between onset of AM and examination varied between 2 and 45 days (mean 12 days). CSF was obtained from 16 of these patients.
Thirteen patients, aged 21–76 years (mean 45 years) had myasthenia gravis (MG) based on the presence of characteristic muscular fatigue, the results from laboratory tests that included determinations of serum acetylcholine receptor (AChR) antibody concentrations and single fibre electromyography (EMG), and the positive response to treatment with choline esterase inhibitors. Seven patients received treatment with immunosuppressive drugs (azathioprine or cyclosporin A). These patients were included as controls with a well defined autoimmune disease.
Eighteen patients, aged 32–77 years (mean 57 years) had non-inflammatory neurological diseases (OND). The diagnoses were cerebrovascular disease (n = 7), polyneuropathy (n = 4), Bells paresis (n = 2), migraine (n = 2), dementia (n = 1), epilepsy (n = 1) and cervical disc herniation (n = 1).
Twenty-three healthy volunteers consisted of staff from the Department, matched with the MS patients regarding sex, age and ethnic background.
Preparation of blood and CSF MNC
MNC were separated from peripheral blood through density gradient centrifugation on Lymphoprep (Nycomed, Oslo, Norway). The cells from the interphase were collected, washed twice with Dulbecco's modified Eagles' medium (Gibco, Paisley, UK), and finally washed once with PBS and counted. CSF was centrifuged for 10 min at 175 g. After discharging the supernatant, cells were counted. Cell viability as measured by trypan blue exclusion was always > 95%. Aliquots containing 1 × 105 blood MNC or at least 1 × 104 CSF MNC were dried onto electrically charged microscope slides (SuperFrost/Plus; Menzel-Gläser, Braunschweig, Germany). Slides were kept at −20°C until hybridization.
In situ hybridization to detect IL-15 mRNA expression in MNC
In situ hybridization (ISH) was performed as previously described [15]. A mixture of two different 48 base long synthetic oligonucleotide probes was used in order to increase the sensitivity of the method. The oligonucleotide sequences were obtained from GenBank using the Oligo 5·0 System Software (IBI, New Haven, CT). The human IL-15 probes (GenBank accession no. U540098 [6]) were complementary to bases 158–205 and 221–268. A constant guanine/cytosine ratio of ≈ 60% was used. Following ISH, slides were rinsed in SSC, dehydrated through gradient ethanol, dipped in Kodak (Paris, France) NTB2 emulsion, and exposed at 4°C for 14 days. The emulsion-coated slides were developed in D19 (Kodak) and fixed in Unifix (Kodak). As negative control probe, the sense sequence identical to bases 400–447 was used, without revealing any positive cells. Coded slides were examined by dark field microscopy for positive cells containing > 15 grains per cell in a star-like distribution (Fig. 1). The intracellular distribution of the grains was always checked by light microscopy. The number of cells used for ISH was not equal to the number of cells ultimately detected on the slides, and to compensate for cell losses the total number of cells on the slides was counted. Cell losses varied between 10% and 50% from cell application to cell counting. The preferential loss of certain cell types is not ruled out. Labelled cells practically always contained more than 15 (usually 50–100) grains, while negative cells almost always contained no or few grains which were then scattered randomly over the cell and not distributed in a star-like fashion. Consequently, it was only rarely difficult to differentiate between IL-15 mRNA-positive and negative cells.
Fig. 1.
Cytokine mRNA-expressing cell seen at light field microscopy, mag. ×640. Positive cells contain more than 15 grains evenly distributed over the cell.
Statistical analysis
Kruskal–Wallis non-parametric anova test was used for multiple comparisons. The non-parametric Mann–Whitney test and Wilcoxon signed rank test for pairs were used for two-group comparisons. Reported P values are two-tailed, and P < 0·05 was considered statistically significant.
RESULTS
IL-15 mRNA-expressing MNC were found in peripheral blood in 21 of 32 (65%) of the MS patients under study (Fig. 2). MS patients had higher numbers of IL-15 mRNA-expressing cells compared with patients with AM (P < 0·02) and healthy controls (P < 0·01) (Table 1). In contrast, there were no differences in the numbers of IL-15 mRNA-positive cells in patients with AM, MG or OND compared with healthy controls. Adopting the mean value + 2 s.d. of the healthy controls as an upper reference limit, 11 of the MS patients (34%) had elevated numbers of IL-15 mRNA-expressing cells in blood, compared with two of the AM patients (9%), one of the MG patients (8%) and none of the OND patients.
Fig. 2.
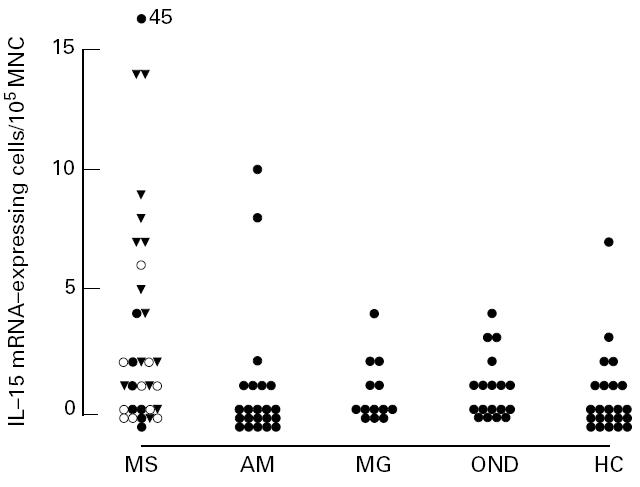
Numbers of IL-15 mRNA-expressing cells per 105 blood mononuclear cells (MNC) in patients with MS, aseptic meningo-encephalitis (AM), myasthenia gravis (MG), non-inflammatory neurological diseases (OND) and healthy controls (HC). •, Remission; ○, exacerbation; ▾, progressive.
Table 1.
Numbers of IL-15 mRNA-expressing cells per 105 blood and cerebrospinal fluid (CSF) mononuclear cells (MNC) from patients with MS and controls
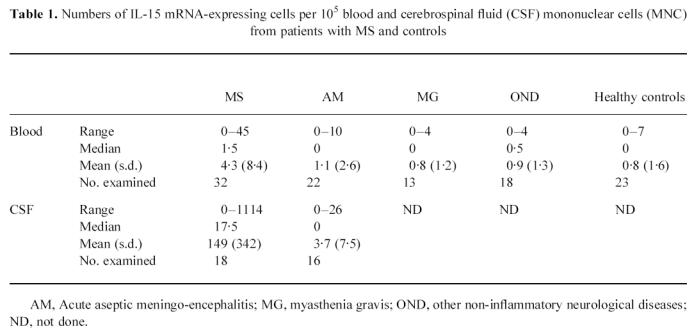
Paired blood and CSF samples were available from 18 MS patients. Eleven patients had clearly elevated numbers of IL-15 mRNA-expressing cells in CSF compared with blood, whereas seven patients had similar, or slightly lower numbers in CSF (Fig. 3). Numbers of IL-15 mRNA-expressing cells were higher in CSF than blood in MS (P < 0·02) (Table 1).
Fig. 3.
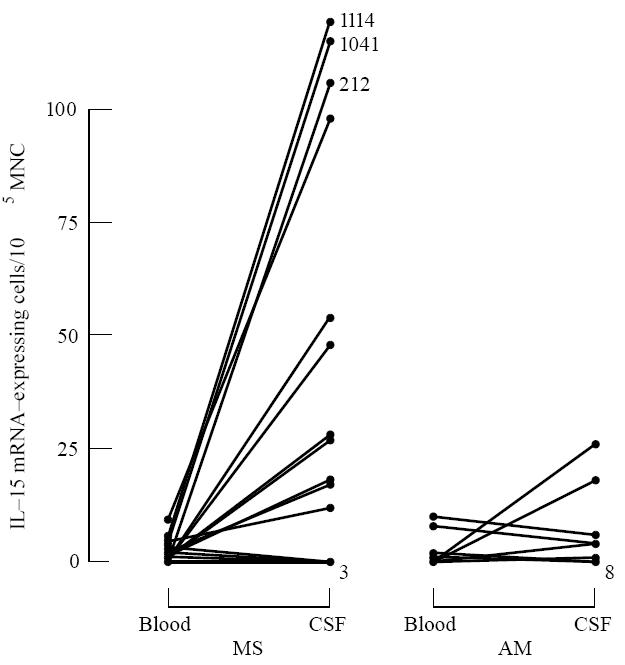
Numbers of IL-15 mRNA-expressing cells per 105 blood or cerebrospinal fluid (CSF) mononuclear cells (MNC) in paired samples from patients with multiple sclerosis (MS) and aseptic meningo-encephalitis (AM). Due to few available CSF MNC, each sample contained 104 CSF MNC.
In AM patients, no augmentation of IL-15 mRNA-expressing cells could be detected in CSF compared with blood. The numbers of IL-15 mRNA-positive cells in CSF were lower in AM compared with MS (P < 0·05).
When subgrouping the MS patients regarding clinical variables, the 14 patients examined during the chronic-progressive phase were found to have higher numbers of IL-15 mRNA-expressing blood MNC compared with the 18 patients examined during the relapsing-remitting phase (P < 0·02). In CSF, there was no difference between patients in the chronic-progressive phase compared with the relapsing-remitting phase, even though the small number of patients examined (five versus 13 patients) preclude any meaningful statistical calculations. No differences were found in numbers of IL-15 mRNA-expressing blood MNC between patients in exacerbation versus remission, slight versus moderate to severe disability (EDSS < 3 versus EDSS ≥ 3) or duration of MS (< 2 years (n = 8) versus > 10 years (n = 11)) (Fig. 2).
DISCUSSION
In MS, abnormal immune responses against a number of CNS myelin antigens have been reported, perpetuated by the production of cytokines from activated T cells and macrophages [16]. Using ISH with radiolabelled oligonucleotide probes, elevated numbers of blood MNC expressing mRNA for the proinflammatory cytokine IL-15 were detected in patients with MS compared with AM patients. MS patients had even higher numbers of IL-15 mRNA-expressing MNC in CSF, i.e. in the immediate vicinity of the diseased organ, compared with blood. When subgrouping the MS patients regarding clinical phase of the disease, patients examined during the chronic progressive phase had higher numbers of IL-15 mRNA-expressing blood MNC compared with patients examined during the relapsing-remitting phase.
Being a proinflammatory cytokine, IL-15 could mediate negative, disease-promoting effects in MS. In analogy with IL-2, IL-15 promotes proliferation of activated CD4+ and CD8+ T cells, as well as γδ T cells [6]. IL-15 stimulates proliferation of NK cells, and induces production of IFN-γ and tumour necrosis factor-alpha (TNF-α) by these cells [8]. Cell-mediated immune responses are further promoted by the induction of cytolytic effector cells such as cytotoxic T cells and lymphokine-activated NK cells [6]. In addition, IL-15 is a selective chemoattractant for T cells, with no effect on monocytes or B cells [11].
In human diseases, a role for IL-15 in the local immune response to infection has been proposed. Patients resistant to Mycobacterium leprae infections, with the potential of self-healing of the disease, had higher levels of IL-15 expression in skin lesions, and rIL-15 augmented T cell responses to the pathogen [17]. Similarly, rIL-15 facilitated the expansion of HIV-specific cytotoxic T cells in HIV-1-infected patients [18, 19]. Patients with rheumatoid arthritis (RA), a destructive inflammatory polyarthritis, had high concentrations of IL-15 in synovial fluid with the capacity to both attract and activate T cells in vitro, suggesting a biological role for IL-15 in the pathogenesis of RA [20].
ISH with radiolabelled oligonucleotide probes is a specific, highly sensitive method for detection of cytokine mRNA expression at the cellular level [15]. Data must, however, be interpreted cautiously, since cytokine mRNA expression may not necessarily equal protein secretion. Another dilemma with cytokine measurements in body fluids, regardless of method used, is to what extent the results reflect ongoing processes in the target organ. In this study, numbers of IL-15 mRNA-expressing MNC were further elevated in CSF compared with peripheral blood, suggesting enhanced IL-15 production also intrathecally. IL-15 is preferentially produced by macrophages, which is the most abundant cell type in MS lesions [21]. Besides macrophages, astrocytes and microglia might be involved in IL-15 production, since human fetal astrocytes and microglia have been shown to express IL-15 mRNA in cell cultures both spontaneously and, to an even higher degree, after stimulation with IFN-γ [22].
Up-regulation of cytokines is clearly not a phenomenon specific for MS, since elevated levels of MNC expressing or secreting cytokines, including IFN-γ, IL-4 and transforming growth factor-beta (TGF-β), have been observed also in patients with AM, HIV infection and stroke [23–25]. In patients with AM, high levels of cells expressing mRNA for these cytokines were also found in the CSF [25]. This might indicate that many of the immune deviations found in MS may reflect an inflammation that is secondary to non-specific tissue damage rather than an inflammatory process specific for MS. Notably in the present study, however, no elevation of IL-15 mRNA-expressing blood MNC was found in patients with AM, MG or OND. Moreover, no augmentation of numbers of IL-15 mRNA-positive MNC was found in CSF from patients with AM, an inflammation of the CNS which, in contrast to MS, is self-limiting. Patients examined during the chronic progressive phase of MS, usually representing a later stage of the disease, also had high numbers of IL-15 mRNA-expressing cells. Taken together, these data suggest that IL-15 could be one factor involved in the perpetuation and progression of the immune deviation in MS, even though this is highly speculative.
In conclusion, our data show elevated numbers of IL-15 mRNA-expressing blood MNC in MS compared with AM and healthy controls. IL-15 mRNA expression was further up-regulated in CSF MNC in MS, which was not seen in patients with AM. These data indicate that IL-15 is up-regulated in MS, and could further suggest a role for proinflammatory cytokines in the pathogenesis of MS lesions.
Acknowledgments
This study was supported by The Swedish Medical Association, The Swedish Society for Medical Research, the Swedish MS Society (NHR), the Swedish Medical Research Council and funds from the Karolinska Institute.
References
- 1.Brosnan C, Raine C. Mechanisms of immune injury in multiple sclerosis. Brain Pathol. 1996;6:243–57. doi: 10.1111/j.1750-3639.1996.tb00853.x. [DOI] [PubMed] [Google Scholar]
- 2.Olsson T. Role of cytokines in multiple sclerosis and experimental autoimmune encephalomyelitis. Eur J Neurol. 1994;1:7–19. doi: 10.1111/j.1468-1331.1994.tb00045.x. [DOI] [PubMed] [Google Scholar]
- 3.Giovannoni G, Hartung H. The immunopathogenesis of multiple sclerosis and Guillain–Barré syndrome. Curr Opin Neurol. 1996;9:165–77. doi: 10.1097/00019052-199606000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Merill J, Benveniste E. Cytokines in inflammatory brain lesions: helpful and harmful. Trends Neurosci. 1996;19:331–8. doi: 10.1016/0166-2236(96)10047-3. [DOI] [PubMed] [Google Scholar]
- 5.Navikas V, Link H. Cytokines and the pathogenesis of multiple sclerosis. J Neurosci Res. 1996;45:322–33. doi: 10.1002/(SICI)1097-4547(19960815)45:4<322::AID-JNR1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 6.Grabstein K, Eisenman J, Shanebeck K, et al. Cloning of a T cell growth factor that interacts with the β-chain of the interleukin-2 receptor. Sci. 1994;264:965–8. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 7.Giri J, Kumaki S, Ahdieh M, et al. Identification and cloning of a novel IL-15 binding protein that is structurally related to the α-chain of the IL-2 receptor. EMBO J. 1995;14:3654–63. doi: 10.1002/j.1460-2075.1995.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carson W, Giri J, Lindemann M, et al. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180:1395–403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armitage R, Macduff B, Eisenmann J, Paxton R, Grabstein K. IL-15 has stimulatory activity for the induction of B cell proliferation and differentiation. J Immunol. 1995;154:483–90. [PubMed] [Google Scholar]
- 10.MacInnes I, Leung B, Sturrock R, Field M, Liew F. Interleukin-15 mediates T cell-dependent regulation of tumor necrosis factor-α production in rheumatoid arthritis. Nature Med. 1997;2:189–95. doi: 10.1038/nm0297-189. [DOI] [PubMed] [Google Scholar]
- 11.Wilkinson P, Liew F. Chemoattraction of human blood T lymphocytes by interleukin-15. J Exp Med. 1995;181:1255–9. doi: 10.1084/jem.181.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akbar A, Borthwick N, Wickremasinghe R, et al. Interleukin-2 receptor common γ-chain signalling cytokines regulate activated T cell apoptosis in response to growth factor withdrawal: selective induction of anti-apoptotic (bcl-2, bcl-xl) but not pro-apoptotic (bax, bcl-xs) gene expression. Eur J Immunol. 1996;106:230–6. doi: 10.1002/eji.1830260204. [DOI] [PubMed] [Google Scholar]
- 13.Poser C, Paty D, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13:227–31. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- 14.Kurtzke J. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurol. 1983;33:1444–52. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 15.Link J, Söderström M, Olsson T, Höjeberg B, Ljungdahl Å, Link H. Increased TGF-β, IL-4 and IFN-γ mRNA expression in mononuclear cells in multiple sclerosis. Ann Neurol. 1994;36:379–86. doi: 10.1002/ana.410360309. [DOI] [PubMed] [Google Scholar]
- 16.Link J. Interferon-γ, interleukin-4 and transforming factor-β mRNA expression in multiple sclerosis and myasthenia gravis. Acta Neurol Scand. 1994;90:S1–S58. [PubMed] [Google Scholar]
- 17.Jullien D, Sieling P, Uyemura K, Mar N, Rea T, Modlin R. IL-15, an immunomodulator of T-cell responses in intracellular infection. J Immunol. 1997;158:800–6. [PubMed] [Google Scholar]
- 18.Seder R, Grabstein K, Berzofsky J, McDyer J. Cytokine interactions in human immunodeficiency virus-infected individuals: roles of interleukin (IL) -2, IL-12 and IL-15. J Exp Med. 1995;182:1067. doi: 10.1084/jem.182.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanai T, Thomas E, Yasutomi Y, Letvin N. IL-15 stimulates the expansion of AIDS virus-specific CTL. J Immunol. 1996;157:3681–7. [PubMed] [Google Scholar]
- 20.McInnes I, Al-Mughales J, Field M, et al. The role of interleukin-15 in T-cell migration and activation in rheumatoid arthritis. Nature Med. 1996;2:175–82. doi: 10.1038/nm0296-175. [DOI] [PubMed] [Google Scholar]
- 21.Lassmann H, Suchanek G, Ozawa K. Histopathology and the blood–cerebrospinal fluid barrier in multiple sclerosis. Ann Neurol. 1994;36:S42–S46. doi: 10.1002/ana.410360713. [DOI] [PubMed] [Google Scholar]
- 22.Lee Y, Satoh J, Walker D, Kim S. Interleukin-15 gene expression in human astrocytes and microglia in culture. Neuro Report. 1996;7:1062–6. doi: 10.1097/00001756-199604100-00022. [DOI] [PubMed] [Google Scholar]
- 23.Wang WZ, Olsson T, Kostulas V, Höjeberg B, Ekre H, Link H. Myelin antigen reactive T cells in cerebrovascular disease. Clin Exp Immunol. 1992;88:157–62. doi: 10.1111/j.1365-2249.1992.tb03056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Navikas V, Link J, Wahren B, Persson C, Link H. Increased levels of IFN-γ, IL-4 and TGF-β mRNA expressing blood mononuclear cells in human HIV infection. Clin Exp Immunol. 1994;96:59–63. doi: 10.1111/j.1365-2249.1994.tb06230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Navikas V, Haglund M, Link J, He B, Lindqvist L, Link H. Cytokine mRNA profiles in mononuclear cells in acute aseptic meningoencephalitis. Infect Immun. 1995;63:1581–3. doi: 10.1128/iai.63.4.1581-1586.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]


