Abstract
In the present study, we describe the potential role of melatonin, a pineal hormone, in regulating the activation of the antigen-specific T cell response. Melatonin encouraged the proliferation of Th cells and improved their ability to secrete IL-4, but down-regulated the levels of IL-2 and interferon-gamma (IFN-γ). Melatonin, however, could not exert any influence on the T cells of unprimed mice. On studying the regulation of subclass of IgG isotype, melatonin specifically enhanced the secretion of antigen-specific IgG1 antibodies and decreased the yield of IgG2a isotype. The results suggest that melatonin possibly acts by selectively activating a Th2-like immune response.
Keywords: melatonin, T cell, IL-4 and IgG1
INTRODUCTION
CD4+T cells can be subdivided into two subsets, Th1 and Th2 cells [1]. Th1 clones produce IL-2, interferon-gamma (IFN-γ), lymphotoxin, etc., and induce cell-mediated immunity, and Th2 clones secrete IL-4, IL-5, IL-10, etc., and are responsible for humoral immunity [2, 3]. Th1 and Th2 cells also differ in their ability to stimulate B cells to secrete various kinds of isotypes. The regulation of IgG isotype switching in vivo will depend upon the types of Th cell and B cell interaction, and the involvement of T cell-derived lymphokines. Th1 clones were shown to elicit IgG2a antibody secretion, whereas Th2 clones induce the production of IgG1 antibodies [4]. Furthermore, the regulation of the subclass of immunoglobulin by lymphokines has also been studied in vitro in a polyclonal system using lipopolysaccharide (LPS). It was shown that IL-4 induces the secretion of IgG1 and IFN-γ enhances the output of IgG2a [5].
Melatonin (N-acetyl-5 methoxytryptamine) is regarded as the major hormone of the pineal gland. It is also secreted by lymphocytes and plays an important role in the immune system [6, 7]. Melatonin belongs to the group of indolamines. Indolamines have been shown to modulate the cytotoxicity of natural killer (NK) cells, modify antibody responses, inhibit the proliferation of lymphocytes activated by mitogen and the production of IFN-γ by human T cells [8, 9]. It has also been reported that administration of melatonin increases the antibody response to various antigens and restores the antibody production in mice immunodepressed by acute restrain stress or by corticosterone treatment. Also, melatonin was found to restore impaired Th cell activity in mice immunodepressed by ageing or by cyclophosphamide treatment [10, 11].
The present study was undertaken to examine the role of melatonin in vivo in ovalbumin-specific T cells by evaluating the secretion of IL-2, IFN-γ, IL-4 and IgG isotypes. Results described here suggest that melatonin possibly acts on Th2-type cells, as evidenced by predominant secretion of IL-4, IgG1 antibody, but not IL-2, IFN-γ and IgG2a subtype production.
MATERIALS AND METHODS
Animals
Inbred female BALB/c mice 8–10 weeks old were obtained from the Institute's Animal House Facility.
Drug, antigen, antibodies
Melatonin (Morepen Laboratories, Parwanoo, India), biotinylated goat anti-mouse IgG1, IgG2a, IgG2b, IgG3 and steptavidin-labelled horseradish peroxidase (HRP; Sera Labs, Crawley Down, UK), ovalbumin (OVA), 2,2-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid)-diammonium salt (ABTS), streptavidin–HRP, hydrogen peroxide and tetramethylbenzidine were procured from Sigma (St Louis, MO). Recombinant murine IL-2, IFN-γ, IL-4, monoclonal anti-mouse IFN-γ and biotinylated polyclonal goat anti-mouse IFN-γ antibodies were purchased from Genzyme (Cambridge, MA). Antibodies to IL-4 (11B11) were purchased from Texstar (Dallas, TX). Anti-IL-2 MoAbs (cocktail of TIB222, HB8794 and CRL 1698) were used as a culture supernatant.
Cell lines and hybridomas
The cell lines and hybridomas used in this study, HT-2 (CRL-1841), TIB222 (PC61.5.3), CRL 1698 (7D4) and HB 8794 (S4B6) were procured from ATCC (Rockville, MD).
Immunization protocol
OVA (2 mg/ml) was dissolved in PBS (0·01 m, pH 7·2) and emulsified in Freund's complete adjuvant (FCA). Emulsion (100 μl) was then injected intraperitoneally in the groups of five female BALB/c mice. Control animals were injected with PBS. After 1 week, a booster dose of the antigen was repeated. Five days before bleeding, the animals were injected subcutaneously daily with melatonin (10, 20 and 50 mg/kg body wt of mice). The control animals were immunized intraperitoneally with 0·1 ml each of placebo (PBS) and ethanol–PBS. After 2 weeks, draining popliteal lymph node (LN) cells from each group were removed and pooled for T cell proliferation and blood was drawn and sera used for the quantification of IL-2, IFN-γ, IL-4 and IgG isotypes.
T cell proliferation
LN cells (1·5 × 105/well) obtained from different groups of five mice (i.e. immunized with OVA and melatonin, 1, 10, 20 and 50 mg/kg body wt, OVA–PBS, OVA–ethanol, PBS, etc.) were cultured in triplicate wells. The cells were challenged in vitro with 100 μg/ml of OVA along with different concentrations of melatonin (1, 10, 20 and 50 μg/ml). The cultures were incubated for 72 h at 37°C/7% CO2. The cells were pulsed with 0·5 μCi 3H-thymidine for 16 h before harvesting by automatic cell harvester (Skatron, Lier, Norway). 3H-thymidine incorporation was measured by standard liquid scintillation counting. Results are expressed as mean ct/min of triplicate cultures.
Lymphokine assay
IL-2 and IL-4 were measured using HT-2 cells
The blood from experimental and control animals was collected 24 h after the last melatonin injection. The sera were separated and levels of IL-2 and IL-4 were measured by their respective abilities to induce the proliferation of HT-2 cells as described earlier [12]. Briefly, 1 × 104/well of HT-2 cells were cultured in 96-well microtitre plates containing medium and various concentrations of serum obtained from the control and experimental animals. For the selective inhibition of IL-2 and IL-4 lymphokines, antibodies to IL-2 (culture SN in 1:12 dilution of CRL 1698, TIB222 and HB 8794) and IL-4 (11B11, 1 μg/ml) were used, respectively. The specificity of the assay for measuring IL-4 was established by inhibiting the proliferation of HT-2 cells induced by the sera obtained from the animals immunized with OVA and challenged with 50 mg of melatonin by anti-IL-4 antibodies. Similarly, the specificity of IL-2 was checked by anti-IL-2 antibodies. The cells were incubated for 16 h at 37°C, pulsed with 0·5 μCi of 3H-thymidine and harvested 8 h later. 3H-thymidine incorporation was measured by liquid scintillation spectrometry. All data are calculated from the mean ct/min of triplicate determinations as U/ml of IL-2 and IL-4 as computed by comparison with the standard curve using recombinant IL-2 or IL-4 (Genzyme).
IFN-γ was measured by ELISA
IFN-γ was estimated using appropriate purified and biotinylated antibody pairs for IFN-γ (Genzyme) according to the manufacturer's protocols. Briefly, 50 μl of the purified anti-mouse IFN-γ MoAbs were adsorbed overnight on polystyrene microtitre plates at 4°C. The samples were followed by biotinylated polyclonal goat anti-mouse IFN-γ. Later on, streptavidin–HRP, followed by hydrogen peroxide and tetramethylbenzidine (Sigma) were used for detection. Titration curves of recombinant IFN-γ (Genzyme) were used as standards for calculating cytokine concentrations in the samples tested. The usual steps of incubation and washing were followed at each step. The limit of detection for IFN-γ was routinely in the range 15–30 pg/ml.
Determination of OVA-specific IgG isotypes by ELISA
The production of OVA-specific antibodies was measured in the sera 24 h after the last injection of melatonin. Ninety-six-well microtitre plates (Costar, Cambridge, MA) were coated overnight with 50 μl of OVA (25 μg/ml) in carbonate-bicarbonate buffer, 0·05 m, pH 9·6 at 4°C. After extensive washing with PBS–Tween 20 (PBS–T) buffer, 50 μl of blocking buffer (3% skimmed milk in PBS–T) were applied to the wells and incubated at 37°C for 90 min. The blocking buffer was removed and log2 dilutions of test and control sera were added. The reaction was allowed to proceed at 37°C for 2 h. The microplates were washed and 50 μl of biotinylated goat anti-mouse IgG1, IgG2a, IgG2b and IgG3 were added. The plates were incubated at 37°C for 1 h. After the usual washing steps, 50 μl of streptavidin–HRP were added to each well and the plates were incubated at 37°C for 1 h. The plates were washed again before adding 50 μl of ABTS and were finally incubated at 37°C for 20 min. The reaction was terminated by the addition of 50 μl of 7% H2SO4. The absorbance was read at 492 nm with a microplate reader (Eurogenetics, Torino, Italy). Antibody titres were expressed as the highest dilution of serum that yielded an optical density (OD) of 0·2 above the control wells.
Statistical analysis
The data were analysed by one-way analysis of variance (anova) followed by Dunnet's t-test. P< 0·05 was considered statistically significant.
RESULTS
Melatonin enhances the proliferation of OVA-specific CD4+ T cells
In the present study, we analysed the stimulatory potential of melatonin on the proliferation of antigen-specific T cells. T cell responsiveness to melatonin was observed in a dose-dependent manner. LN cells of BALB/c mice immunized with 50 mg/kg body wt of melatonin showed maximum proliferation (P< 0·001) (Fig. 1). In contrast, a 1-mg dose of melatonin stimulated T cells to lesser degree. On evaluating the in vitro stimulatory capacity of melatonin, it was noticed that T cell proliferation peaked when 12·50 μg/ml of melatonin was incorporated into the cultures. Higher doses brought about a decline in T cell responses. Control cultures containing the cells obtained from the OVA-unimmunized animals, immunized with PBS, ethanol–melatonin, or melatonin only did not show significant T cell proliferation.
Fig. 1.

Melatonin augmented the proliferation of ovalbumin (OVA)-specific T cells. Different groups comprising five animals were immunized with OVA, followed by melatonin administration daily for 5 days before lymph node (LN) cells were harvested (inset legend). Th cells (1.5 × 106/ml) were cultured in vitro with 100 μg/ml OVA and varied doses of melatonin. After 72 h, 3H-thymidine was added, and its incorporation was measured 16 h later by liquid scintillation spectroscopy. The control cultures consisting of cells ± melatonin, no OVA (1592 ± 305 ct/min), cells + medium only (952 ± 586 ct/min) and LN cells of animals immunized with PBS–ethanol (1163 ± 763 ct/min) could not induce significant levels of T cell proliferation. Data are the mean ct/min of three determinations, the s.d. values were generally < 10% of the actual ct/min.
Melatonin increases the production of IL-4 and inhibits the secretion of IL-2 and IFN-γ
The role of melatonin on the in vivo secretion of IL-2, IFN-γ and IL-4 was evaluated in the sera of animals immunized with OVA. It is of particular interest to mention here that melatonin significantly (P< 0·001) enhanced the levels of IL-4 (from 1·96 to 16·8 U/ml) and decreased the production of IL-2 (from 4·2 to 0·53 U/ml) and IFN-γ (from 26 to 12 pg/ml) compared with animals administered OVA only (Fig. 2a,b). The greatest stimulation for the production of IL-4 was provided by 10 mg of melatonin inoculated in the animals. In contrast, 50 mg of melatonin showed the maximum inhibition in production of IFN-γ (Fig. 2b), but the variation in the dose of melatonin had little effect on the levels of IL-2. No detectable amount of lymphokines was observed in the case of control animals inoculated with PBS, melatonin–ethanol or melatonin alone.
Fig. 2.
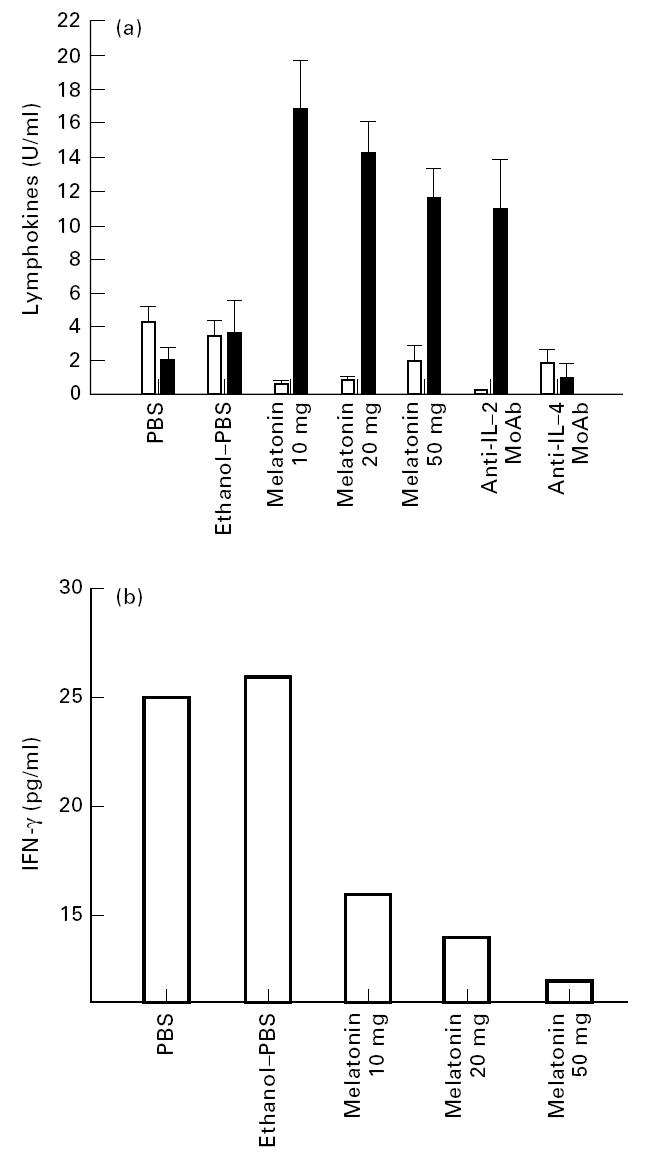
(a) Melatonin enhances IL-4 and down-regulates the production of IL-2. The levels of IL-4 (▪) and IL-2 (□) were monitored in the serum of animals immunized with ovalbumin (OVA) and followed by melatonin and control, PBS and PBS–ethanol. Sera were collected 24 h after the last injection of melatonin and assayed for IL-4 and IL-2 using HT-2 cells. IL-2 activity was assayed in the presence of 11B11 MoAb (600 ng/ml) and IL-4 secretion was measured in the presence of anti-IL-2 MoAb (cocktail of 1:12 dilution of culture SN of CRL 1698, HB 8794 and TIB222). The specificity of IL-4 and IL-2 was established by blocking the proliferation of HT-2 cells by their respective MoAbs (for details see Materials and Methods). All data calculated as U/ml are from the mean ± s.d. of triplicate determinations. (b) Melatonin decreases the secretion of IFN-γ. The level of IFN-γ was measured in the serum by ELISA. Sera were collected from the immunized animals as mentioned in the legend to (a). All data calculated from the mean ± s.d. of triplicate determinations as pg/ml were computed by comparison with the standard curve using recombinant IFN-γ (Genzyme).
Melatonin induces the secretion IgG1 but not IgG2a isotype antibodies
The impact of melatonin on the production of OVA-specific IgG isotype was monitored in sera of the animals. Interestingly, among the different isotypes, i.e. IgG1, IgG2a, IgG2b and IgG3 analysed, significant increases (P< 0·001) in the secretion of OVA-specific IgG1 antibodies were detected in the sera of OVA-primed mice administered with melatonin (antibody titre 65 536) compared with animals immunized with OVA alone (antibody titre 3248) (Fig. 3). The animals immunized with 10 mg of melatonin elicited greatest secretion of IgG1 isotype. On increasing the dose of melatonin (20–50 mg/kg body wt), the yield of IgG1 antibodies decreased. Further, melatonin down-regulated the level of IgG2a isotype (antibody titre 960) compared with animals inoculated with OVA (antibody titre 3686) in the absence of melatonin. No excessive changes were noticed in the case of IgG2b and IgG3 isotypes. No detectable level of IgG isotypes was seen in the control group of mice inoculated with melatonin–ethanol, melatonin only or PBS.
Fig. 3.
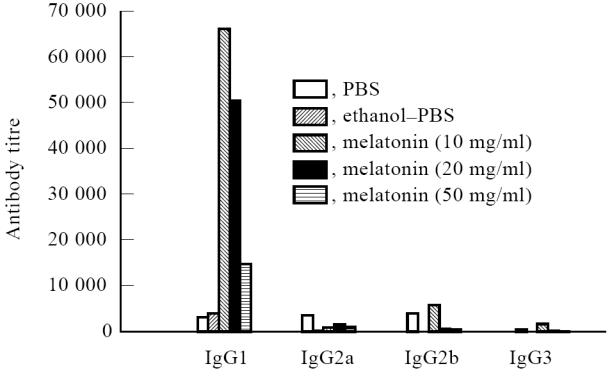
Melatonin increases the secretion of IgG1 isotype. Sera from animals immunized with melatonin were analysed for the production of ovalbumin (OVA)-specific IgG isotype by ELISA. The inset legend indicates the in vivo immunization of the animals with OVA, and 5 days before bleeding the animals were injected subcutaneously daily with different doses of melatonin. Antibody titres are expressed as the highest dilution that yielded an optical density of 0.2 above the control wells.
DISCUSSION
The induction of cell-mediated (CMI) or humoral immunity (HI) to protein antigens is dependent on the activation of Th1 and Th2 cells, respectively [13–18]. The maturation and differentiation of naive CD4+ T cells are influenced by a number of factors, including antigen dose, the nature of peptide/MHC complex, exposure to cytokines and costimulatory molecules, the kind of adjuvants and the type of antigen-presenting cell (APC) [19–23]. The cytokine milieu present during the priming of naive T cells determines the ultimate pathway of differentiation. Th1 cell development is positively influenced by IL-12 and IFN-γ, while the cytokines IL-4 and IL-10 are thought to favour Th2 differentiation [24].
Melatonin is not only produced by the pineal gland but can also be synthesized by macrophages/monocytes [6, 7]. It plays an important role in modulating the immune system. Treatment of B cells with melatonin resulted in an increased production of antibodies. Similarly, the function of T cells was shown to be enhanced with melatonin treatment [10, 11].
In this study we have attempted to analyse the role of melatonin on the activity of OVA-specific T cells by monitoring the proliferation and lymphokine secretion and on B cells by estimating the production of IgG isotypes. Three major findings have emerged from this study. Melatonin predominantly enhanced: (i) proliferation of antigen-specific Th cells; (ii) production of OVA-reactive IgG1 isotype; and (iii) secretion of IL-4. Levels of IL-2 and IFN-γ were decreased in melatonin-treated mice.
Thus melatonin could convert antigen-specific Th clones in vivo to predominantly secrete IL-4. It could be argued that the original OVA-primed Th cells were the mixture of Th1 and Th2 cells (Fig. 2), the latter of which might have received a stimulus generated by melatonin leading to clonal expansion of Th2 cells engineered by IL-4. It is of interest to mention here that melatonin could not influence the secretion of IL-4 by naive T cells of the animals unprimed with OVA. It may be concluded that melatonin works only on antigen-activated T cells.
The regulation of isotype secretion by Th clones occurs as two events. The first involves Th cell and B cell interaction, the other involves T cell-derived cytokines. The regulation of isotype switching in vivo will depend upon the types of Th cells that are activated, and whether lymphokines are transferred between T and B cells. Th1 and Th2 cells also differ in their ability to stimulate B cells to secrete various kinds of IgG isotypes [4]. Th1 clones were shown to elicit IgG2a antibodies, whereas Th2 clones induced the production of IgG1 isotype. Enhanced secretion of IL-4 induced by melatonin dramatically improved the signals delivered to B cells, leading to increased production of IgG1. It may be recalled that it has also been reported earlier that IL-4 induces the production of IgG1, and IFN-γ primarily activates B cells to secrete IgG2a [5]. It may be concluded from these experiments that melatonin also augments the ability of Th cells to help B cells, as evidenced by augmentation of the levels of IgG1 secretion. Preferential production of IgG1 antibodies is of particular interest, since this isotype binds to mast cell receptors and can mediate mast cell degranulation that may confer increased resistance to nematode parasites and may contribute to its ability to induce anaphylaxis [25, 26].
Such considerations which could selectively enhance Th1 or Th2 cells are pertinent in designing strategies by using drugs such as melatonin, which could regulate the activation of the Th subtype and production of IgG1 isotype, that will subserve different biological functions and hence induce an optimal immune response to a particular type of pathogen.
Acknowledgments
We are grateful to Ms Sangita Mukhopadhyay and Dr Satyajit Rath at National Institute of Immunology, New Delhi for helping with the estimation of IFN-γ, Mr Dinesh Kumar for secretarial help, and Morepen Laboratories Ltd, Parwanoo, India, for supplying melatonin.
References
- 1.Mosmann TR, Cherwinski H, Bond MW, Gieldin HA, Coffman RL. Two types of murine T cell clones. 1. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–57. [PubMed] [Google Scholar]
- 2.Bottomly KA. A functional dichotomy in CD4+T lymphocytes. Immunol Today. 1988;9:268–74. doi: 10.1016/0167-5699(88)91308-4. [DOI] [PubMed] [Google Scholar]
- 3.Boom WH, Liano D, Abbas AK. Heterogeneity of helper/inducer T lymphocytes. II. Effects of interleukin-4 and interleukin-2 producing T cell clones on resting B lymphocytes. J Exp Med. 1988;167:1350–63. doi: 10.1084/jem.167.4.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevens TL, Bossie A, Sanders VM, Fernandez-Botran R, Coffman RL, Mosmann TR, Vitetta ES. Regulation of antibody isotype secretion by subset of antigen specific helper T cells. Nature. 1988;334:2525–8. doi: 10.1038/334255a0. [DOI] [PubMed] [Google Scholar]
- 5.Snapper CM, Paul WE. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–7. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 6.Finocchiaro LME, Nahmod VE, Launay JM. Melatonin biosynthesis and metabolism in peripheral blood mononuclear leukocytes. Biochem J. 1991;280:727–31.. doi: 10.1042/bj2800727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finocchiaro LME, Arzt ES, Fernandez-Castelo S, Criscuolo M, Finkelman S, Nahmod VE. Serotonin and melatonin synthesis in peripheral blood mononuclear cells: stimulation by interferon-γ as part of an immunomodulatory pathway. J Interferon Res. 1988;8:705–10. doi: 10.1089/jir.1988.8.705. [DOI] [PubMed] [Google Scholar]
- 8.Hellstrand K, Hermodsson S. Role of serotonin in the regulation of human natural killer cell cytotoxicity. J Immunol. 1987;139:869–75. [PubMed] [Google Scholar]
- 9.Hellstrand K, Hermodsson S. Monocyte-mediated suppression of IL-2-induced NK-cell activation: regulation by 5-HT1A-type serotonin receptors. Scand J Immunol. 1990;32:183–8. doi: 10.1111/j.1365-3083.1990.tb02908.x. [DOI] [PubMed] [Google Scholar]
- 10.Kuribayashi K, Gillis S, Kern DE, Henry CS. Murine NK cell culture: effects of interleukin-2 and interferon on cell growth and cytotoxic reactivity. J Immunol. 1981;126:2321–7. [PubMed] [Google Scholar]
- 11.Caroleo MC, Doria G, Niatico G. Melatonin restores immunodepression in aged and cyclophosphamide-treated mice. Ann NY Acad Sci. 1994;719:343–52. doi: 10.1111/j.1749-6632.1994.tb56841.x. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Botran R, Krammer PH, Diamanstein T, Uhr JW, Vitteta ES. B-cell stimulatory factor-1 promotes growth of helper T cell lines. J Exp Med. 1986;164:580–93. doi: 10.1084/jem.164.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janeway CA, Jr, Carding S, Jones B, et al. CD4+ T cells: specificity and function. Immunol Rev. 1988;101:39–80. doi: 10.1111/j.1600-065x.1988.tb00732.x. [DOI] [PubMed] [Google Scholar]
- 14.Mosmann TR, Coffman RL. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 15.Killar L, Macdonald G, West J, Woods A, Bottomly K. Cloned, Ia-restricted T cells that do not produce interleukin 4 (IL-4)/B cell stimulatory factor 1 (BSF-1) fail to help antigen-specific B cells. J Immunol. 1987;138:1674–9. [PubMed] [Google Scholar]
- 16.Romagnani S. Lymphokine production by human T cells in disease states. Annu Rev Immunol. 1994;12:227–57. doi: 10.1146/annurev.iy.12.040194.001303. [DOI] [PubMed] [Google Scholar]
- 17.Scot P, Kaufmann SHE. The role of T-cell subsets and cytokines in the regulation of infection. Immunol Today. 1991;12:346–8. doi: 10.1016/0167-5699(91)90063-Y. [DOI] [PubMed] [Google Scholar]
- 18.Scot PA, Sher A. Immunoparasitology. In: Paul WE, editor. Fundamental immunology. 3. New York: Raven Press; pp. 1179–210. [Google Scholar]
- 19.Secrist H, DeKruyff H, Umetsu DT. Interleukin 4 production by CD4+ T cells from allergic individuals is modulated by antigen concentration and antigen-presenting cell type. J Exp Med. 1995;181:1081–9. doi: 10.1084/jem.181.3.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evavold BD, Sloan-Lancaster J, Allen PM. Tickling the TCR. Selective T-cell functions stimulated by altered peptide ligands. Immunol Today. 1993;14:602–9. doi: 10.1016/0167-5699(93)90200-5. [DOI] [PubMed] [Google Scholar]
- 21.Agrewala JN, Vinay DS, Joshi A, Mishra GC. A 150-kDa molecule of macrophage membrane stimulates interleukin-2 and interferon-γ production and proliferation of ovalbumin-specific CD4+ T cells. Eur J Immunol. 1994;24:2092–7. doi: 10.1002/eji.1830240924. [DOI] [PubMed] [Google Scholar]
- 22.Agrewala JN, Owais M, Gupta CM, Mishra GCP. Antigen incorporation into liposomes results in the enhancement of IL-4 and IgG1 secretion: evidence for preferential expansion of Th-2 cells. Cytokines Mol Ther. 1996;2:59–65. [PubMed] [Google Scholar]
- 23.Paul WE, Seder RA. Lymphocyte responses and cytokines. Cell. 1994;76:241–51. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 24.Seder RA, Paul WE. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen BW, Lind P, Hansen B, Reimert CM, Nansen P, Schiotz P. Immune response to nematode exoantigens: sensitizing antibodies and basophil histamine release. Allergy. 1994;49:427–35. doi: 10.1111/j.1398-9995.1994.tb00835.x. [DOI] [PubMed] [Google Scholar]
- 26.Miyajima I, Dombrowicz D, Martin TR, Ravetch JV, Kinet JP, Galli SJ. Systemic anaphylaxis in the mouse can be mediated largely through IgG1 and Fc gammaRIII. Assessment of the cardiopulmonary changes, mast cell degranulation, and death associated with active or IgE- or IgG1-dependent passive anaphylaxis. J Clin Invest. 1997;99:901–14. doi: 10.1172/JCI119255. [DOI] [PMC free article] [PubMed] [Google Scholar]


