Abstract
In order to understand the mechanism of unresponsiveness towards Mycobacterium leprae antigens in leprosy, we evaluated the role of M. leprae sonicate antigens in regulating the expression of the costimulatory molecules B7-1, CD28, intercellular adhesion molecule-1 (ICAM-1), LFA-1α, LFA-1β and Mac-1 on the lymphocytes of both leprosy patients and healthy subjects. It was observed that the expression of B7-1 and CD28 was significantly decreased but the levels of ICAM-1 and LFA-1α were increased in patients with untreated borderline leprosy (BL)/lepromatous leprosy (LL) disease. No remarkable change was noticed in the case of borderline tuberculoid (BT) leprosy or treated BL/LL patients. Further, a striking finding was that lymphocytes from healthy subjects cultured with a particularly high dose of M. leprae sonicate antigens down-regulated the expression of B7-1 and CD28 molecules, but up-regulated the display of ICAM-1 and LFA-1α. Furthermore, proliferation induced by M. leprae sonicate was inhibited only by anti-B7-1 antibody. Mycobacterium leprae antigen-induced suppression of the proliferation of lymphocytes of healthy volunteers and LL patients was reversed by culturing the lymphocytes with purified protein derivative (PPD). It may be concluded from the findings in this study that down regulation of B7-1 and CD28 in BL/LL leprosy patients may be responsible for a defective T cell signalling by the B7-1/CD28 pathway caused by M. leprae antigens. This may lead to clonal inactivation of M. leprae-reactive T cells, consequently the bacilli grow without restriction in macrophages.
Keywords: B7-1, CD28, costimulation, leprosy
INTRODUCTION
Leprosy is a disease that encompasses an immunological spectrum. At the lepromatous pole, patients specifically lack cell-mediated immunity (CMI) to antigens of Mycobacterium leprae and fail to restrict the growth of the pathogen. At the tuberculoid end of the spectrum, the patients exhibit CMI to antigens of M. leprae and develop one or a few sharply defined lesions that contain only few acid-fast bacilli. The immunological state of these patients is generally assessed by using a lepromin skin test. The test is positive in tuberculoid patients but negative in lepromatous individuals. The weight of evidence points to a defect in the T cell–macrophage interaction as being the crucial event underlying the hyporesponsiveness characteristic of multibacillary leprosy. CMI, which is regulated by specifically sensitized T lymphocytes, is clearly required for protection and resistance to leprosy [1, 2].
Optimum activation of T helper cells requires not only T cell receptor (TCR) occupancy by the antigen–MHC complex, but also a set of costimulatory signals (i.e. intercellular adhesion molecule-1 (ICAM-1), LFA-1, LFA-3, vascular cell adhesion molecule-1 (VCAM-1), B7, etc.) provided by the antigen-presenting cells (APC) [3–11]. The significance of accessory cell molecules in T cell activation has gained considerable impetus following the observation that occupancy of TCR alone can render T cells tolerant [12]. Costimulatory molecules are critical in ensuring the optimum activation of T cells. The binding of accessory molecules such as ICAM-1 and B7-1/2 expressed on APC to their co-receptor molecules LFA-1 and CD28 on T cells, respectively, plays an important role in T cell adhesion and in the initiation of signal transduction events [4]. The ability of the APC to deliver the costimulatory signals to T cells can be regulated by the intracellular pathogens. Expression of costimulatory molecules is inducible by a lipopolysaccharide, a component of the cell wall of Gram-negative bacteria [11]. In contrast, intracellular pathogens not only enhance T cell activation but also modulate the accessory function of the APC in a negative way. Leishmania major-infected macrophages show diminished antigen presentation capacity. Leishmania donovani results in the failure to trigger expression of B7-1 and of the heat-stable antigen (HSA) [12–14]. Recently, the selective regulation of ICAM-1 and B7-1 in tuberculosis and visceral leishmaniasis has also been demonstrated. Up-regulation of costimulatory molecules plays a critical role not only in eliciting CMI but also in the production of cytokines in response to antigen stimulation [15, 16].
In leprosy, from the tuberculoid to the lepromatous pole, there is a linear depression in CMI to M. leprae antigens. Therefore the present study was undertaken to evaluate whether inadequate expression of costimulatory molecules, such as B7-1, CD28, ICAM-1, LFA-1α, LFA-1β and Mac-l, in different types of leprosy is responsible for this anomaly. The findings in the present study provide insight into the mechanism by which M. leprae antigens depress CMI by regulating the expression of costimulatory molecules.
MATERIALS AND METHODS
Subjects
Leprosy patients were recruited from the Postgraduate Institute of Medical Education and Research (Chandigarh, India). Patients were diagnosed by the clinicopathological criteria of Ridley & Jopling [17, 18]. Seven healthy laboratory volunteers, nine borderline leprosy (BL)/lepromatous leprosy (LL) and 10 borderline tuberculoid (BT) leprosy patients were selected for the study. Of the nine BL/LL cases, three were untreated and the others had undergone chemotherapy for periods varying from 2 to 5 years to render them bacillary-negative. Similarly, in the case of the BT patients, three were untreated and seven had been treated by multi-drug therapy (MDT) as recommended by the WHO. All the treated leprosy patients were bacteriological index (BI)-negative. None of the patients showed clinical features of a reaction. A group of healthy laboratory volunteers was used as a control.
Antibodies
Anti-B7-1 and anti-CD28 antibodies were obtained from Pharmingen and antibodies to ICAM-1, LFA-1α, LFA-1β and Mac-1 were procured from Bender Medsystems (Vienna, Austria). Anti-CD4 MoAb was used as culture supernatant (SN) generated by hybridoma CRL 8002 obtained from ATCC (Rockville, MD). Rabbit anti-mouse immunoglobulin-FITC was bought from Sigma (St Louis, MO). Mycobacterium leprae sonicate (Mls) was a kind gift from IMMLEP Bank, WHO.
Antibody inhibition assay
Peripheral blood mononuclear cells (PBMC) were isolated by differential centrifugation over Ficoll–Hypaque (Pharmacia, Uppsala, Sweden). PBMC (1 × 105/well) obtained from healthy volunteers and from untreated BT/TT, treated BT/TT and BL/LL leprosy patients were cultured in 200 μl of RPMI 1640 (Gibco, Grand Island, NY) pH 7·2 supplemented with 10 mm sodium bicarbonate, 100 μg/ml penicillin, 75 μg/ml streptomycin containing 10% AB serum and with different concentrations of M. leprae sonicate (0·01–100 μg/ml) and purified protein derivative (PPD; 100 U/ml) in triplicate wells in 96-well microtitre plates (Costar, Boston, MA). For antibody blocking experiments PBMC were preincubated at 4°C for 1 h with 1 μg/ml of antibodies to B7-1, CD28, ICAM-1, LFA-1α, LFA-1β and Mac-1 before the addition of Mls (10 μg/ml) or PPD (100 U/ml). The antibodies were present throughout the culture period. The control cultures were kept where PBMC were cultured with antibodies against costimulatory molecules B7-1, CD28, ICAM-1, LFA-1α, LFA-1β and Mac-1 in the absence of Mls or PPD. PBMC incubated with isotype-matched antibodies and Mls or PPD were also used as control. The involvement of CD4+ T cells in the proliferative responses was confirmed by incubating Mls (10 μg/ml) or PPD (100 U/ml) with PBMC of healthy subjects incubated either with anti-CD4 MoAb (culture SN in 1:10 dilution of CRL 8002), or with anti-CD4 MoAb and baby rabbit complement (BRC)-treated lymphocytes. After 72 h 3H-TdR was added and the cells were harvested 16 h later by an automatic PHD sample harvester (Watertown, MA), and monitored for β-emission by liquid scintillation counting. Results are expressed as the ct/min (mean ± s.d.) of the total patient population employed in the study.
Incubation of PBMC isolated from healthy individuals with M. leprae sonicate for FACS analysis
PBMC (1 × 106/well) isolated from healthy volunteers were cultured in 24-well plates (Costar) with M. leprae sonicate (10 and 50 μg/ml) in 1 ml of RPMI 1640 containing 10% AB serum. The cultures were kept at 37°C for 48 h in 7% CO2.
Fluorescence analysis of PBMC
FACS analysis was either performed on freshly isolated PBMC of leprosy patients and healthy subjects or cultured with M. leprae sonicate. The PBMC were incubated with anti-B7-1, CD28, ICAM-1, LFA-1α, LFA-1β and Mac-1 MoAbs (5 μg/ml) at 4°C for 1 h. The cells were then washed × 5 with PBS (0·01 m, pH 7·2) and labelled with rabbit anti-mouse immunoglobulin-FITC by incubating at 4°C for 1 h and then washed at least five times. The cells were subsequently fixed in 0·1% paraformaldehyde in PBS. Isotype-matched antibodies were used as control.
Cells (1 × 104) from each suspension were acquired on Lysis II software of FACScan (Becton Dickinson, Mountain View, CA). Debris in the cell suspension was excluded from the analysis by suitable gating which allowed the collection of data only from those light-scattering events of cells. Analysis of mean fluorescence intensity (MFI) was done on histograms where abscissa and ordinate denote log FITC fluorescence and relative cell count, respectively. The data presented are a percentage change in MFI and are expressed as a mean ± s.d. of patient population as a whole, calculated by comparing the expression of costimulatory molecules with their expression on the lymphocytes of healthy subjects i.e. % change in MFI = (MFI of the experiment (i.e. leprosy patient) × 100)/(MFI of the control (i.e. healthy subjects)).
RESULTS
T cell proliferation induced by M. leprae antigens could be specifically inhibited by the antibodies directed against the costimulatory molecules B7-1, CD28, ICAM-1, LFA-1α, LFA-1β and Mac-1
The dose responsiveness showed the optimum proliferation of the PBMC of healthy volunteers using 10 μg/ml of Mls. Further, a concentration of 50 μg/ml of Mls inhibited T cell proliferation (data not shown). For all subsequent cultures, a dose of either 10 or 50 μg/ml of Mls, as indicated, was chosen. Figure 1a shows that Mls (10 μg/ml)-induced proliferation of lymphocytes was significantly blocked by anti-B7-1 MoAbs. Nearly 79% reduction in 3H-thymidine uptake compared with that of control cultures containing no MoAb was observed. In contrast, incubation of PBMC with Mls and anti-CD28 antibodies increased the proliferation by 33% compared with control cultures provided with no MoAb against costimulatory molecules. No change was detected when anti-ICAM-1, anti-LFA-1α, anti-LFA-1β and anti-Mac-1 MoAbs were used to neutralize the signals. Further, anti-B7-1, anti-ICAM-1 and anti-LFA-1α MoAbs significantly blocked T cell responses to PPD (Fig. 1b). Interestingly, inhibition induced in the proliferation (6915 ± 1651 ct/min) by a high dose of Mls (50 μg/ml) was neutralized significantly when PPD (42 896 ± 3699 ct/min) was incorporated into the same cultures. When the cells were incubated with a high dose of Mls and PPD (100 U/ml), DNA synthesis was checked not only by anti-B7-1, but also by anti-ICAM-1 and anti-LFA-1α MoAbs (Fig. 1c). The observed proliferation was mainly associated with CD4+ T cells, as evidenced by the fact that (i) addition of anti-CD4 MoAb into the culture wells inhibited the proliferation of PBMC incubated with Mls (10 μg/ml) or PPD (100 U/ml); and (ii) treatment of PBMC with anti-CD4 MoAb and BRC significantly abolished the proliferative response. The lymphocytic activity remained unaffected in the control cultures where PBMC were incubated with antibodies against costimulatory molecules but in the absence of Mls or PPD, and in the cultures containing isotype-matched MoAbs in the presence of Mls or PPD.
Fig. 1.
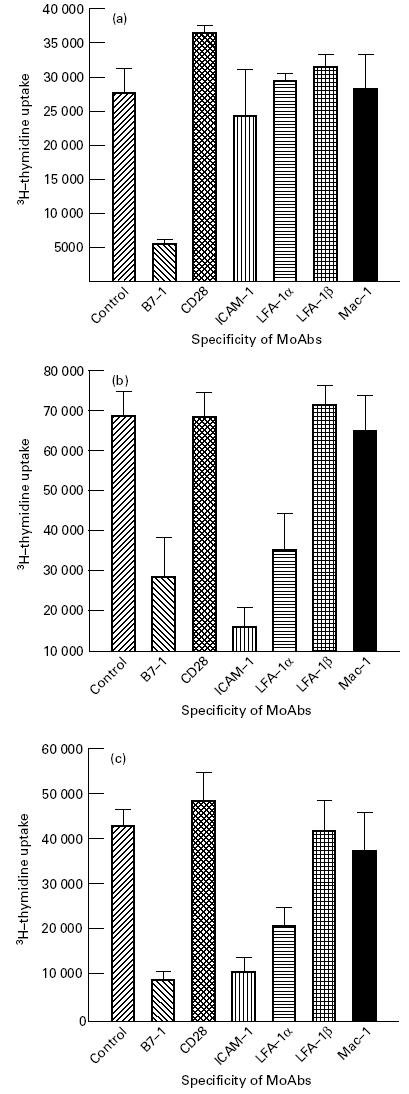
Effect of anti-B7-1, anti-CD28, anti-intercellular adhesion molecule-1 (ICAM-1), anti-LFA-1α, anti-LFA-1β and Mac-1 MoAbs in the inhibition of proliferation of lymphocytes of healthy subjects induced by Mycobacterium leprae sonicate (Mls) and purified protein derivative (PPD). Peripheral blood mononuclear cells (PBMC) isolated from healthy volunteers were cultured either with Mls (10 μg/ml) (a), PPD (100 U/ml) (b), Mls (50 μg/ml) +PPD (100 U/ml) (c). MoAbs against the accessory molecules were added in the cultures to monitor the inhibition of proliferation. 3H-TdR was added 72 h later and the cells were harvested after 16 h. The radioactivity incorporated was monitored by liquid scintillation counting. The lymphocytes cultured with 50 μg/ml Mls showed 6915 ± 1651 ct/min. The control cultures containing isotype-matched MoAbs could not inhibit the Mls or PPD-induced T cell proliferation. The blocking of the proliferation of CD4+ T cells was achieved either by incorporation of anti-CD4 MoAb in the cultures containing PBMC and Mls (3924 ± 1822 ct/min) and PPD (2764 ± 352 ct/min), or treating the PBMC with anti-CD4 MoAb and complement (1049 ± 211 ct/min). Results are expressed as mean ± s.d.; n = 7.
Similarly, the effect of MoAbs was also determined, using PBMC derived from three untreated BL/LL patients. None of the patients responded to Mls (10 μg/ml) (Fig. 2a). However, PPD (100 U/ml) induced a significant growth of lymphocytes. Antibodies against B7-1, ICAM-1 and LFA-1α blocked PPD-specific proliferation remarkably. Furthermore, Mls abrogated slightly the induction by PPD of proliferation of lymphocytes from untreated BL/LL patients. The proliferation in the cultures containing Mls along with PPD was also blocked by anti-B7-1, anti-ICAM-1 and anti-LFA-1α MoAbs. The regulation of Mls-induced proliferation was also studied using lymphocytes of three untreated BT patients. The induction of proliferation by Mls (10 μg/ml) was inhibited only by anti-B7-1 MoAbs but not by MoAbs to other costimulatory molecules (Fig. 2b).
Fig. 2.
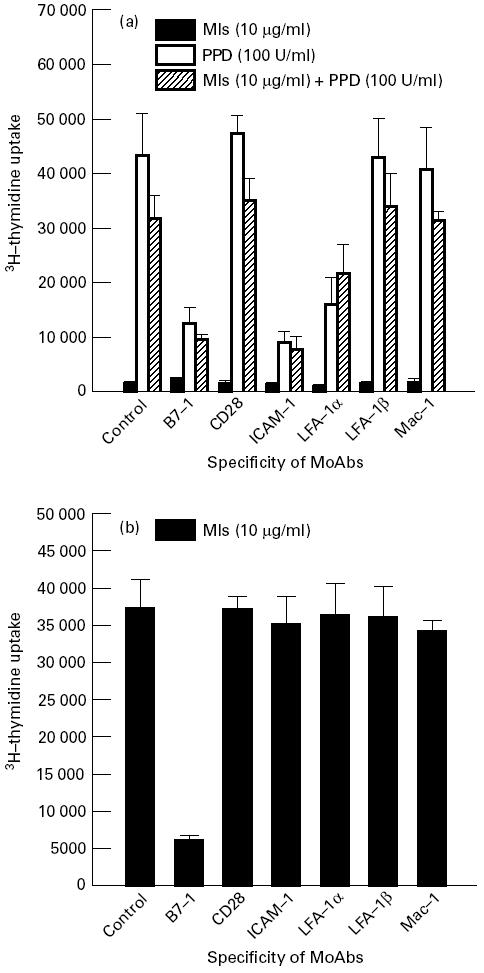
Effect of anti-B7-1, anti-CD28, anti-intercellular adhesion molecule-1 (ICAM-1), anti-LFA-1α, anti-LFA-1β and Mac-1 MoAbs in the inhibition of proliferation of lymphocytes of borderline leprosy (BL)/lepromatous leprosy (LL) and borderline tuberculoid (BT) leprosy patients. Peripheral blood mononuclear cells (PBMC) were isolated from untreated BL/LL (a) and BT (b) patients. The culture conditions were the same as for Fig. 1. The results expressed are mean ± s.d.; n = 3.
Expression of CD28, B7-1, ICAM-1, LFA-1α, LFA-1β and Mac-1 in leprosy patients
Compared with lymphocytes obtained from healthy subjects, no significant change in surface expression of B7-1, CD28, ICAM-1, LFA-1α, LFA-1β and Mac-1 was observed in the PBMC from BT patients. This correlates very well with the reported intact CMI at this pole of leprosy. However, expression of B7-1 and CD28 were both decreased, by an average of 63% and 45%, respectively (Fig. 3a,b), in untreated BL/LL patients; this suggests that these costimulatory molecules are involved in the suppression of CMI. Further, levels of ICAM-1 (57%) and LFA-1α (39%) were elevated (Fig. 3c,d), but those of LFA-1β and Mac-1 (Fig. 3e,f) remained unchanged. Furthermore, no significant change in the expression of the costimulatory molecules was observed in the case of treated BT and BL/LL leprosy patients. No change in the expression of costimulatory molecules was demonstrated in the control lymphocytes incubated with isotype-matched antibodies.
Fig. 3.
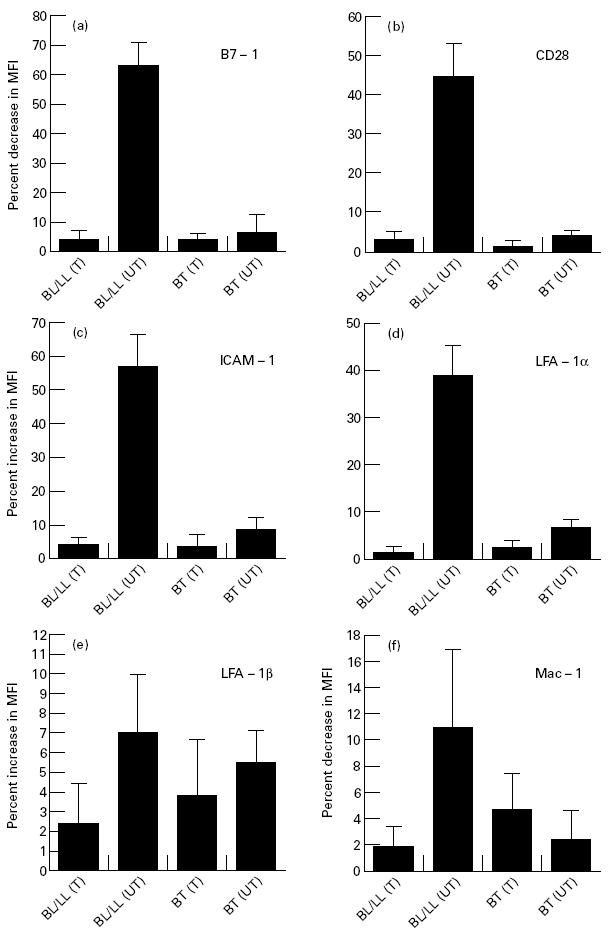
Expression of B7-1, CD28, intercellular adhesion molecule-1 (ICAM-1), LFA-1α, LFA-1β and Mac-1 on freshly isolated lymphocytes of leprosy patients. The lymphocytes were stained using MoAbs against the costimulatory molecules (5 ng/ml) and secondary FITC-labelled antibodies. Data shown are represented as percent change in the mean fluorescence intensity (MFI) of the costimulatory molecules expressed on the surface of the lymphocytes obtained from the leprosy patients (mean ± s.d.; borderline leprosy (BL)/lepromatous leprosy (LL) treated (T), n = 3; BL/LL untreated (UT), n = 6; borderline tuberculoid (BT) leprosy treated (T), n = 7; BT untreated (UT), n = 3), compared with the expression of costimulatory molecules on the lymphocytes of healthy subjects (mean ± s.d.; n = 7). No staining was viewed in the controls containing isotype-matched MoAbs.
Mls antigens down-regulate the expression of B7-1 and CD28 but up-regulate the expression of ICAM-1 and LFA-1α on the surface of lymphocytes from healthy subjects
The belief that the decrease in the expression of B7-1 and CD28 molecules was mediated by M. leprae antigens has been further strengthened by the fact that inhibition of the expression of these molecules was recognized in the lymphocytes from healthy subjects incubated with Mls. Interestingly, a decrease in the expression of B7-1 and CD28 and up-regulation of ICAM-1 and LFA-1α was only observed with 50 μg/ml concentration of Mls, but not with 10 μg/ml. It may be mentioned here that a 50 μg/ml dose of Mls also inhibited the proliferation of PBMC. The regulation of LFA-1β and Mac-1 remained unchanged, as evidenced by no shift in the MFI profile (Fig. 4). Surprisingly, the expression of each of B7-1, ICAM-1 and LFA-1α was enhanced remarkably when the lymphocytes were incubated with Mls in the presence of PPD. Similar effects were also observed with PPD alone. The expression of costimulatory molecules in the control cultures containing PBMC incubated either with medium alone or with isotype-matched MoAbs remained unaltered.
Fig. 4.
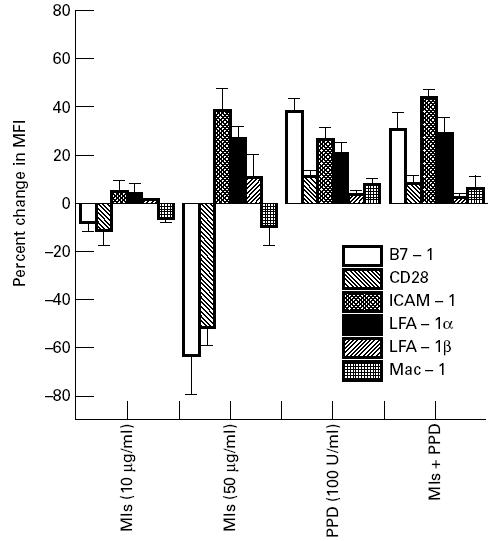
Mycobacterium leprae sonicate (Mls) alters the expression of B7-1, CD28, intercellular adhesion molecule-1 (ICAM-1) and LFA-1α on the lymphocytes of healthy volunteers. The lymphocytes were cultured with Mls or purified protein derivative (PPD) for 48 h and labelled with the MoAbs against the costimulatory molecules for FACScan. Staining was done as described in the legend to Fig. 3. Data are presented as percentage difference in mean fluorescence intensity (MFI) of the expression of costimulatory molecules compared with the lymphocytes cultured in the absence of Mls or PPD (mean ± s.d.; n = 7).
DISCUSSION
Leprosy exhibits a clinical spectrum that is precisely paralleled by differences in immunological reactivity. At one pole of the disease patients with tuberculoid leprosy have intact CMI with one or few skin lesions in which bacilli can only rarely be seen. At the lepromatous end, there is a selective anergy of T cells to M. leprae antigens, and therefore the host is unable to mount a CMI sufficient to eliminate the infection. In this form of the disease, macrophages primarily in the nerves, skin and mucous membrane get heavily infiltrated by the bacilli. Lepromatous leprosy patients, however, have been shown to exhibit a very strong tuberculin reaction and are not prone to other infections which are known to inflict immunosuppressed individuals [17, 18]. Several mechanisms have been proposed for the unresponsiveness towards M. leprae in LL patients, but no definite conclusions can yet be drawn [19–22].
Recognition of the peptide–MHC class II complex on the surface of APC by the antigen-specific CD4+ T cells is not sufficient for their optimum activation and secretion of lymphokines, but leads to T cell anergy [12, 23, 24]. In order to avoid antigen-specific tolerance, T cells also need a second signal delivered by costimulatory molecules also expressed on the surface of APC. While considerable literature regarding the antigen processing and delivery of the first signal by the M. leprae-infected macrophages is available, little is known about the functional relevance of costimulatory molecules responsible for suppression in leprosy. Therefore the current study was undertaken to evaluate the significance of costimulatory molecules in immune suppression in different types of leprosy. Further, the effect of Mls on the expression of costimulatory molecules on the lymphocytes obtained from the healthy individuals was also monitored.
Using MoAbs against the costimulatory molecules B7-1, CD28, ICAM-1, LFA-1α, LFA-1β and Mac-1, we showed by FACScan that only the expression of B7-1 and CD28 is crucial in M. leprae-specific interactions of macrophage–T cell. Six major findings have emerged from this study: (i) compared with healthy subjects, the expression of B7-1 and CD28 declined but that of ICAM-1 was increased in untreated BL/LL patients; (ii) no significant change in the expression of costimulatory molecules, B7-1, CD28, ICAM-1, LFA-1α, LFA-1β and Mac-1, was observed in untreated BT or treated BT and BL/LL patients; (iii) down-regulation of B7-1 and CD28 and up-regulation of ICAM-1 and LFA-1α were demonstrated in the lymphocytes of healthy subjects cultured with high but not low concentration of Mls; (iv) up-regulation by PPD of B7-1 expression on the lymphocytes of healthy volunteers cultured with high dose of Mls; (v) the induction by Mls of lymphocyte proliferation was blocked by anti-B7-1 antibodies, but in contrast, PPD-induced growth was checked by anti-B7-1, anti-ICAM-1 and anti-LFA-1α MoAbs; (vi) inhibition of lymphocyte proliferation induced by a high dose of Mls was neutralized by co-incubation with PPD. These data, together, support the proposition that M. leprae impedes the immune response by reducing the costimulatory activity of its host cells.
The reduction in the expression of B7-1 and CD28 molecules in the case of BL/LL patients may be related to the impaired delivery of costimulatory signals to antigen-specific T cells by M. leprae-laden macrophages. It is a well established fact that the engagement of TCR alone, in the absence of costimulatory signals, can direct CD4+ T cells towards apoptosis [25], thus affecting the antigen-specific CMI and, in turn, the outcome of the disease.
We also observed an increased expression of ICAM-1 and LFA-1α in untreated BL/LL patients. Numerous references are available in the literature demonstrating the costimulatory function of ICAM-1 and LFA-1α in T cell activation. The increase in the expression of ICAM-1 and LFA-1α may be responsible for the multiple ligand–receptor interactions that may be necessary for the multiplex of T cell functions [26]. It is demonstrated that only combinations of anti-CD2 and anti-CD28 MoAbs induce IL-1 production by T cells, although separately they are able to induce T cell proliferation and interferon-gamma (IFN-γ) secretion [27]. A similar situation may exist for ICAM-1, where B7–CD28 ligation is necessary to bring out some of the effects of the ICAM-1–LFA-1α interaction. It has in fact been shown that the ICAM-1 signalling pathway is coupled to the B7–CD28 signalling pathway [28]. Finally, it has been demonstrated that as a consequence of this coupling, the responsiveness of CD28 to B7 is increased by costimulation via ICAM-1 [29], which in turn leads to the death of activated T cells. It may be possible that, upon interaction with M. leprae-laden macrophages displaying a low number of B7, the primary antigen-specific T cells in the repertoire become anergized. On the other hand, since ICAM-1 co-stimulates antigen-specific activated T cells, it is also possible that, upon receiving costimulatory signals from infected macrophages displaying a high number of ICAM-1, the activated antigen-specific T cells are led to death [29]. Taken together, our results indicate that multiple mechanisms of T cell unresponsiveness may be operative in leprosy.
The belief that the reduction in the expression of B7-1 in BL/LL patients is mediated by M. leprae antigens has been further substantiated by the FACS analysis showing the down-regulation in the expression of B7-1 and CD28 molecules in lymphocytes of healthy subjects cultured with Mls. Furthermore, we also observed inhibition of the proliferation of lymphocytes cultured with Mls by anti-B7-1 and CD28 MoAbs. Interestingly, the dose of Mls appeared to be critical in modulating the immune response. A high dose (50 μg/ml) of the Mls was inhibitory, whereas a low dose (10 μg/ml) induced the proliferation of lymphocytes of healthy donors and BT patients but not of the BL/LL patients. A high dose of Mls also decreased the levels of B7-1 and CD28 on the lymphocytes obtained from healthy persons. Our observation that a high dose of Mls is associated with inhibition of T cell proliferation and reduced expression of B7-1 and CD28 may also have implications for the pathogenesis of leprosy. It has also been reported [30] that infection of mice with a high dose of Mycobacterium results in DTH anergy. This has led to the hypothesis that the infection load may regulate the immune response [31]. Compared with healthy subjects, no significant change in the expression of B7-1, ICAM-1, CD28, LFA-1α, LFA-1β and Mac-1 was demonstrated in untreated and treated BT patients. This correlates very well with the presence of a low antigenic load and a largely intact CMI at this pole of leprosy.
It has been reported that costimulatory molecules are important for the initiation and maintenance of an immune response against certain diseases [32, 33]. Several intracellular pathogens, such as Mycobacteria, Listeria, Leishmania, Trypanosoma [15, 16, 35–39], have been shown to adopt various strategies for influencing the host immune response at the level of costimulatory signalling. The regulatory effects of costimulatory molecules on T cell activation range from inhibition of T cell proliferation and cytokine production in the case of L. donovani [16] to strong augmentation of Th2 cell proliferation in the case of L. major and Trypanosoma cruzi [37, 39]. An inhibition of the expression of B7-1 on macrophages and failure to deliver a co-stimulus have also been reported in the case of tuberculosis, which is also a mycobacterial disease [15]. Interaction of B7-1 with its counter-receptor CD28 may influence commitment of precursors to Th1 or Th2 lineage and thereby result in a different clinical outcome. Preferential activation of Th1 cells augments CMI and can regulate infection to intracellular pathogens such as M. leprae [39–41].
We also studied the role of PPD in regulating the expression of costimulatory molecules. The PPD-mediated T cell responses of healthy volunteers were abrogated by anti-B7-1, ICAM-1 and LFA-1α MoAbs. Surprisingly, lymphocytes of healthy subjects proliferated vigorously in response to the inhibitory dose of Mls in the presence of PPD. Furthermore, the blocking of lymphoproliferation with anti-B7-1 antibody and restoration of the expression of B7-1 in the presence of PPD indicate that PPD functions as an adjuvant and neutralizes the inhibitory effect of Mls by up-regulating the expression of B7-1. Retention of responsiveness to non-M. leprae antigens, e.g. PPD in BL/LL patients, may be due to enhanced expression of B7-1 by PPD or through ICAM-1/LFA-1 or non-B7-1/CD28 costimulatory pathway [42–44]. It may be pointed out here that PPD may be augmenting the expression of B7-1 by stimulating the secretion of IFN-γ by T cells. It has been reported earlier that expression of B7-1 can be induced by lipopolysaccharide and IFN-γ [45–47].
Since M. leprae is an obligate parasite, resident within macrophages, selective regulation of B7-1 may be viewed as a novel immune evasion strategy adopted by this parasite. The defect in the B7-1 and CD28 signalling pathway may play a critical role in clonal inactivation of M. leprae-specific T cells and in the progression of leprosy. Finally, these observations suggest the possibility of immunotherapy for LL patients by selective regulation of the B7-1 and CD28 costimulatory molecules.
Acknowledgments
The authors are grateful to all the donors for providing their blood for the study, Dr U. Sengupta for helping in obtaining M. leprae sonicate from IMMLEP, WHO Bank, Professors A. L. Brown, A. Mehmood and Dr R. Kishore for helping in preparation of the manuscript. We are also thankful to Mr Dinesh Verma for secretarial assistance.
References
- 1.Boom BR, Mehra V. Immunological unresponsiveness in leprosy. Immunol Rev. 1984;80:5–28. doi: 10.1111/j.1600-065x.1984.tb00493.x. [DOI] [PubMed] [Google Scholar]
- 2.Pancholi P, Mirza A, Bhardwaj N, Steinman RM. Sequestration from immune CD4+ T cells of mycobacterial growing in human macrophages. Science. 1993;260:984–6. doi: 10.1126/science.8098550. [DOI] [PubMed] [Google Scholar]
- 3.Clevers H, Alarcon B, Wileman T, Terhorst C. The T cell receptor/CD3 complex: a dynamic protein ensemble. Annu Rev Immunol. 1988;6:629–31. doi: 10.1146/annurev.iy.06.040188.003213. [DOI] [PubMed] [Google Scholar]
- 4.Damle NK, Klussman K, Linsley PS, Aruffo A. Differential costimulatory effects of adhesion molecules B7, ICAM-1, LFA-3 and VCAM-1 on resting and antigen-primed CD4+T lymphocytes. J Immunol. 1992;148:1989–92. [PubMed] [Google Scholar]
- 5.Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425–34. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 6.Marlin SD, Springer TA. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1) Cell. 1987;51:813–9. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- 7.van Seventer GA, Shimizu Y, Horgan KJ, Shaw S. The LFA-1 ligand and ICAM-1 provides an important costimulatory signal for T cell receptor-mediated activation of resting T cells. J Immunol. 1990;144:4579–86. [PubMed] [Google Scholar]
- 8.de Fougerolles AR, Springer TA. Intercellular adhesion molecule 3, a third adhesion counter-receptor for lymphocyte function-associated molecule 1 on resting lymphocytes. J Exp Med. 1992;175:185–90. doi: 10.1084/jem.175.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bluestone JA. New perspective of CD28-B7-mediated T cell costimulation. Immunity. 1995;2:555–9. doi: 10.1016/1074-7613(95)90000-4. [DOI] [PubMed] [Google Scholar]
- 10.Linsley PS, Brady W, Grosmaire L, Aruffo A, Damle NK, Ledbetter JA. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991;173:721–30. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lieu Y, Janway Jr CA. Microbial induction of co-stimulatory activity for CD4 T-cell growth. Int Immunol. 1991;3:323–32. doi: 10.1093/intimm/3.4.323. [DOI] [PubMed] [Google Scholar]
- 12.Mueller DL, Jenkins MK, Schwartz Clonal expansion versus clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:455–80. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- 13.Kaye PM, Rogers NJ, Curry AJ, Scott JC. Deficient expression of co-stimulatory molecules on Leishmania-infected macrophages. Eur J Immunol. 1994;24:2850–4. doi: 10.1002/eji.1830241140. [DOI] [PubMed] [Google Scholar]
- 14.Corry DB, Reiner SL, Linsley PS, Locksley RM. Differential effects of blockade of CD28-B7 on the development of Th1 or Th2 effector cells in experimental Leishmaniasis. J Immunol. 1994;153:4142–8. [PubMed] [Google Scholar]
- 15.Saha B, Das G, Vohra H, Ganguly NK, Mishra GC. Macrophage–T cell interaction in experimental mycobacterial infection. Selective regulation of costimulatory molecules of Mycobacterium-infected macrophages and its implication in the suppression of cell-mediated immune response. Eur J Immunol. 1994;24:2618–24. doi: 10.1002/eji.1830241108. [DOI] [PubMed] [Google Scholar]
- 16.Saha B, Das G, Vohra H, Ganguly NK, Mishra GC. Macrophage–T cell interaction in experimental visceral leishmaniasis: failure to express costimulatory molecules on leishmania-infected macrophages and its implication in the suppression of cell-mediated immunity. Eur J Immunol. 1995;25:2492–8. doi: 10.1002/eji.1830250913. [DOI] [PubMed] [Google Scholar]
- 17.Ridley DS. Histological classification and the immunological spectrum of leprosy. Bull WHO. 1974;51:451–65. [PMC free article] [PubMed] [Google Scholar]
- 18.Ridley DS, Jopling WH. Classification of leprosy according to immunity. A five-group system. Int J Lepr. 1966;34:255–73. [PubMed] [Google Scholar]
- 19.Salgame PR, Birdi TJ, Mahadevan PR, Antia NH. Role of macrophages in defective CMI in lepromatous leprosy. I. Factors affecting protein synthesis and lymphocyte transformation. Int J Lepr. 1980;48:171–82. [PubMed] [Google Scholar]
- 20.Satish M, Bhutani LK, Sharma AK, Nath I. Monocyte-derived soluble suppressor factor(s) in patients with leprosy. Infect Immun. 1983;42:890–6. doi: 10.1128/iai.42.3.890-899.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horwitz MA, Levis WR, Cohn ZA. Defective production of monocyte-activating cytokines in lepromatous leprosy. J Exp Med. 1984;159:666–78. doi: 10.1084/jem.159.3.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehra V, Convit J, Rubinstein A, Bloom BR. Activated suppressor T cells in leprosy. J Immunol. 1982;129:1946–51. [PubMed] [Google Scholar]
- 23.Janeway Jr CA, Bottomly K. Signals and signs for lymphocyte responses. Cell. 1994;76:275–82. doi: 10.1016/0092-8674(94)90335-2. [DOI] [PubMed] [Google Scholar]
- 24.Jenkins MK. The ups and downs of T cell costimulations. Immunity. 1994;1:443–9. doi: 10.1016/1074-7613(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Janeway CA. Interferon γ plays a critical role in induced cell death of effector T cell: a possible third mechanism of self-tolerance. J Exp Med. 1990;172:1735–9. doi: 10.1084/jem.172.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Seventer GA, Shimiza Y, Shaw S. Role of multiple accessory molecules in T cell activation. Curr Opin Immunol. 1991;3:294–303. doi: 10.1016/0952-7915(91)90027-x. [DOI] [PubMed] [Google Scholar]
- 27.Cerdan C, Martin Y, Braily H, Courcoul M. IL-1α is produced by T lymphocytes activated via the CD2 plus CD28 pathways. J Immunol. 1991;146:560–4. [PubMed] [Google Scholar]
- 28.Damle NK, Klussman K, Linsley PS, Arruffo A, Ledbetter JA. Costimulation with integrin ligands intercellular adhesion molecule-1 or vascular cell adhesion molecule-1 augments activation-induced death of antigen-specific CD4+T lymphocytes. J Immunol. 1993;151:2368–79. [PubMed] [Google Scholar]
- 29.Damle NK, Klussman K, Linsley PS, Aruffo A, Ledbetter JA. Differential regulatory effects of intercellular adhesion molecule-1 on costimulation by the CD28 counter-receptor B7. J Immunol. 1992;149:2541–8. [PubMed] [Google Scholar]
- 30.Orme IM, Collins FM. Passive transfer of tuberculosis sensitivity from anergic mice. Infect Immun. 1984;46:850–3. doi: 10.1128/iai.46.3.850-853.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orme IM, Anderson P, Boom WH. T cell response to Mycobacterium tuberculosis. J Infect Dis. 1993;167:1481–97. doi: 10.1093/infdis/167.6.1481. [DOI] [PubMed] [Google Scholar]
- 32.Springer TA, Dustin ML, Kishimoto TK, Marlin SD. The lymphocyte function associated LFA-1, CD2 and LFA-3 molecules: cell adhesion receptors of the immune system. Ann Rev Immunol. 1987;5:223–52. doi: 10.1146/annurev.iy.05.040187.001255. [DOI] [PubMed] [Google Scholar]
- 33.Anderson DC, Schmalstieg FC, Finegold MJ, et al. The severe and moderate phenotypes of heritable Mac-1, LFA-1 deficiency; their quantitative deficiation and relation to leukocyte dysfunction and clinical features. J Infect Dis. 1985;152:668–89. doi: 10.1093/infdis/152.4.668. [DOI] [PubMed] [Google Scholar]
- 34.Sullivan L, Sano S, Pirmez C, et al. Expression of adhesion molecules in leprosy lesions. Infect Immun. 1991;59:4154–60. doi: 10.1128/iai.59.11.4154-4160.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramirez GML, Rom WN, Ciotoli C, Talbot A, Martiniuk F, Cronstein B, Reibman J. Mycobacterium tuberculosis alters expression of adhesion molecules on monocytic cells. Infect Immun. 1994;62:2515–20. doi: 10.1128/iai.62.6.2515-2520.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chakkalath HR, Titus RG. Leishmania major parasitized macrophages augment Th2-type activation. J Immunol. 1994;153:4378–87. [PubMed] [Google Scholar]
- 37.Fruth U, Solioz N, Louis JA. Leishmania major interferes with antigen presentation by infected macrophages. J Immunol. 1993;150:1857–64. [PubMed] [Google Scholar]
- 38.Frosch S, Kuntzlin D, Fleischer B. Infection with Trypanosoma cruzi selectively upregulates B7-2 molecules on macrophages and enhances their costimulatory activity. Infect Immun. 1997;65:971–7. doi: 10.1128/iai.65.3.971-977.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuchroo VK, Das MP, Brown JA, et al. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707–18. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 40.Bottomly K. A functional dichotomy in CD4+ lymphocytes. Immunol Today. 1988;9:268–74. doi: 10.1016/0167-5699(88)91308-4. [DOI] [PubMed] [Google Scholar]
- 41.Scot P, Kaufmann SHE. The role of T-cell subsets and cytokines in the regulation of infection. Immunol Today. 1991;12:346–8. doi: 10.1016/0167-5699(91)90063-Y. [DOI] [PubMed] [Google Scholar]
- 42.Altmann DM, Hogg N, Trowsdale J, Wilkinson D. Cotransfection of ICAM-1 and HLA-DR reconstitutes human antigen-presenting cell function in mouse L cells. Nature. 1989;338:512–4. doi: 10.1038/338512a0. [DOI] [PubMed] [Google Scholar]
- 43.St. Pierre Y, Watts TH. Characterization of the signalling function of MHC class II molecules during antigen presentation by B cells. J Immunol. 1991;147:2875–82. [PubMed] [Google Scholar]
- 44.Dang LH, Michalek MT, Takei F, Benaceraff B, Rock KL. Role of ICAM-1 in antigen presentation demonstrated by ICAM-1 deficient mutants. J Immunol. 1990;144:4082–91. [PubMed] [Google Scholar]
- 45.Freedman AS, Freeman G, Rhynhart K, Nadler LM. Selective induction of B7/BB-1 on IFN-γ stimulated monocytes: a potential mechanism for amplification of T cell activation through the CD28 pathway. Cell Immunol. 1991;137:429–37. doi: 10.1016/0008-8749(91)90091-o. [DOI] [PubMed] [Google Scholar]
- 46.Dockrell HM, Stoker NG, Lee M, et al. T-cell recognition of the 18-kilodalton antigen of Mycobacterium leprae. Infect Immun. 1989;57:1979–83. doi: 10.1128/iai.57.7.1979-1983.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jurcevic S, Hills A, Pasvol Davidson RN, Ivanyi J, Wilkinson RJ. T cell responses to a mixture of Mycobacterium tuberculosis peptides with complementary HLA-DR binding profiles. Clin Exp Immunol. 1996;105:416–21. doi: 10.1046/j.1365-2249.1996.d01-791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


