Abstract
IgE antibodies play a crucial role in allergic type I reactions. Only IL-4 and IL-13 are able to induce an immunoglobulin isotype switch to IgE in B cells. A major question is to what extent these cytokines contribute to the production of IgE in allergic patients. To address this question we used an in vitro culture system in which the production of IgE is dependent on endogenously produced IL-4 and IL-13. In cultures of purified T and B cells from allergic asthma patients and non-atopic controls, T cells were polyclonally stimulated to obtain IL-4, IL-13 and subsequently IgE secretion. The absolute amount of IgE produced was not significantly different between patients and controls. When neutralizing IL-4 antibodies were included during culture, the production of IgE was dramatically inhibited in both patients and controls (production of IgE was reduced to 12%). However, neutralization of IL-13 led to a significantly stronger inhibition of IgE production in the patient group: production of IgE was reduced to 23 ± 3% versus50 ± 10% in the control group. Corresponding with these results, we also observed a higher production of IL-13 by the patients, while the production of IL-4 was not significantly different. A more detailed analysis of the production of IL-13 revealed that patients' T cells were less sensitive to a negative signal controlling IL-13 production. Our results indicate that, at least in vitro, IgE production in allergic asthma patients is more dependent on IL-13 than in non-atopics, due to enhanced IL-13 production and to enhanced IgE production in response to IL-13.
Keywords: IL-4, IL-13, IgE, allergy, asthma
INTRODUCTION
Allergic asthma is a multifactorial disease, influenced by genetic and environmental factors, and is characterized by bronchial hyperresponsiveness, the presence of IgE antibodies to inhalant allergens and often also by enhanced total serum IgE levels. A switch recombination of antibodies to IgE requires two signals from activated T cells: the expression of the ligand for CD40 and the secretion of IL-4 or IL-13. Both IL-4 and IL-13, independently of each other, are able to induce IgE antibody production [1–3]. Other shared activities of IL-4 and IL-13 include induction of CD23 expression on B cells [1, 3] and monocytes [4, 5] and the inhibition of cytokine secretion by lipopolysaccharide (LPS)-activated monocytes, IL-1α, IL-1β, IL-6, IL-8, IL-10, IL-12, tumour necrosis factor-alpha (TNF-α), interferon-alpha (IFN-α), MIP-1α, granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF) [4, 6], whereas IL-1Ra is up-regulated. However, while IL-4 is also a growth factor for T cells, IL-13 has no such activity [7, 8], although both IL-13 and IL-4 are able to induce stat-6 activation in peripheral blood T cells [9]. Stat-6 activation is required for development of Th2 responses, as shown in stat-6-deficient mice [10], although IL-13 failed to induce Th2 differentiation in human naive T cells [11]. The overlapping activities of IL-4 and IL-13 are reflected in the shared use of receptor chain(s) [12]: the IL-4 receptor α-chain is also used by IL-13 [13]. In addition, two human IL-13 binding subunits (IL-13Rα1 and IL-13Rα2) have recently been described, which may also be used by IL-4 [14–17]. T cells only express the IL-13Rα1 chain, but this expression is rapidly lost after activation [17], which may explain the lack of prolonged biological activity of IL-13 on T cells.
IL-13 is produced predominantly by T cells; in addition, human basophils and mast cells are also able to secrete IL-13 [18, 19]. In contrast to IL-4, which is produced by peripheral blood CD4+ memory T cells, IL-13 is produced by CD4+ as well as CD8+ T cells and by both naive and memory T cells [7, 20–22]. The regulation of production of IL-13 is totally different from IL-4. This is illustrated by the fact that stimulation of T cells with anti-CD28 and phorbol myristate acetate (PMA) results in optimal induction of IL-13, with almost no IL-4 secretion [22]. The local tissue expression of IL-13 has been shown in nasal mucosa of allergic rhinitis patients [23] and in bronchoalveolar lavage fluid from allergic asthma patients after pulmonary allergen challenge [24], whereas normal controls failed to express IL-13. In peripheral blood mononuclear cells (PBMC) from atopic dermatitis patients increased spontaneous IL-13, but not IL-4 mRNA has been found [25]. Also in skin lesions from atopic dermatitis patients an increased number of IL-13 mRNA-positive cells was identified [26]. The role of IL-13 in the induction of IgE has been investigated in nephrotic syndrome patients with enhanced total serum IgE. The spontaneous in vitro production of IgE was shown to be dependent on IL-13 [27], whereas in the same study atopic dermatitis patients produced IgE to spontaneously secreted IL-4. Little is known about the relative contribution of IL-4 and IL-13 to the production of IgE in allergic asthma patients. The IgE-inducing capacity of T cell supernatants from patients with asthma or allergic rhinitis (n = 5) could not be completely inhibited by anti-IL-4 [28]. We previously reported that the in vitro production of IgE in allergic asthma/rhinitis patients is not related to the production of IL-4, whereas normal controls showed a good correlation between these parameters [29]. In addition, we found no abnormalities in the production of IL-4 (and IFN-γ) in these patients. We therefore hypothesized that in these patients the production of IgE is more dependent on IL-13 than in non-atopic controls.
MATERIALS AND METHODS
Patients and control subjects
Fifteen allergic asthma patients, four females and 11 males (mean age 35 years, range 21–54 years) were recruited from the Outpatient Department of Pulmonology. Asthma was defined according to the criteria of the American Thoracic Society [30]. Patients with asthma had a history of paroxysms of dyspnoea, and coughing, but were studied during a stable phase of the disease. The median forced expiratory volume in 1 s (FEV1) was 87% of predicted value (range 57–114%). The median provocative concentration causing a 20% fall in FEV1 (PC20) was 2·4 mg histamine/ml (range 0·01–16 mg/ml, n = 11). Histamine threshold was not performed in patients with spirometry-induced bronchoconstriction and predicted percent FEV1 < 70% at the test day (n = 4). Patient selection was based on a positive skin prick test and a positive radioallergosorbent test (RAST) for at least one inhalant allergen and a total IgE of > 500 U/ml established during the previous 3 years. Geometric mean of serum total IgE was 1068 U/ml. Excluded were patients treated with systemic corticosteroids, antihistamines and theophylline, and patients who had received hyposensitization therapy. Most patients received low-dose inhaled corticosteroids (< 800 μg) and inhaled beta-2-agonists. No medication was taken 12 h before blood sampling. The study was approved by the local medical ethics committee, and written consent was given by all patients. Healthy subjects without symptoms or a history of allergy, six females and nine males (mean age 34 years, range 24–51 years), without specific IgE for common inhalant allergens (RAST-negative) and serum IgE levels < 40 U/ml were selected as controls.
Cell isolation
Mononuclear cells from patients and controls were isolated from peripheral blood by a density separation over Percoll (Pharmacia Fine Chemicals AB, Uppsala, Sweden) with a specific density of 1·078 kg/l. T and B cells were isolated from these cells by negative depletion with magnetic beads (Dynabeads M450; Dynal, Oslo, Norway), after labelling of the cells with MoAbs: natural killer (NK) cells and monocytes were removed using CLB-FcR gran1 (anti-CD16) and CLB-CD14 (CLB, Amsterdam, The Netherlands), respectively. Isolated cells contained < 1% CD14+ or CD16+ cells.
Cell cultures
For IgE, IL-13 and IL-4 secretion, isolated T and B cells were cultured in Iscove's modified Dulbecco's medium (IMDM) at 5000 cells/200 μl well, with 10% fetal calf serum (FCS) supplemented with penicillin 100 U/ml, streptomycin 100 μg/ml, 5 × 10−5 m 2-mercaptoethanol (2-ME) and 20 μg/ml human transferrin (Behringwerke, Marburg, Germany) in round-bottomed 200-μl wells (Greiner, Nuertingen, Germany). Anti-CD2 antibodies (CLB-T11.1/1 and CLB-T11.2/1) were used at 1 μg/ml. Human rIL-2, Escherichia coli-derived (a kind gift of J. Farrar, Hoffman-La Roche, Nutley, NJ) was used at 100 U/ml. Human rIL-4, E. coli-derived (a kind gift from M. Schreier, Sandoz, Basel, Switzerland) was used at 20 ng/ml. Human rIL-13 (Prepotech, London, UK) was used at 50 ng/ml. For neutralization of IL-4, MoAb 5B5 [31] was used and for IL-13 neutralization CLB-IL13/2 was used, both at 5 μg/ml. After 8 days of culture, supernatants were harvested and assayed for IgE and cytokine contents. To study detailed regulation of IL-13 production, the cells were cultured at 40 000/200 μl well and additionally stimulated with anti-CD28 (CLB-CD28/1) at 5 μg/ml and PMA (Sigma, St Louis, MO) at 1 ng/ml.
Cytokine and IgE measurements
IL-13 ELISA
The ELISA was performed as described before [22]. Briefly, MoAb CLB-IL13/1 was coated overnight at 1 μg/ml in 0·1 m carbonate buffer pH 9·6 on flat-bottomed microtitre plates (Nunc, Roskilde, Maxisorb). All subsequent incubations were in 100-μl volumes at room temperature. The plates were washed twice with PBS, 0·02% (v/v) Tween 20 and incubated for 30 min with PBS, containing 2% (v/v) pasteurized and homogenized cow's milk as a blocking step. After washing, biotinylated purified MoAb CLB-IL13/2 was added (final concentration 0·5 μg/ml) together with IL-13 containing samples diluted in high performance ELISA (HPE) buffer (CLB) for 1·5 h. Thereafter the plates were washed five times and incubated with poly-streptavidin-horseradish peroxidase (poly-HRP; CLB), 1/10 000 diluted (according to the manufacturer's instructions) in PBS containing 2% (v/v) cow's milk for 0·5 h, washed and developed with a solution of 100 μg/ml of 3,5,3′,5′-tetramethylbenzidine (Merck, Darmstadt, Germany) with 0·003% (v/v) H2O2 in 0·11 m sodium acetate pH 5·5 (100 μl/well). The reaction was stopped by adding an equal volume of 2 m H2SO4 to the wells. Plates were read at 450 nm in a Titertek Multiskan reader. Background absorbance at 540 nm was subtracted. rhIL-13 (Prepotech; calibrated to the WHO International Standard Preparation, Ampoule 94/622; NIBSC, South Mimms, UK) was used as a standard. The detection limit was 0·5 pg/ml.
IL-4 ELISA
The assay was performed identically to the IL-13 ELISA and has been described before [31]. For coating MoAb CLB-IL4/5 was used at 1 μg/ml and for detection biotinylated MoAb CLB-IL4/1 was used at 1 μg/ml. rhIL-4, a kind gift from M. Schreier, calibrated to the WHO International Standard Preparation (Ampoule 88/656, NIBSC), was used as a standard; the detection limit was 1 pg/ml.
IgE ELISA
The ELISA was performed identically to the IL-13 ELISA. Plates were coated with mouse anti-human IgE (MoAb 3B8-B1; CLB) at 1 μg/ml. For detection biotinylated mouse anti-human-IgE (CLB-MH25) was used at 0·25 μg/ml. The results obtained with this ELISA were identical to our previously described radioimmunoassay (RIA) for IgE measurements [29]. Normal human serum IgE, calibrated to the 2nd WHO International Reference Preparation of Human IgE (ampoule 75/502; NIBSC) was used as a standard. As in previous studies, serum IgE levels are expressed in IU/ml, while culture supernatants are expressed in ng/ml, using the same standard (1 IU= 2·4 ng [32]). The detection limit was 10 pg/ml (corresponding to 4 × 10−3 IU/ml).
Statistical analysis
Comparison of cytokine and IgE production between patients and controls was performed using the Mann–Whitney test (one-tailed). Correlation coefficients were calculated by the Spearman rank test.
RESULTS
Allergic asthma patients produce more IgE in response to exogenous IL-13
IgE production by B cells is dependent on stimulation through membrane-expressed CD40, and the soluble factors IL-4 or IL-13. To study these T and B cell functions in allergic asthma patients, we used PBMC depleted for CD14+ and CD16+ cells, to rule out the influence of monocytes and NK cells. After depletion, the number of T and B cells did not differ between patients and controls (78% and 80% of the cells were CD3+, respectively, and 17% of the cells were CD19+ in both groups, not shown) After activation with anti-CD2 and IL-2, T cells expressed the ligand for CD40 (not shown) and secreted IL-4 and IL-13, which resulted in the induction of IgE synthesis (Figs 1 and 4). Costimulation with anti-CD3 instead of anti-CD2 gave essentially the same results, but cytokine and IgE production was less efficient during CD3 stimulation ([33] and data not shown). Therefore we decided to use anti-CD2 as a costimulus in all experiments. Without any further additions, the production of IgE was approximately two-fold higher in the allergic asthma group (Fig. 1, patients produced 15·2 ng IgE/ml, versus6·2 ng IgE/ml by controls), but this difference did not reach statistical significance (P = 0·08). Graded amounts of IL-4, IL-13, anti-IL-4 and anti-IL-13 were used to determine saturating concentrations of these cytokines and antibodies to induce or inhibit IgE, respectively (not shown). They were subsequently used to analyse maximum production of IgE in response to exogenous IL-4, in the presence of neutralizing monoclonal anti-IL-13 and maximum production of IgE to exogenous IL-13 in the presence of neutralizing monoclonal anti-IL-4 (Fig. 1). Maximum production of IgE in response to exogenous IL-13 was higher in patients: while patients produced 12·2 ng IgE/ml in response to IL-13 (when IL-4 was neutralized), controls produced 5·7 ng IgE/ml (P = 0·03). The maximum IgE response to exogenous IL-4 (when IL-13 was neutralized) was not different between patients and controls. The same was true for the combination of exogenous IL-4 + IL-13. The IgE response obtained in the presence of exogenous IL-4 was not different from the IgE response to the combination of exogenous IL-4 and IL-13. Therefore, saturating amounts of IL-13 and IL-4 did not act additively or synergistically in the induction of IgE in either patients or controls.
Fig. 1.
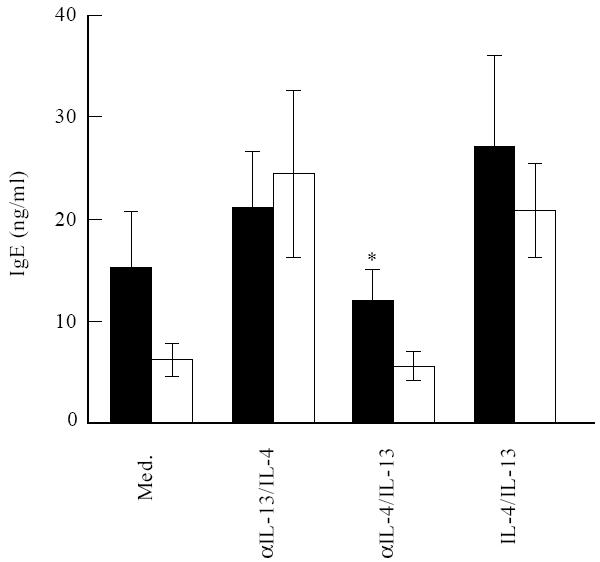
Allergic asthma patients produce more IgE to exogenous IL-13. Freshly isolated T and B cells from 15 patients and 15 control individuals were stimulated with anti-CD2 and IL-2 in the absence or presence of combinations of IL-4 (20 ng/ml), IL-13 (50 ng/ml), anti-IL-4 (5 μg/ml) and anti-IL-13 (5 μg/ml), as indicated. After 8 days of culture IgE levels were measured. Results are expressed as mean ± s.e.m. *P = 0.03. ▪, Patients; □, controls.
Fig. 4.
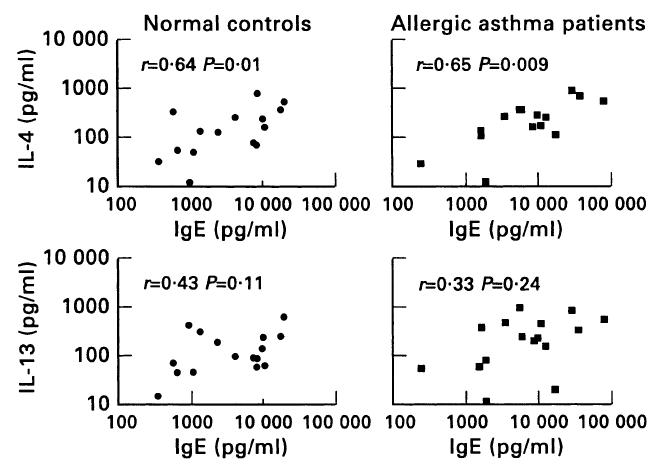
Correlation of IL-4 and IL-13 with IgE production in allergic asthma patients and normal controls. Cells were stimulated as in Figs 1 and 3. Each point represents an individual patient or control individual as indicated.
IgE production is more dependent on endogenous IL-13 in allergic asthma patients than in normal controls
The experimental design of this study was based on the hypothesis that the production of IgE by patients with allergic asthma is more dependent on IL-13 than IgE production by healthy controls. To study the relative contribution of endogenous IL-4 and IL-13 to the synthesis of IgE, we blocked the activity of IL-13 or IL-4 or both by neutralizing MoAb (Fig. 2). Neutralization of IL-4 resulted in an equal inhibition of IgE production in patients and controls (production of IgE was reduced to 12%). In allergic patients the production of IgE was more dependent on IL-13 than IgE production by controls: only 23% of the IgE response was observed after neutralization of IL-13 in allergic asthma patients, whereas 50% was still produced in the normal control group (P = 0·02). When the activity of both IL-13 and IL-4 was neutralized, this resulted in an almost complete inhibition of IgE production in both patients and controls (the production of IgE was reduced to 10% and 5%, respectively).
Fig. 2.
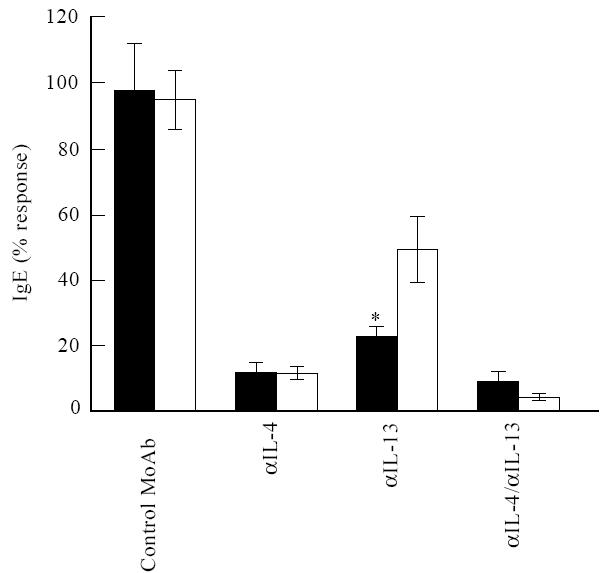
Role of endogenous IL-4 and IL-13 in the induction of IgE. Freshly isolated T and B cells from 14 patients and 14 control individuals were stimulated with anti-CD2 and IL-2 in the absence or presence of neutralizing IL-4 (5 μg/ml) and IL-13 MoAb (5 μg/ml) or both. IgE production was measured after 8 days of culture. Results are expressed as mean ± s.e.m. *P =0.02. ▪, Patients; □, controls.
IL-13 production is enhanced in allergic asthma patients
We next investigated whether the stronger dependence of IgE production in patients on IL-13 was reflected in a higher production of IL-13 in these cultures. This was indeed the case, as patients produced 385 pg IL-13/ml, whereas controls produced 175 pg/ml (P = 0·03, Fig. 3). The production of IL-4 was higher in the patient group (334 pg IL-4/ml), but was not significantly different from the control group (226 pg IL-4/ml). Corresponding to the fact that IL-4 contributed most to the production of IgE, we found a good correlation between the production of IgE and IL-4 secretion in both the patient (r = 0·65, P = 0·009) and the control group (r = 0·64, P = 0·01). Correlation of IgE with IL-13 secretion was not statistically significant (Fig. 4).
Fig. 3.
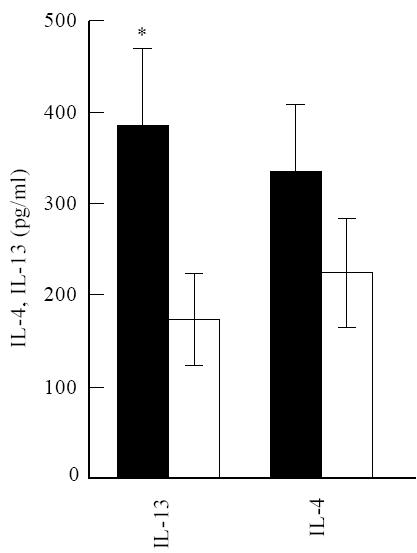
IL-13 production is enhanced by allergic asthma patients. The same conditions were used as in Fig. 1. The mean production of IL-4 and IL-13 ± s.e.m. is shown for 15 patients and controls. *P =0.03. ▪, Patients; □, controls.
IL-13 production is differentially regulated in allergic asthma patients
IL-13 production in the allergic asthma patients was further studied by using different stimulation conditions. A signal provided by CD2 or CD3 stimulation comprises a positive as well as a negative component for the induction of IL-13 secretion [22]. The positive signal might very well be protein kinase C (PKC) activation, while the negative signal can be mimicked by a calcium ionophore (data not shown) and is reversed by cyclosporin A (CsA) [22]. The strength of the negative signal can be examined. For this purpose T cells need to be stimulated for optimal induction of IL-13 secretion; this is established by stimulation with anti-CD28 and PMA ([22] and Fig. 5). Additional stimulation with anti-CD3 or anti-CD2 provides the negative component for IL-13 production. Because production of IL-13 after stimulation of T cells with anti-CD2 and IL-2 was higher in allergic patients, we next analysed whether the negative signal provided by CD2 stimulation was less pronounced in the patient group. For this purpose T cells were stimulated with anti-CD28 and PMA to obtain optimal IL-13 production. To examine the inhibition of IL-13 production by a T cell receptor signal, a calcium-inducing signal was provided by CD2 antibodies. The percentage response of IL-13 production after addition of the CD2 stimulus was higher in the patient group (Fig. 6, P = 0·003), indicating that patients' T cells were less sensitive to this negative signal.
Fig. 5.
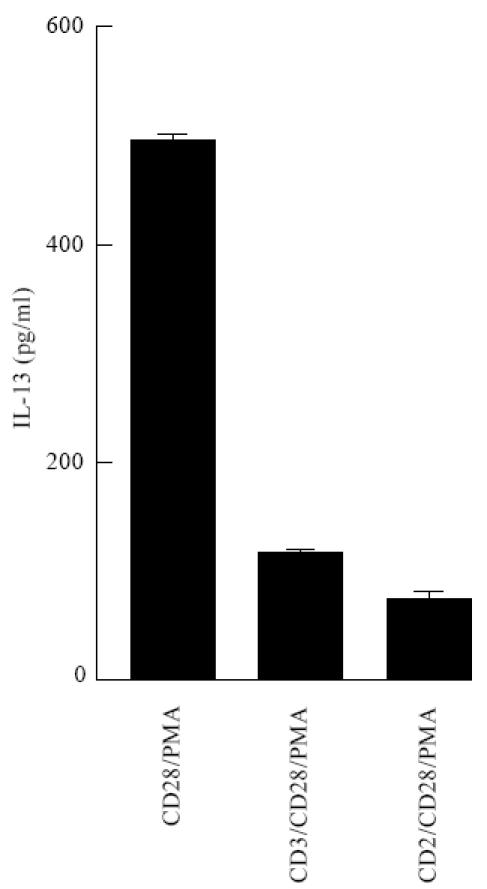
Optimal IL-13 production induced by anti-CD28 and phorbol myristate acetate (PMA) stimulation is inhibited by CD2 or CD3 costimulation. Purified T cells from a non-allergic donor were stimulated as indicated at 40 000 cells/200 μl wells. After 3 days of culture, supernatants were assayed for IL-13 content. One representative experiment out of three is shown.
Fig. 6.
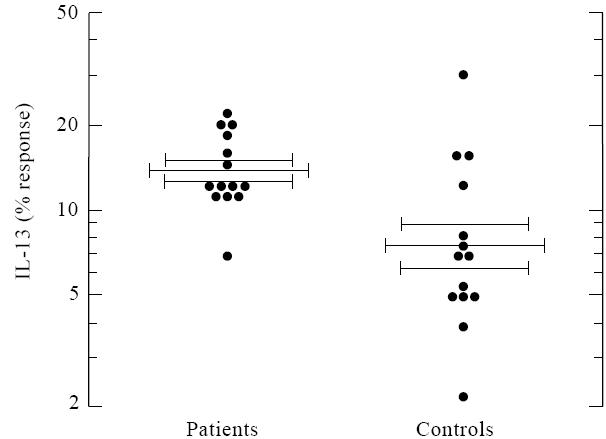
Inhibition of optimal anti-CD28/phorbol myristate acetate (PMA)-induced IL-13 production by costimulation with anti-CD2. Patients are less sensitive to inhibition of IL-13 production by CD2 stimulation. T cells from patients or controls were stimulated with anti-CD28 and PMA, with or without additional anti-CD2. The percent response of IL-13 production was calculated after addition of anti-CD2. Results are expressed as the mean percent IL-13 response ± s.e.m. *P =0.005.
DISCUSSION
In this study we chose to analyse general T and B cell function in allergic asthma patients, and not allergen-induced T cell differentiation, which is already known to lead to Th2-biased responses [34, 35] or allergen-specific IgE-producing B cells which are no longer influenced by IL-4 or IL-13 [36]. This was achieved by culturing very low cell concentrations, thus reducing the influence of allergen-specific T cells or already switched B cells. Frequencies of allergen-specific T cells are low and have been found from 0·8% [37] to < 0·1% [38]. The frequency of already switched B cells is also very low—even in hyper IgE patients only 0·2% of B cells secrete IgE [39]. Thus is it unlikely that at the low cell concentrations we used, allergen-specific T or B cells were of significant influence. Indeed, no spontaneous production of cytokines or IgE was found in this situation (not shown). In addition, the production of IgE in polyclonally stimulated cultures was dependent on endogenously produced IL-4 and IL-13, allowing us to study the relative contribution of these two cytokines to the production of IgE. The production of IgE was shown to be similarly and almost completely inhibited by IL-4-neutralizing antibodies in patients and controls. Surprisingly, also after neutralization of IL-13 a major inhibition of IgE production was found (77% and 50% inhibition in patients and controls, respectively). In agreement with others [1], we found that optimal concentrations of IL-4 and IL-13 acted neither synergistically nor additively in the induction of IgE. Possibly, at limiting levels of endogenous IL-4 and IL-13, as is to be expected in the cultures, there were additive and perhaps even synergistic effects between IL-4 and IL-13 at the B cell level. Alternatively, neutralization of IL-4 may lead to reduced production of IL-13 [20], although IL-13 has no IL-4-promoting activity [11].
Production of IgE and IL-4 were correlated in both patients and controls. In a previous study we found no correlation between the production of IgE and IL-4 in atopic patients, while normal donors showed a good correlation between these parameters [29]. This could be the result of an extremely heterogeneous patient population in the earlier study, consisting of four allergic asthma, nine allergic rhinitis (with or without allergic asthma), four allergic bronchopulmonary aspergillosis patients and two atopic dermatitis patients, of which one also showed symptoms of asthma. These are all clinically distinct entities. In the current study a far more homogeneous population was studied, consisting of allergic asthma patients only. Also, in the previous study nine out of 19 patients showed serum IgE levels below 500 IU/ml, while of the present patient population all patients showed serum IgE levels > 500 IU/ml. Therefore different mechanisms may lead to disturbed synthesis of IgE in the different patient groups. In addition, NK cells could have influenced the results of the previous study, while in the present study T and B cells, depleted for NK cells (and monocytes), were used.
The most striking observation was that IgE production in the allergic asthma group was more dependent on endogenously produced IL-13 than in the control group. This can be explained by the fact that patients showed both an enhanced production of IgE to exogenous IL-13 and an enhanced production of IL-13, indicating an increased sensitivity to IL-13 as well as a greater availability of IL-13. The enhanced production of IL-13 is in agreement with the reported enhanced expression of IL-13 mRNA [40] and enhanced IL-13 protein production [41] (although not significantly different from the non-atopic controls) in stimulated PBMC from allergic asthma/rhinitis patients. Also in these reports no difference was found for IL-4 protein production.
We previously analysed the regulation of IL-13 in normal individuals: IL-13 turned out to be an unusual cytokine in terms of its regulation of production [22]. Optimal IL-13 production occurs after stimulation of T cells with anti-CD28 and PMA. Additional stimulation of the T cell receptor (TCR) complex by CD3 or CD2 antibodies results in a reduced production of IL-13. This reduction can be overcome by an inhibitor of calcium-dependent responses, CsA. However, stimulation through CD2 also provides a positive signal, because anti-CD2 in combination with IL-2 or anti-CD28 induces IL-13 secretion, whereas IL-2 or anti-CD28 alone fail to induce IL-13 secretion [22]. Here we show that allergic asthma patients are less sensitive to the inhibitory signal provided by CD2 stimulation.
Interestingly, a linkage of bronchial hyperreactivity and elevated IgE levels in asthma families with polymorphic markers on chromosome 5q31-33 has been observed [42–44]. This chromosomal region contains a number of cytokine genes known to affect IgE production and pulmonary inflammation, such as IL-12, IL-4, IL-5, IL-3, GM-CSF, IL-9 and IL-13 [45, 46]. This finding, together with the aberrant regulation of IL-13 production in allergic asthma patients, makes IL-13 an interesting candidate gene that may contribute to the inheritance of allergic asthma. In addition to its IgE-inducing capacity, IL-13 up-regulates vascular cell adhesion molecule-1 (VCAM-1) expression on endothelial cells, which induces the adherence of eosinophils [47]. Therefore IL-13 may also contribute to pulmonary inflammation by recruiting eosinophils.
Acknowledgments
This work was financially supported by ‘The Netherlands Asthma Foundation’.
References
- 1.Punnonen J, Aversa G, Cocks BG, et al. Interleukin 13 induces interleukin 4-independent IgG4 and IgE synthesis and CD23 expression by human B cells. Proc Natl Acad Sci USA. 1993;90:3730–4. doi: 10.1073/pnas.90.8.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cocks BG, De Waal Malefyt R, Galizzi JP, et al. IL-13 induces proliferation and differentation of human B cells activated by the CD40 ligand. Int Immunol. 1993;5:657–63. doi: 10.1093/intimm/5.6.657. [DOI] [PubMed] [Google Scholar]
- 3.Defrance T, Carayon P, Billian G, et al. Interleukin 13 is a B cell stimulating factor. J Exp Med. 1994;179:135–43. doi: 10.1084/jem.179.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Waal Malefyt R, Figdor CG, Huijbens R, et al. Effects of IL-13 on phenotype, cytokine production, and cytotoxic function of human monocytes. J Immunol. 1993;151:6370–81. [PubMed] [Google Scholar]
- 5.McKenzie ANJ, Culpepper JA, De Waal Malefyt R, et al. Interleukin 13, a T-cell-derived cytokine that regulates human monocyte and B-cell function. Proc Natl Acad Sci USA. 1993;90:3735–9. doi: 10.1073/pnas.90.8.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minty A, Chalon P, Derocq J-M, et al. Interleukin-13 is a new human lymphokine regulating inflammatory and immune responses. Nature. 1993;362:248–50. doi: 10.1038/362248a0. [DOI] [PubMed] [Google Scholar]
- 7.De Waal Malefijt R, Abrams JS, Zurawski SM, et al. Differential regulation of IL-13 and IL-4 production by human CD8+ and CD4+ Th0, Th1, and Th2 T cell clones and EBV-transformed B cells. Int Immunol. 1995;7:1405–16. doi: 10.1093/intimm/7.9.1405. [DOI] [PubMed] [Google Scholar]
- 8.Zurawski G, De Vries JE. Interleukin 13, an interleukin 4-like cytokine that acts on monocytes and B cells, but not on T cells. Immunol Today. 1994;15:19–26. doi: 10.1016/0167-5699(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 9.Köhler I, Alliger P, Minty A, et al. Human interleukin-13 activates the interleukin-4-dependent transcription factor NF-IL4 sharing a DNA binding motif with an interferon-gamma-induced nuclear binding factor. FEBS Letters. 1994;345:187–92. doi: 10.1016/0014-5793(94)00438-2. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan MH, Schindler U, Smiley ST, et al. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–9. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 11.Sornasse T, Larenas PV, Davis KA, et al. Differentiation and stability of T helper 1 and 2 cells derived from naive human neonatal CD4+ T cells, analyzed at the single cell level. J Exp Med. 1996;184:473–83. doi: 10.1084/jem.184.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Callard RE, Matthews DJ, Hibbert L. IL-4 and IL-13 receptors: are they one and the same? Immunol Today. 1996;17:108–10. doi: 10.1016/0167-5699(96)80600-1. [DOI] [PubMed] [Google Scholar]
- 13.Zurawski SM, Chomarat P, Djossou O, et al. The primary binding subunit of the human interleukin-4 receptor is also a component of the interleukin-13 receptor. J Biol Chem. 1995;270:13869–78. doi: 10.1074/jbc.270.23.13869. [DOI] [PubMed] [Google Scholar]
- 14.Obiri NI, Debinski W, Leonard WJ, et al. Receptor for interleukin 13. Interaction with interleukin 4 by a mechanism that does not involve the common gamma chain shared by receptors for interleukins 2, 4, 7, 9, and 15. J Biol Chem. 1995;270:8797–804. doi: 10.1074/jbc.270.15.8797. [DOI] [PubMed] [Google Scholar]
- 15.Aman MJ, Tayebi N, Obiri NI, et al. cDNA cloning and characterization of the human interleukin 13 receptor alpha chain. J Biol Chem. 1996;271:29265–70. doi: 10.1074/jbc.271.46.29265. [DOI] [PubMed] [Google Scholar]
- 16.Obiri NI, Leland P, Murata T, et al. The IL-13 receptor structure differs on various cell types and may share more than one component with IL-4 receptor. J Immunol. 1997;158:756–64. [PubMed] [Google Scholar]
- 17.Gauchat JF, Schlagenhauf E, Feng NP, et al. A novel 4-kb interleukin-13 receptor alpha mRNA expressed in human B, T, and endothelial cells encoding an alternate type-II interleukin-4/interleukin-13 receptor. Eur J Immunol. 1997;27:971–8. doi: 10.1002/eji.1830270425. [DOI] [PubMed] [Google Scholar]
- 18.Li H, Sim TC, Alam R. IL-13 released by and localized in human basophils. J Immunol. 1996;156:4833–8. [PubMed] [Google Scholar]
- 19.Pawankar R, Oduka M, Yssel H, et al. Nasal mast cells in perennial allergic rhinitics exhibit increased expression of the Fc-epsilonRI, CD40L, IL-4, and IL-13, and can induce IgE synthesis in B cells. J Clin Invest. 1997;99:1492–9. doi: 10.1172/JCI119311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung T, Wijdenes J, Neumann C, et al. Interleukin-13 is produced by activated human CD45RA+ and CD45RO+ T cells: modulation by interleukin-4 and interleukin-12. Eur J Immunol. 1996;26:571–7. doi: 10.1002/eji.1830260311. [DOI] [PubMed] [Google Scholar]
- 21.Brinkmann V, Kristofic C. TCR-stimulated naive human CD4+45RO− T cells develop into effector cells that secrete IL-13, IL-5, and IFN-gamma, but no IL-4, and help efficient IgE production by B cells. J Immunol. 1995;154:3078–87. [PubMed] [Google Scholar]
- 22.van der Pouw Kraan Tctm, Boeije LCM, Troon JTM, et al. Human IL-13 production is negatively influenced by CD3 engagement. Enhancement of IL-13 production by Cyclosporin A. J Immunol. 1996;156:1818–23. [PubMed] [Google Scholar]
- 23.Pawankar RU, Okuda M, Hasegawa S, et al. Interleukin-13 expression in the nasal mucosa of perennial allergic rhinitis. Am J Respir Crit Care Med. 1995;152:2059–67. doi: 10.1164/ajrccm.152.6.8520776. [DOI] [PubMed] [Google Scholar]
- 24.Huang SK, Xiao HQ, Kleine-Tebbe J, et al. IL-13 expression at the sites of allergen challenge in patients with asthma. J Immunol. 1995;155:2688–94. [PubMed] [Google Scholar]
- 25.Katagiri K, Itami S, Hatano Y, et al. Increased levels of IL-13 mRNA, but not IL-4 mRNA, are found in vivo in peripheral blood mononuclear cells (PBMC) of patients with atopic dermatitis. Clin Exp Immunol. 1997;108:289–94. doi: 10.1046/j.1365-2249.1997.d01-1015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamid Q, Naseer T, Minshall EM, et al. In vivo expression of IL-12 and IL-13 in atopic dermatitis. J Allergy Clin Immunol. 1996;98:225–31. doi: 10.1016/s0091-6749(96)70246-4. [DOI] [PubMed] [Google Scholar]
- 27.Kimata H, Fujimoto M, Furusho K. Involvement of interleukin (IL)-13, but not IL-4, in spontaneous IgE and IgG4 production in nephrotic syndrome. Eur J Immunol. 1995;25:1497–501. doi: 10.1002/eji.1830250604. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Polla B, Hauser C, et al. T cells from atopic individuals produce IgE-inducing activity incompletely blocked by anti-interleukin-4 antibody. Eur J Immunol. 1992;22:829–33. doi: 10.1002/eji.1830220330. [DOI] [PubMed] [Google Scholar]
- 29.Van der Pouw Kraan CTM, Aalberse RC, Aarden LA. IgE production in atopic patients is not related to IL-4 production. Clin Exp Immunol. 1994;97:254–9. doi: 10.1111/j.1365-2249.1994.tb06077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Thoracic Society. Medical section of the American lung association. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. Am Rev Respir Dis. 1987;136:225–44. doi: 10.1164/ajrccm/136.1.225. [DOI] [PubMed] [Google Scholar]
- 31.Van der Pouw Kraan T, Rensink I, Aarden L. Characterisation of monoclonal antibodies to human IL-4: application in an IL-4 ELISA and differential inhibition of IL-4 bioactivity on B cells and T cells. Eur Cytokine Netw. 1993;4:343–9. [PubMed] [Google Scholar]
- 32.Bazaral M, Hamburger RN. Standardization and stability of immunoglobulin E (IgE) J Allergy Clin Immunol. 1972;49:189–91. doi: 10.1016/0091-6749(72)90113-3. [DOI] [PubMed] [Google Scholar]
- 33.Van der Pouw Kraan T, Van Kooten C, Rensink Hjam, et al. IL-4 production by human T cells. Differential regulation of IL-4 versus IL-2 production. Eur J Immunol. 1992;22:1237–41. doi: 10.1002/eji.1830220519. [DOI] [PubMed] [Google Scholar]
- 34.Parronchi P, Macchia D, Piccinni MP, et al. Allergen- and bacterial antigen-specific T-cell clones established from atopic donors show a different profile of cytokine production. Proc Natl Acad Sci USA. 1991;88:4538–42. doi: 10.1073/pnas.88.10.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wierenga EA, Snoek M, Jansen HM, et al. Human atopen-specific types 1 and 2 T helper cell clones. J Immunol. 1991;147:2942–9. [PubMed] [Google Scholar]
- 36.Dolecek C, Steinberger P, Susani M, et al. Effects of IL-4 and IL-13 on total and allergen-specific IgE production by cultured PBMC from allergic patients determined with recombinant pollen allergens. Clin Exp Allergy. 1995;25:879–89. doi: 10.1111/j.1365-2222.1995.tb00031.x. [DOI] [PubMed] [Google Scholar]
- 37.Khirwadkar K, Schmitz M, Kabelitz D. Frequency analysis of allergen-reactive T-lymphocytes in indviduals allergic against the house-dust mite Dermatophagoides pteronyssinus. Int Arch Allergy App Immunol. 1992;98:6–12. doi: 10.1159/000236158. [DOI] [PubMed] [Google Scholar]
- 38.Burastero SE, Fenoglio D, Crimi E, et al. Frequency of allergen-specific T lymphocytes in blood and bronchial response to allergen in asthma. J Allergy Clin Immunol. 1993;91:1075–81. doi: 10.1016/0091-6749(93)90222-2. [DOI] [PubMed] [Google Scholar]
- 39.King CL, Poindexter RW, Ragunathan J, et al. Frequency analysis of IgE-secreting B lymphocytes in persons with normal or elevated serum IgE levels. J Immunol. 1991;146:1478–83. [PubMed] [Google Scholar]
- 40.Esnault S, Benbernou N, Lavaud F, et al. Differential spontaneous expression of mRNA for IL-4, IL-10, IL-13, IL-2, and interferon-gamma (IFN-gamma) in peripheral blood mononuclear cells (PBMC) from atopic patients. Clin Exp Immunol. 1996;103:111–8. doi: 10.1046/j.1365-2249.1996.00911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levy F, Kristofic C, Heusser C, et al. Role of IL-13 in CD4 T cell-dependent IgE production in atopy. Int Arch Allergy Immunol. 1997;112:49–58. doi: 10.1159/000237431. [DOI] [PubMed] [Google Scholar]
- 42.Marsh DG, Neely JD, Breazeale DR, et al. Linkage analysis of IL4 and other chromosome 5q31.1 markers and total serum immunoglobulin E concentrations. Science. 1994;264:1152–6. doi: 10.1126/science.8178175. [DOI] [PubMed] [Google Scholar]
- 43.Meyers DA, Postma DS, Panhuysen CIM, et al. Evidence for a locus regulating total serum IgE levels mapping to chromosome 5. Genomics. 1994;23:464–70. doi: 10.1006/geno.1994.1524. [DOI] [PubMed] [Google Scholar]
- 44.Postma DS, Bleecker ER, Amelung PJ, et al. Genetic susceptibility to asthma-bronchial hyperresponsiveness coinherited with a major gene for atopy. New Eng J Med. 1995;333:894–900. doi: 10.1056/NEJM199510053331402. [DOI] [PubMed] [Google Scholar]
- 45.Panhuysen CIM, Meyers DA, Postma DS, et al. The genetics of asthma and atopy. Allergy. 1995;50:863–9. doi: 10.1111/j.1398-9995.1995.tb02491.x. [DOI] [PubMed] [Google Scholar]
- 46.Trinchieri G, Scott P. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions. Res Immunol. 1995;146:423–651. doi: 10.1016/0923-2494(96)83011-2. [DOI] [PubMed] [Google Scholar]
- 47.Bochner BS, Klunk DA, Sterbinsky SA, et al. IL-13 selectively induces vascular cell adhesion molecule-1 expression in human endothelial cells. J Immunol. 1995;154:799–803. [PubMed] [Google Scholar]


