Abstract
It has been postulated that an impaired immune response may contribute to the progression of human papillomavirus (HPV)-associated preneoplastic lesions. Based on this hypothesis, we evaluated the cytokine production in the blood of patients with squamous intraepithelial lesions (SIL) of the uterine cervix. The levels of type-1 (interferon-gamma (IFN-γ) and IL-12) and type-2 (IL-4 and IL-10) cytokines were measured in whole blood culture supernatants of patients with low- and high-grade SIL and control women. There was no difference in IL-4 and IFN-γ levels between patients with SIL and the control group. In contrast, the ratio of IL-12/IL-10 levels was significantly lower in patients with SIL compared with the control group. A lower IL-12/IL-10 ratio in women with SIL was also observed when peripheral blood mononuclear cell (PBMC) culture supernatants and plasma samples were analysed. In patients, neither the lower expression of the CD3ε chain nor the higher frequency of HLA-DRB1*1501 expression could be correlated with abnormal cytokine production. These results suggest that a part of the cytokine network, namely IL-10 and IL-12, is perturbed in patients with SIL. A better knowledge of the role of these cytokines in regulating the growth of HPV-associated SIL might have practical implications for the development of vaccines or immunomodulatory strategies in the treatment of cervical cancers.
Keywords: IL-10, IL-12, cervical squamous intraepithelial lesion, human papillomavirus, blood culture
INTRODUCTION
Cervical cancer is the second leading cause of cancer mortality in women worldwide [1]. There is a great interest in studying the factors influencing tumour progression. Preneoplastic stages, so-called squamous intraepithelial lesions (SIL) [2], of cervix cancer are well defined in this type of tumour. One of the factors associated with cervical neoplasia is the human papillomavirus (HPV) infection. More than 90% of cervical cancers contain HPV DNA sequences [3], but HPV infection is not sufficient for the acquisition of the fully transformed cell phenotype. Other factors intrinsic to the host contribute to the process of tumourigenesis. For example, it has been demonstrated that immunodeficiency is associated with a rapid progression of cervical cancer [4, 5]. In this context, the general or local immune status might play a key role in regulating the growth of HPV-transformed cells. In particular, cell-mediated immunity is important in controlling both HPV infections and HPV-associated neoplasms [6].
Cell-mediated immunity is regulated by cytokines that are secreted, in part, by T helper (Th) cells. According to their cytokine production pattern, Th cells are classified into two distinct subsets of cells which are termed Th1 and Th2. Th1 cells produce IL-2, interferon-gamma (IFN-γ) and lymphotoxin. These cells promote cell-mediated immunity and are required for effective responses to intracellular pathogens (such as viruses) and tumour cells. Th2 cells produce IL-4, IL-5, IL-6 and IL-10 and favour humoral immunity against extracellular pathogens and allergic responses [7]. The regulation of immune responses is also controlled by cytokines produced by non-T cells. These cytokines are referred to as type-1 and type-2 cytokines. For instance, IL-12 is produced by antigen-presenting cells (monocytes and dendritic cells) and B cells and is a major factor required for the efficient differentiation of Th1 cells [8].
The predominance of the type-2 cytokine profile, associated with a diminished type-1 profile, has been demonstrated in patients with different neoplasms [9–13]. This type 1 to type 2 shift might facilitate the tumour progression by subverting cellular immune surveillance mechanisms [1]. In favour of this hypothesis, several alterations in the cellular immune response have been reported in the blood of patients with (pre)neoplastic cervical lesions, such as impaired mitogen response [14], lower expression of the TCR ζ-chain [15] and abnormal cytokine production after cell activation [16, 17].
Several studies suggested that the deficiency of the immune response could be associated with the expression of a particular HLA haplotype [18]. A particular HLA/T cell receptor (TCR) combination can give rise to either a Th1 or a Th2 response according to the avidity effect (affinity and ligand density), in analogy to what is involved in repertoire development [19]. HPV-derived peptides presented in the context of different HLA alleles might therefore lead to a preferential Th1 or Th2 differentiation.
The purpose of this study was to analyse the basal production of type-1 cytokines (IFN-γ and IL-12) and type-2 cytokines (IL-4 and IL-10) in the blood of patients with SIL. A lower IL-12/IL-10 ratio was observed in the blood of patients with SIL compared with control donors. IL-4 and IFN-γ levels did not differ between the two groups. The proportion of cell subpopulations in the blood was also the same in both groups, although a decreased cell surface expression of the CD3ε chain and a higher frequency of HLA-DRB1*1501 expression were observed in patients with SIL. The results of this study performed in women with preneoplastic lesions might be of potential interest in understanding the mechanism of tumour progression and to identify prognostic factors of the disease.
MATERIALS AND METHODS
Patients
Peripheral blood samples (10 ml) of patients with low (n = 7) and high (n = 20) grade SIL of the uterine cervix were obtained before surgical procedure. HPV DNA was detected in biopsy specimens by polymerase chain reaction (PCR) with degenerated oligonucleotides hybridizing in L1 open reading frame [20]. HPV DNA was detected in 87% of low-grade SIL and in 100% of high-grade SIL biopsy specimens. Blood samples from women undergoing plastic surgery or minor intervention unrelated to cervical pathology were also obtained before surgery and used as controls. The SIL patients (32·8 ± 9·3 years old) and the control donors (31·6 ± 9·7 years old) were age-matched.
This study protocol was approved by the Ethics Committee of the University Hospital of Liège.
Cultures
Blood (300 μl) was diluted 10-fold in culture medium consisting of RPMI 1640 (Gibco BRL, Gent, Belgium) supplemented with 1% non-essential amino acids (Gibco), sodium pyruvate (1 mm; Gibco), 30 U/ml penicillin-streptomycin (Gibco). The blood cultures were incubated at 37°C during 24 h. For some experiments, Staphylococcus aureus cells diluted 5 × 10−4 (SAC; Sansorbin; Calbiochem, La Jolla, CA) or 5 μg/ml of Escherichia coli lipopolysaccharides (LPS; Difco, Detroit, MI) were added to the culture.
The peripheral blood mononuclear cells (PBMC) were prepared by centrifugation on Lymphoprep (Nycomed, Oslo, Norway), washed three times and counted. Cells were cultured (1·25 × 106 cells/ml) in culture medium with the addition of 5% pooled heat-inactivated human AB serum. For some experiments, PBMC were cultivated in the presence of 10 ng/ml of purified anti-CD3 (OKT3 hybridoma; ATCC, Rockville, MD).
ELISA assays
The cytokines IL-4, IL-10, IL-12 and IFN-γ were measured by using specific immunoassays from Medgenix Diagnostics (Fleurus, Belgium) [21]. The anti-IL-12 antibody used recognized both IL-12 p40 and IL-12 p70. Recombinant human IL-4, IL-10, IL-12 and IFN-γ were used as reference standards.
Surface phenotype of cells
Double- and triple-staining were performed with fluorescent conjugated antibodies. The anti-CD3 (peridinin chlorophyll (PerCP)) MoAb, anti-CD4 (FITC) MoAb, anti-CD8 (PerCP) MoAb, anti-CD45RA (FITC) MoAb, anti-CD19 (PerCP) MoAb, anti-CD28 (PE) MoAb and anti-HLA-DR (FITC) MoAb were purchased from Becton Dickinson (Erembodegem, Belgium). The anti-CD45RO (PE) MoAb and anti-CD25 (PE) MoAb were obtained from Dako (Glostrup, Denmark) and the anti-CD16 (FITC) MoAb from Ortho (Raritan, NJ). The phenotype was performed on whole blood or PBMC samples following previously published protocols [22, 23]. The cells were analysed for fluorescence intensity on a FACScan (Becton Dickinson).
HLA determination
The HLA phenotype (HLA-A, -B, -C, -DQ) was determined by microlymphocytotoxicity [24]. HLA-DR expression was analysed by DNA typing reverse dot blot (INNOLIPA; Innogenetics, Zwijndrecht, Belgium) [25].
Statistical analysis
The non-parametric Mann–Whitney or Kruskall Wallis test was applied by using the Instat Mac 2·01 software (GraphPad Software, San Diego, CA).
RESULTS
The ratio of IL-12/IL-10 production is decreased in patients with cervical preneoplastic lesions
The production of type-1 (IFN-γ and IL-12) and type-2 (IL-4 and IL-10) cytokines in whole blood culture samples of patients with low- and high-grade cervical SIL was analysed by ELISA assays. Blood samples from women undergoing minor surgical procedures unrelated to cervical pathology were used as controls.
After 24 h of culture, the level of IL-4 was near the limit of detection and no difference was observed between patients with cervical lesions and the control group. The production of IFN-γ was also similar for patients with SIL (35 ± 30 U/ml) and for the control group (28 ± 23 U/ml). In contrast, we noticed a significant difference in the production of IL-10 and IL-12 between the two groups (Fig. 1). Generally, IL-10 production (76 ± 58 pg/ml) increased and IL-12 production (136 ± 96 pg/ml) decreased in patients with SIL compared with the control donors (respectively, 29 ± 18 pg/ml and 204 ± 68 pg/ml) (Fig. 1a,b). When the results were expressed as a ratio between the IL-12 and IL-10 levels for individual patients the differences were more pronounced (Fig. 1c).
Fig. 1.
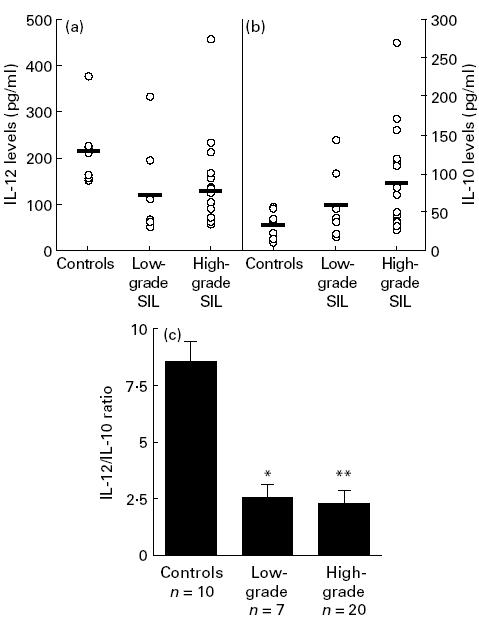
IL-12 (a) and IL-10 (b) levels and IL-12/IL-10 ratio (c) in whole blood cultures. The cytokine levels were detected by ELISA assays on whole blood culture supernatants from 10 control donors, seven patients with low-grade squamous intraepithelial lesions (SIL) and 20 patients with high-grade SIL. The IL-12/IL-10 ratio is expressed by mean ±s.e.m. *P < 0·05; **P < 0·01.
To rule out the possibility that these results were related to the technique of whole blood culture, we made the same analyses using PBMC culture supernatants and plasma. As shown in Fig. 2a, we observed the same pattern of IL-10 and IL-12 production in PBMC culture supernatants (24 h). Similar results were obtained using plasma (Fig. 2b).
Fig. 2.
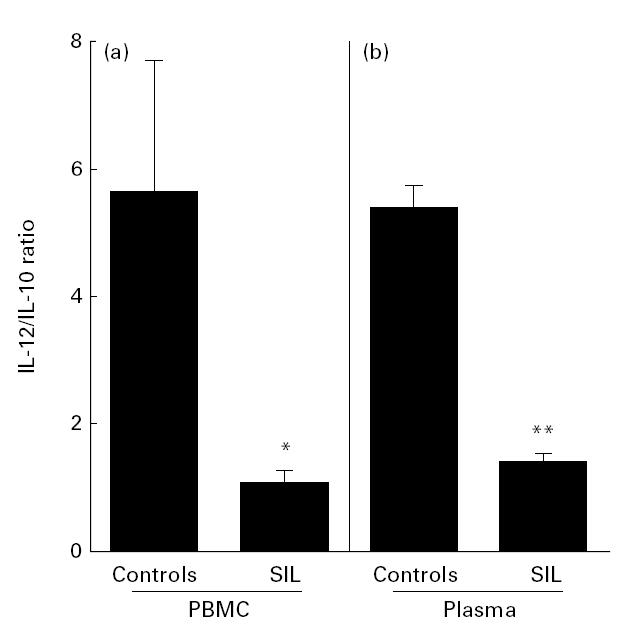
IL-12/IL-10 ratio in peripheral blood mononuclear cell (PBMC) culture supernatant (a) and plasma (b). The cytokine levels were detected by ELISA assays. The IL-12/IL-10 ratio is expressed as mean ± s.e.m. of five control donors and 10 patients with squamous intraepithelial lesions (SIL). *P < 0·05; **P < 0·01.
The low IL-12/IL-10 ratio in patients with SIL is not due to a disparity in the proportions of different cell populations
As we observed an abnormal cytokine production in the blood of patients, we analysed the phenotype of the cells present in the blood. The number of leucocytes was in the range of the normal population (7·86 ± 2·5 × 103 cells/mm3). Table 1 shows the proportion of the different cell populations in the blood detected by flow cytometry. No significant difference was observed in the percentage of lymphocytes, monocytes and CD4+ T cells. The proportions of other lymphocyte subpopulations (CD8+ T cells, naive and memory T cells, B cells and natural killer (NK) cells) were also similar in controls and patients with SIL (data not shown).
Table 1.
Percentage of the different cell populations in the blood of controls and patients with cervical squamous intraepithelial lesions (SIL)*

The IL-12 deficiency in SIL patients is maintained following cell stimulation
Cytokine production after 24 h stimulation with SAC (5 × 10−4) and LPS (5 μg/ml) was assayed (Fig. 3). The difference in IL-12 levels between patients with SIL and control donors persisted after both types of stimulation (Fig. 3a). In contrast, the production of IL-10 by both groups was not significantly different following stimulation (Fig. 3b). The IL-12/IL-10 ratio remained lower after stimulation for SIL patients compared with the control group (P < 0·01, data not shown).
Fig. 3.
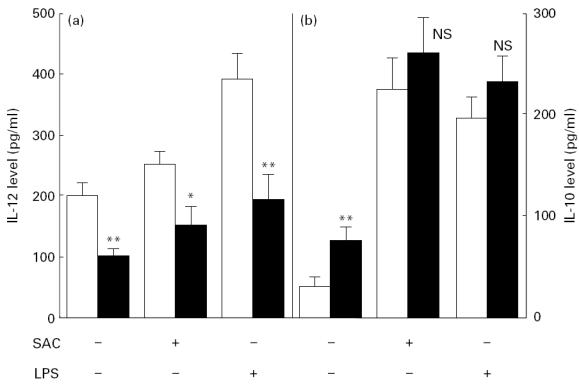
IL-12 (a) and IL-10 (b) levels in whole blood culture supernatant after 24h of stimulation in the presence of Staphylococcus aureus cells (SAC; 5×10−4) or lipopolysaccharide (LPS; 5μg/ml). Cytokine levels were detected by ELISA assays on whole blood culture supernatants. Results are expressed as the mean±s.e.m. of 10 control donors (□) and 10 patients with squamous intraepithelial lesions (SIL) (▪). *P<0.05; **P<0.01. NS, Not significant.
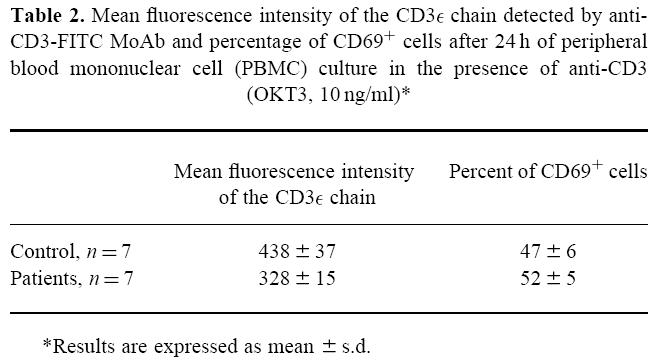
As T lymphocytes are able to produce IL-10, we also analysed the stimulation response of T cells. Despite a lower expression of the CD3ε chain on T cells from SIL patients (P < 0·05) (Table 2), cell activation via the TCR did not seem to be affected, since the percentage of activated cells detected by CD69 expression was similar in SIL patients and controls after 24 h of PBMC culture in the presence of anti-CD3 MoAb (OKT3, 10 ng/ml) (Table 2). Similar results were observed on analysis of the percentage of cells expressing the IL-2 receptor (CD25) using the same conditions of culture (data not shown). No correlation was observed between CD3ε expression and IL-10 level (data not shown).
Table 2.
Mean fluorescence intensity of the CD3ε chain detected by anti-CD3-FITC MoAb and percentage of CD69+ cells after 24 h of peripheral blood mononuclear cell (PBMC) culture in the presence of anti-CD3 (OKT3, 10 ng/ml)*
The low IL-10/IL-12 ratio is not associated with higher frequency of HLA-DRB1*1501 haplotype expression
Analysis of the HLA phenotype (HLA-A, -B, -C, -DR and -DQ) was performed in patients with SIL. The proportion of the different alleles was comparable to that which is found in the normal Caucasian population, except for the HLA-DRB1*1501 allele. A higher percentage of SIL patients (43%) expressed HLA-DRB1*1501 compared with the normal population (29%) [26]. We also compared the IL-12/IL-10 ratio in patients expressing HLA-DRB1*1501 and non-HLA-DRB1*1501 molecules (Fig. 4c). We did not find any correlation between the expression of HLA-DRB1*1501 allele and the IL-12/IL-10 ratio. In the same way, no correlation was observed between IL-10 and IL-12 levels (Fig. 4a,b).
Fig. 4.
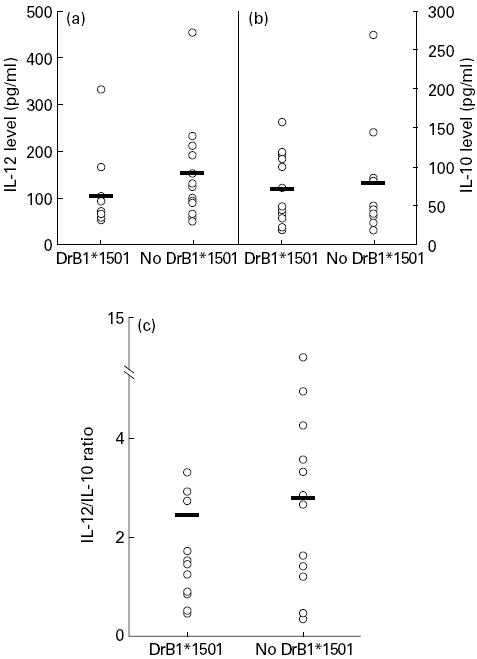
IL-12 (a), IL-10 (b) levels and IL-12/IL-10 ratio (c) in squamous intraepithelial lesion (SIL) patients expressing or not HLA-DRB1*1501. Cytokine levels were detected by ELISA assays on whole blood culture supernatants. Expression of HLA-DRB1*1501 allele was detected by DNA typing reverse dot blot.
DISCUSSION
The main finding of this study is the demonstration of a diminished IL-12/IL-10 ratio in the blood of patients with HPV-associated cervical preneoplastic lesions. IL-10 and IL-12 have been heavily implicated in the outcome of tumour or viral infection. IL-10 could be considered an immunosuppressive cytokine, since it can inhibit type-1 cell differentiation. IL-10 is one of the most potent inhibitors of IL-12 production by monocytes and dendritic cells [27]. IL-10 mRNA and protein have been found in excess in several cancers [12, 13, 28, 29]. Increased IL-10 production has been shown not only by tumour-infiltrating lymphocytes [30] but also by tumour cells themselves [11]. In contrast, IL-12 plays a key role in the development of anti-tumour immune response, as shown by in vitro studies [31] or in in vivo mouse models [32, 33]. In the field of viral infection, lower IL-12 production levels have been found in patients with HIV infection [34] and IL-12 appears to be required for resistance against some viral infections, such as cytomegalovirus infection in mice [35].
The presence of small preneoplastic lesions in the cervix and the immunological alterations in the peripheral blood could be linked, given the fact that patients with localized cervical HPV infection can generate a systemic antibody response directed against HPV proteins [36]. Several immune impairments have been detected in the blood of patients with cervical lesions [14, 15], and we have chosen to focus our study on cytokine production. In patients with cervical SIL or carcinoma, abnormal cytokine production after cell activation has already been reported. Th1 cytokine production in response to HPV-16 E6 and E7 peptides is diminished in PBMC culture from patients with high-grade cervical SIL and cancer [37]. Elsässer-Beile and collaborators [16] have shown that blood cultures of patients with cervical carcinoma exhibit a decreased production of IFN-γ after activation with phytohaemagglutinin A (PHA). In a recent study, PBMC from patients with local and invasive lesions produced decreased amounts of IL-2 and IFN-γ and higher levels of IL-4 and IL-10 after 48 h of stimulation with PHA in comparison with healthy controls [17].
Our results, together with other recent publications, suggest the presence of a type 1 to type 2 shift in patients with SIL. At present, it is unknown whether this Th1-to-Th2 shift leads to the development of neoplasia by subverting immune surveillance mechanisms, or whether the shift is a consequence of tumour development or viral infection. One argument against the hypothesis that the shift from a type-1 to a type-2 response is the cause of the progression to neoplasia is that patients with allergic disorders, who generally display high Th2 responses, do not appear to have higher rates of viral infection or cancer [1, 38]. In contrast, studies showing that cancer development is associated with a particular HLA haplotype expression [39–42] argue that genetic factors could play a role in tumour progression by influencing the immune response. We observed an increased frequency of women expressing HLA-DRB1*1501 in our group of patients with cervical SIL. A similar association has been previously reported [40]. However, we could not find an association between the IL-12/IL-10 ratio and HLA-DRB1*1501 allele expression. In fact, it is possible that an innate defect in the immune response is increased by HPV infection. Furthermore, HPV could modify cytokine production by infected epithelial cells ([43] and unpublished observations). It is interesting to note that the Th2 cytokine IL-6 is constitutively released by HPV-16-harbouring keratinocytes [44] and expressed at higher levels in invasive cervical carcinoma [45]. Moreover, in our group of patients there were abnormal levels of cytokine mRNA in their cervical biopsy when tested using semiquantitative reverse transcriptase PCR. In particular, increased IL-10 mRNA and decreased IL-12 mRNA expression was observed in high-grade SIL compared with low-grade SIL (S. L. Giannini, personal communication).
To the best of our knowledge, this is the first report of abnormal basal cytokine production in the blood of patients with SIL. Studies are in progress to determine whether the evaluation of the IL-12/IL-10 ratio in the blood could serve as a prognostic factor of cervical preneoplastic lesions.
Acknowledgments
This work was supported by the Belgian Fund for Medical Scientific Research, the Centre de Recherche Interuniversitaire en Vaccinologie, with a grant from the Walloon Region and SmithKline Beecham Biologicals and the Centre Anticancéreux près l'Université de Liège. P.D. and M.M. are research associates of the Belgian National Fund for Scientific Research. The authors wish to acknowledge L.-M. Dupuis, E. Franzen-Detrooz and H. Piron for their excellent technical assistance, C. Bouillenne from the Histocompatibility Laboratory for HLA typing and P. Marchand for providing blood samples. Antibodies for ELISA assays were kindly provided by Medgenix.
References
- 1.Wu T-C, Kurman RJ. Analysis of cytokine profiles in patients with human papillomavirus-associated neoplasms. J Natl Cancer Inst. 1997;89:185–7. doi: 10.1093/jnci/89.3.185. [DOI] [PubMed] [Google Scholar]
- 2.NCI Workshop. The 1988 Bethesda system for reporting cervical/vaginal cytological diagnoses. JAMA. 1989;262:931–4. [PubMed] [Google Scholar]
- 3.Bosch FX, Manos MM, Munoz N, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995;87:796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 4.Benton C, Shahidullah H, Hunter JAA. Human papillomavirus in the immunosuppressed. Papillomavirus Rep. 1992;3:23–26. [Google Scholar]
- 5.Petry KU, Scheffel D, Bode U, et al. Cellular immunodeficiency enhances the progression of human papillomavirus-associated cervical lesions. Int J Cancer. 1994;57:836–40. doi: 10.1002/ijc.2910570612. [DOI] [PubMed] [Google Scholar]
- 6.Wu TC. Immunology of the human papilloma virus in relation to cancer. Curr Opin Immunol. 1994;6:746–54. doi: 10.1016/0952-7915(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 7.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1,Th2 and more. Immunol Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 8.Scott P. IL-12: initiation cytokine for cell-mediated immunity. Sciences. 1993;260:496–7. doi: 10.1126/science.8097337. [DOI] [PubMed] [Google Scholar]
- 9.Pisa P, Halapi E, Pisa EK, et al. Selective expression of interleukin 10, interferon γ, and granulocyte-macrophage colony-stimulating factor in ovarian cancer biopsies. Proc Natl Acad Sci USA. 1992;89:7708–12. doi: 10.1073/pnas.89.16.7708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clerici M, Ferrario E, Trabattoni D, et al. Multiple defects of T helper cell function in newly diagnosed patients with Hodgkin's disease. Eur J Cancer. 1994;30A:1464–70. doi: 10.1016/0959-8049(94)00305-o. [DOI] [PubMed] [Google Scholar]
- 11.Huang M, Wang J, Lee P, et al. Human non-small cell lung cancer cells express a type 2 cytokine pattern. Cancer Res. 1995;55:3847–53. [PubMed] [Google Scholar]
- 12.Huettner C, Paulus W, Roggendorf W. Messenger RNA expression of the immunosuppressive cytokine Il-10 in human gliomas. Am J Pathol. 1995;146:317–22. [PMC free article] [PubMed] [Google Scholar]
- 13.Krüger-Krasagakes S, Krasagakis K, Garbe C, et al. Expression of interleukin 10 in human melanoma. Br J Cancer. 1994;70:1182–5. doi: 10.1038/bjc.1994.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy S, Kopersztych S, Musatti CC, Souen JS, Salvatore CA, Mendes NF. Cellular immunity in squamous cell carcinoma of the uterine cervix. Am J Obstet Gynecol. 1978;130:160–4. doi: 10.1016/0002-9378(78)90360-5. [DOI] [PubMed] [Google Scholar]
- 15.Kono K, Ressing ME, Brandt RMP, et al. Decreased expression of signal-transducing ζ chain in peripheral T cells and natural killer cells in patients with cervical cancer. Clin Cancer Res. 1996;2:1825–8. [PubMed] [Google Scholar]
- 16.Elsässer-Beile U, von Kleist S, Sauther W, Gallati H, Schulte Mönting J. Impaired cytokine production in whole blood cell cultures of patients with gynaecological carcinomas in different clinical stages. Br J Cancer. 1993;68:32–36. doi: 10.1038/bjc.1993.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clerici M, Merola M, Ferrario E, et al. Cytokine production patterns in cervical intraepithelial neoplasia: association with human papillomavirus infection. J Natl Cancer Inst. 1997;89:245–50. doi: 10.1093/jnci/89.3.245. [DOI] [PubMed] [Google Scholar]
- 18.Davies DH, Stauss HJ. The significance of human leukocyte antigen associations with cervical cancer. Papillomavirus Report. 1997;8:43–50. [Google Scholar]
- 19.Ashton-Rickardt PG, Bandeira A, Delaney JR, et al. Evidence for differential avidity model of T cell selection in the thymus. Cell. 1994;76:651–63. doi: 10.1016/0092-8674(94)90505-3. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs MV, De Rodo Husman AM, Van den Bruck AJC, Snijders PJF, Meijer Cjlm, Walboomer JMM. Group-specific differentiation between high- and low-risk human papillomavirus genotypes by general primer-mediated PCR and two cocktails of oligonucleotide probes. J Clin Microbiol. 1995;33:901–5. doi: 10.1128/jcm.33.4.901-905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Groote D, Zangerle PF, Gevaert Y, et al. Direct stimulation of cytokines (IL-1β,TNF-α, IL-6, IL-2, IFN-γ, and GM-CSF) in whole blood. I. Comparison with isolated PBMC stimulation. Cytokine. 1992;4:239–48. doi: 10.1016/1043-4666(92)90062-v. [DOI] [PubMed] [Google Scholar]
- 22.Terstappen Lwmm, Buescher S, Nguyen M, Reading C. Differentiation and maturation of growth factor expanded human hematopoietic progenitors assessed by multidimensional flow cytometry. Leukemia. 1992;6:1001–10. [PubMed] [Google Scholar]
- 23.Jacobs N, Moutschen MP, Boniver J, Greimers R, Schaaf-Lafontaine N. Efficient immunoselection of cytolytic effectors with a magnetic cell sorter. Res Immunol. 1993;144:141–50. doi: 10.1016/0923-2494(93)80069-b. [DOI] [PubMed] [Google Scholar]
- 24.Terasaki PI, Bernoco D, Park MS, Ozturk G, Iwaki Y. Microdroplet testing for HLA-A,-B,-C and -D antigens. Am J Clin Pathol. 1978;69:103–20. doi: 10.1093/ajcp/69.2.103. [DOI] [PubMed] [Google Scholar]
- 25.Buyse I, Decorte R, Cuppens H, et al. Rapid DNA typing of class II HLA antigen using polymerase chain reaction and reverse dot blot hybidization. Tissue Antigens. 1993;41:1–14. doi: 10.1111/j.1399-0039.1993.tb01970.x. [DOI] [PubMed] [Google Scholar]
- 26.Baur MP. Berlin: Springer-Verlag; 1984. Histocompatibility testing. [Google Scholar]
- 27.Koch F, Stanzl U, Jennewein P, et al. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J Exp Med. 1996;184:741–6. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakagomi H, Pisa P, Pisa EK, et al. Lack of interleukin-2 (IL-2) expression and selective expression of IL-10 mRNA in human renal cell carcinoma. Int J Cancer. 1995;63:366–71. doi: 10.1002/ijc.2910630311. [DOI] [PubMed] [Google Scholar]
- 29.Huang M, Sharma S, Mao JT, Dubinett SM. Non-small cell lung cancer-derived soluble mediators and prostaglandin E2 enhance peripheral blood lymphocyte IL-10 transcription and protein production. J Immunol. 1996;157:5512–20. [PubMed] [Google Scholar]
- 30.Kim J, Moldin RL, Moy RL, Dubinett SM, McHugh T, Nickoloff BJ, Uyemura K. IL-10 production in cutaneous basal and squamous cell carcinomas. A mechanism for evading the local T cell immune response. J Immunol. 1995;155:2240–7. [PubMed] [Google Scholar]
- 31.Kuge S, Watanabe K, Makino K, et al. Interleukin-12 augments the generation of autologous tumor-reactive CD8+ cytotoxic T lymphocytes from tumor-infiltrating lymphocytes. Jpn J Cancer Res. 1995;86:135–9. doi: 10.1111/j.1349-7006.1995.tb03030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tahara H, Zeh Iiihj, Storkus WJ, et al. Fibroblasts genetically engineered to secrete interleukin 12 can suppress tumor growth and induce antitumor immunity to a murine melanoma in vivo. Cancer Res. 1994;54:182–9. [PubMed] [Google Scholar]
- 33.Yu W-G, Yamamoto N, Takenaka H, et al. Molecular mechanisms underlying IFN-γ-mediated tumor growth inhibition induced during tumor immunotherapy with rIL-12. Int Immunol. 1996;8:855–65. doi: 10.1093/intimm/8.6.855. [DOI] [PubMed] [Google Scholar]
- 34.Chehimi J, Starr SE, Frank I, et al. Impaired interleukin 12 production in human immunodeficiency virus-infected patients. J Exp Med. 1994;179:1361–6. doi: 10.1084/jem.179.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orange JS, Wang B, Terhost C, Biron CA. An absolute and restricted requirement for IL-12 in natural killer cell IFN-γ production and antiviral defense. Studies of natural killer and T cell responses in contrasting viral infections. J Immunol. 1996;156:1138–42. [PubMed] [Google Scholar]
- 36.Viscidi RP, Kotloff KL, Clayman B, Russ K, Shapiro S, Shah KV. Prevalence of antibodies to human papillomavirus (HPV) type 16 virus-like particles in relation to cervical HPV infection among College women. Clin Diagn Lab Immunol. 1997;4:122–6. doi: 10.1128/cdli.4.2.122-126.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsuki T, Hildesheim A, Schiffman MH, et al. Interleukin 2 production in vitro by peripheral lymphocytes in response to human papillomavirus-derived peptides: correlation with cervical pathology. Cancer Res. 1996;56:3967–74. [PubMed] [Google Scholar]
- 38.McKee WD, Arnold CA, Perlman MD. A double-blind study of comparative incidence of malignancy and allergy. J Allergy. 1967;39:294–301. doi: 10.1016/0021-8707(67)90093-7. [DOI] [PubMed] [Google Scholar]
- 39.Vandenvelde C, De Foor M, Van Beers D. HLA-DQB1*03 and cervical intraepithelial neoplasia grades I–III. Lancet. 1993;341:442–3. doi: 10.1016/0140-6736(93)93044-2. [DOI] [PubMed] [Google Scholar]
- 40.Apple RJ, Erlich HA, Klitz W, Manos MM, Becker TM, Wheler CM. HLA DR-DQ associations with cervical carcinoma show papillomavirus-type specificity. Nature Genet. 1994;6:157–62. doi: 10.1038/ng0294-157. [DOI] [PubMed] [Google Scholar]
- 41.Gregoire L, Lawrence WD, Kukuruga D, Eisenbrey AB, Lancaster WB. Association between HLA-DQB1 alleles and risk for cervical cancer in African-American women. Int J Cancer. 1994;57:504–7. doi: 10.1002/ijc.2910570411. [DOI] [PubMed] [Google Scholar]
- 42.Sastre-Garau X, Loste M-N, Vincent-Salomon A, et al. Decreased frequency of HLA-DRB1*13 alleles in French women with HPV-positive carcinoma of the cervix. Int J Cancer. 1996;69:159–64. doi: 10.1002/(SICI)1097-0215(19960621)69:3<159::AID-IJC1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 43.Woodworth CD, Simpson S. Comparative lymphokine secretion by cultured normal human cervical keratinocytes, papillomavirus-immortalized, and carcinoma cell lines. Am J Pathol. 1993;56:3967–74. [PMC free article] [PubMed] [Google Scholar]
- 44.Malejczyk J, Malejczyk M, Urbanski A, et al. Constitutive release of IL6 by human papillomavirus type 16 (HPV16)-harboring keratinocytes: a mechanism augmenting the NK-cell-mediated lysis of HPV-bearing neoplastic cells. Cell Immunol. 1991;136:155–64. doi: 10.1016/0008-8749(91)90390-w. [DOI] [PubMed] [Google Scholar]
- 45.Tatour E, Gey A, Sastre-Garau X, et al. Analysis of interleukin 6 expression in cervical neoplasia using a quantative polymerase chain reaction assay: evidence for enhanced interleukin 6 gene expression in invasive carcinoma. Cancer Res. 1994;54:6243–8. [PubMed] [Google Scholar]


