Abstract
Nitric oxide (NO) is a mediator of inflammatory injury which is inhibited by glucocorticoids and is implicated in rheumatoid (RA) and adjuvant arthritis (AA). The glucocorticoid-induced anti-inflammatory molecule lipocortin 1 is expressed in RA synovium, but the effects of lipocortin 1 on synovial inflammation have been little studied. We investigated the effects of glucocorticoids and lipocortin 1 on inducible NO synthase (iNOS) and glucocorticoids on the induction of lipocortin 1 in AA synovial macrophages. NO production was measured by Griess assay in supernatants of day 14 AA rat synovial explants and of synovial macrophages purified from enzyme-digested synovium and treated with lipopolysaccharide (LPS) 1 μg/ml, dexamethasone (DEX) 10−7 m, and anti-lipocortin 1 MoAb. iNOS and lipocortin 1 expression were detected by flow cytometry using specific MoAb. Cell surface lipocortin was determined by Western blot. NO was produced by all AA synovial explants and NO was released by cultured synovial macrophages (14·5 ± 2·1 μmol/24 h). iNOS was detected in synovial macrophages (ED-1+) by permeabilization flow cytometry. LPS increased synovial macrophage NO release (P < 0·0001) and iNOS expression (P = 0·04). DEX inhibited constitutive (P = 0·002) and LPS-induced (P < 0·001) NO release and iNOS expression (P = 0·03). DEX inhibition of synovial macrophage NO was associated with induction of cell surface and intracellular lipocortin 1. Anti-lipocortin 1 MoAb treatment reduced the inhibition of NO release by DEX (P = 0·002), but had no effect on iNOS expression. These findings demonstrate a role for lipocortin 1 in the inhibition by glucocorticoids of AA synovial macrophage iNOS activity.
Keywords: lipocortin 1, macrophage, nitric oxide, adjuvant arthritis, glucocorticoid
INTRODUCTION
The production by inflamed synovium in human rheumatoid arthritis (RA) and animal models of inflammatory effector molecules such as prostaglandin E2 (PGE2), reactive oxygen species (ROS), and reactive nitrogen species such as nitric oxide (NO), is well described [1––5]. NO is produced by the action of nitric oxide synthase (NOS) on arginine, through constitutive and inducible pathways which are catalysed by separate enzymes.
Constitutive production of low concentrations of NO is transient and NO produced in this way is likely to act predominantly as a signalling molecule [6]. Greater concentrations of NO are produced by the action of inducible NOS (iNOS), produced by cells such as macrophages, synovial fibroblasts, osteoblasts and chondrocytes under the influence of endotoxin and proinflammatory cytokines such as IL-1 and tumour necrosis factor-alpha (TNF-α) [7––9]. Considerable evidence suggests that NO is implicated in joint inflammation and cartilage degradation in RA [3, 4, 10–12]. As such, therapies targeted at molecules such as NO have the potential to be of use in RA. Glucocorticoids, already in widespread use in the treatment of RA, potentially influence many aspects of joint inflammation, including antigen presentation, lymphocyte proliferation, cytokine production, and soluble mediator molecule release [13]. Glucocorticoids are known to inhibit inducible NO production [8, 14], and have been shown in vitro to inhibit NO production in human synovial fibroblasts and osteoblast cell lines [4, 15]. The effects of glucocorticoids on NO production in inflamed synovium have not been well studied, although inhibition of nitrate excretion has been reported among RA patients receiving glucocorticoid therapy [16]. Clearly, given the recent description of reduced rates of joint erosion among patients with RA treated with glucocorticoids [17], demonstration of effects of glucocorticoids on NO production in relevant animal models of RA would allow further exploration of this phenomenon.
Certain anti-inflammatory effects of glucocorticoids are mediated by the annexin, lipocortin 1. Lipocortin 1 is present in peripheral blood monocytes and tissue macrophages in man, where it is synthesized both constitutively and in response to glucocorticoids [18––20]. The anti-inflammatory role of lipocortin 1 has been demonstrated in numerous in vivo studies using recombinant protein and/or neutralizing antibodies [21––24]. Most recently, the role of lipocortin 1 in the modulation of inflammation by endogenous glucocorticoids has been demonstrated [25, 26]. Although the mechanisms of the anti-inflammatory effects of lipocortin 1 are incompletely understood, inhibitory effects on the production of mediators such as PGE2 are well described [26, 27], and a role for lipocortin 1 in glucocorticoid inhibition of inducible NO has also been reported [28]. We thus decided to investigate the inhibition of synovial macrophage NO production by glucocorticoids, and the potential role of lipocortin 1 in this phenomenon.
MATERIALS AND METHODS
Induction of adjuvant arthritis
Inbred male Sprague-Dawley (SD) rats (approximately 50 days of age; 200 ± 20 g) were used for all experiments. Animals were fed laboratory chow and tap water ad libitum and housed six to a cage. Adjuvant arthritis (AA) was induced in SD rats by intradermal injection of 150 μl of pulverized Mycobacterium tuberculosis (Difco Labs, Detroit, MI) at a concentration of 10 mg/ml in heavy squalane (Sigma, St Louis, MO) at the base of the tail. Animals developed clinically apparent arthritis by days 10–12 after injection, and were killed on day 14. This study was approved by the institutional animal research ethics committee.
Synovial tissue explant culture
Synovial tissue surgically excised from hind foot joints of day 14 AA rats was cultured in 3 cm tissue culture dishes in 2 ml RPMI supplemented with 2% heat-inactivated fetal calf serum (FCS; ICN Biomedicals, Seven Hills, Australia). After 24 h, culture supernatants were aspirated and stored at −20°C before assay for nitrite production as below. Results were corrected for wet weight.
Synovial macrophage culture
Synovial tissue was surgically excised from knee and hind foot joints of day 14 AA rats and rinsed in Dulbecco's modified Eagles' medium (DMEM; ICN, Costa Mesa, CA), supplemented with penicillin 50 U/ml, streptomycin 50 mg/ml and l-glutamine 2 mm (Gibco, Gaithersburg, MD), and 10% FCS. After mincing, synovial tissue was pooled and added to 1 ml of Dispase (2·4 U/ml) (Boehringer Mannheim Australia, Castle Hill, Australia) with 1 ml serum-free DMEM. The synovial tissue was digested for 60 min at 37°C with constant stirring. After washing with DMEM, a single-cell suspension was obtained by mesh filtration. This method routinely yielded 2–3 × 106 synovial cells per animal. Synovial macrophages were obtained from synovial cell suspensions by adherence. Synovial cell suspensions at a concentration of 106 cells/ml in DMEM/10% FCS were placed in 3·5-cm Petri dishes for 1 h at 37°C and 5% CO2 in a humidified incubator. After 1 h, non-adherent cells were removed by washing and adherent cells removed by chilling plates on ice followed by pipetting. Cells were cultured in DMEM/10% FCS, at 37°C and 5% CO2, in a humidified incubator overnight and medium replaced before treatment. Cells obtained by this method were >95% macrophages as shown by non-specific esterase staining and were >90% viable by trypan blue exclusion. Synovial macrophages were cultured in 24-well plates (Costar, Cambridge, MA) at 105 cell/well in DMEM/10% FCS. Cells were treated with lipopolysaccharide (LPS; Sigma) 1 μg/ml, dexamethasone (Sigma) 10−7 m, and/or the anti-lipocortin 1 MoAb, 1-B, kindly donated by Dr J. Browning (Biogen Inc, Cambridge, MA) [29]. The endotoxin content of antibody preparations used in in vitro studies was <0·05 U/ml, by Limulus assay (E-toxate; Sigma). Control wells were treated with purified mouse IgG.
Synovial macrophage nitric oxide production
Nitrite in culture supernatants was measured after 24 h of culture by the Griess reaction, as previously described [30]. Briefly, the Griess reagent was prepared as follows: 1·5% sulfanilamide (p-aminobenzene-sulfonamide; Sigma) in 1 m hydrochloric acid (HCl; Ajax Chemicals, Sydney, Australia) was mixed with 0·15% naphthylethyldiamine (N-(1-napthyl)ethyl-diamine dihydrochloride; Sigma) at a ratio of 1:1. Equal volumes (100 μl) of Griess reagent and supernatant were mixed in a 96-well plate and the absorbance was read at 530 nm. Nitrite concentration was determined using sodium nitrite (Ajax Chemicals).
Induction of lipocortin 1 in synovial and peritoneal macrophages
Synovial macrophages were obtained from synovial tissue as described above. Peritoneal macrophages were eluted by lavage of the cavity, purified by adherence and macrophage phenotype confirmed by flow cytometric identification of a rat macrophage marker (ED-1). Macrophages were separately seeded into 24-well plates at 0·2 × 106 cells per well in 1 ml of DMEM/10% FCS and incubated overnight. The medium was changed to 1 ml of DMEM/2% FCS. Dexamethasone at a concentration of 10−7 m or ethanol vehicle were added directly to each well and cells were incubated for 3 h and 24 h. To obtain cell surface lipocortin 1, cell monolayers were washed with 200 μl of PBS containing 10 mm EDTA [20]. Total protein was concentrated 10-fold by centrifuge cryo-evaporation (Jouan, St Nazaire, France) and resuspended in PBS for Western blot analysis. Intracellular lipocortin 1 content was measured by flow cytometry.
Western blot analysis
Aliquots of sample material were loaded to a 1·5-mm 12% Tris–HCL gel (BioRad, Hercules, CA). SDS–PAGE was performed by conventional methods. The proteins were transferred electrophoretically onto a nitrocellulose membrane. Following electrophoresis, the membrane was blotted and then incubated with anti-lipocortin 1 MoAb (1 μg/ml) and horseradish peroxidase-conjugated rabbit anti-mouse IgG (Sigma). The blot was finally developed using a chemiluminescence system (Amersham, North Ryde, Australia). The molecular masses of the positive bands were determined by comparison with the migration of prestained molecular mass standards (BioRad).
Flow cytometric detection of macrophage lipocortin 1 and iNOS
Intracellular expression of macrophage phenotype antigen, lipocortin 1, and iNOS was detected using a permeabilization flow cytometric method, as described [31]. For double-labelling of intracellular macrophage antigen and lipocortin 1, cells were fixed by suspension in 2% paraformaldehyde, permeabilized by suspension in a solution of 0·2% saponin (Sigma) in PBS, then incubated sequentially with ED-1 MoAb (or control MoAb) and FITC-conjugated goat anti-mouse IgG made up in 0·2% saponin/PBS. Cells were subsequently incubated sequentially with the 1-B anti-lipocortin 1 MoAb (or control MoAb) biotinylated with biotin-N-hydroxysuccinimide ester (Vector, Peterborough, UK) according to the manufacturer's instructions, and PE-streptavidin (Vector, Burlingame, CA) also made up in 0·2% saponin/PBS. Cultured synovial macrophage iNOS and lipocortin 1 were single-labelled using a specific mouse anti-iNOS MoAb (Transduction Labs, Lexington, KT) and anti-lipocortin 1 MoAb. Permeabilization was reversed by washing in PBS. Intracellular antigen fluorescence was not affected by enzyme digestion treatments (data not shown). The labelled cells were analysed on a EPICS 752 flow cytometer (Coulter, Hialeah, FL).
RESULTS
To confirm the production of NO by AA synovium, supernatants from cultured synovial biopsies from animals killed at day 14 of disease evolution were assayed for nitrite by Griess reaction. All tissues tested produced NO, and the mean (±s.e.m.) NO concentration in cultured AA synovial explant supernatants (per 100 mg wet weight) was 13·6 ± 3·1 mm. Cultured synovial macrophages from AA rats produced NO without stimulation (14·5 ± 2·1 nmol/105 cells per 24 h) (Fig. 1), suggesting that these cells retained aspects of their in vivo activation status.
Fig. 1.
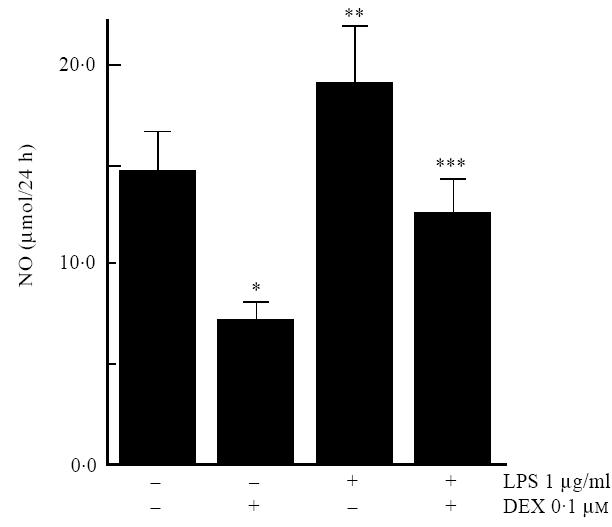
Synovial macrophages from rats with adjuvant arthritis were cultured for 24 h, and nitric oxide (NO) production analysed by measurement of nitrite in culture supernatants. Dexamethasone 10−7m (DEX) inhibited constitutive NO production (*P = 0·002). LPS 1 μg/ml induced NO production (**P = 0·001), and this induction was also inhibited by DEX (***P<0·001
The effects of glucocorticoids on synovial macrophage NO production were examined in experiments using cultured AA synovial macrophages. Dexamethasone 10−7 m significantly inhibited constitutive NO production (P = 0·002) (Fig. 1). LPS 1 μg/ml stimulated marked and statistically significant increases in NO production (P < 0·001), which was significantly inhibited by dexamethasone 10−7 m (P < 0·001) (Fig. 1).
Examination of AA synovial macrophages in double-labelling flow cytometry experiments labelled with an anti-lipocortin 1 MoAb and the macrophage phenotype antigen MoAb ED-1 revealed that AA synovial macrophages contained lipocortin 1. ED-1 labelled 36 ± 3% of synovial cells, and lipocortin 1 fluorescence was detected in 66 ± 8% of ED-1+ cells (Fig. 2). The effects of dexamethasone on the induction of cell surface and intracellular lipocortin 1 were examined in synovial macrophages from AA rats, and peritoneal macrophages from the same animals were also examined. Cells were cultured in the absence or presence of dexamethasone 10−7 m. In comparison with control treatment, synovial macrophage surface lipocortin 1 was increased within 3 h of treatment with dexamethasone, and increased further at 24 h (Fig. 3). In contrast, dexamethasone induction of intracellular lipocortin 1 content was not detected until 24 h of treatment in both synovial (mean fluorescence intensity (MFI) 499 ± 54 versus 399 ± 74, P < 0·05) and peritoneal macrophages (MFI 459 ± 64 versus 323 ± 80, P < 0·05) (Fig. 4).
Fig. 2.
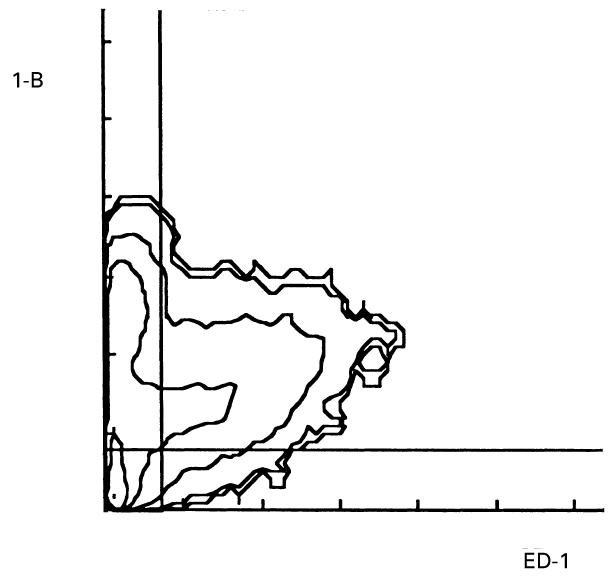
Intracellular lipocortin 1 in adjuvant arthritis (AA) synovial macrophages. Single-cell suspensions were produced by enzyme digestion of tissue from the joints of rats with AA, and intracellular lipocortin 1 detected by permeabilization flow cytometry. Shown is a fluorescence contour map of macrophage phenotype antigen (ED-1, abscissa) and lipocortin 1 (1-B, ordinate), representative of data from nine separate experiments. The negative cutoff lines shown were determined with a negative MoAb control. Cells in the upper right quadrant are positive for both ED-1 and 1-B, i.e. are macrophages expressing lipocortin 1 protein; 66±8% of ED-1+ synovial macrophages exhibited lipocortin 1 fluorescence.
Fig. 3.
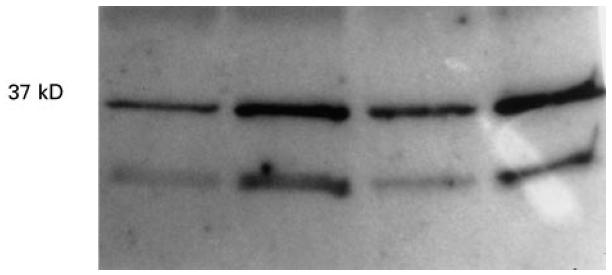
Synovial macrophage cell surface lipocortin 1. Synovial macrophages from rats with adjuvant arthritis (AA) were exposed to dexamethasone 10−7m for 3 h and 24 h, cell surface lipocortin 1 eluted with 10 mm EDTA, and detected by Western blotting using a specific anti-lipocortin 1 MoAb. Lanes 1 and 2 show 3 hour control- and dexamethasone-treated cell surface eluates, lanes 3 and 4 show 24 hour control- and dexamethasone-treated cell surface eluates. A 37-kD band representing full-length lipocortin 1 was increased at 3 h and increased further at 24 h by dexamethasone. A smaller 33-kD fragment of lipocortin 1 is also shown to be increased by dexamethasone.
Fig. 4.
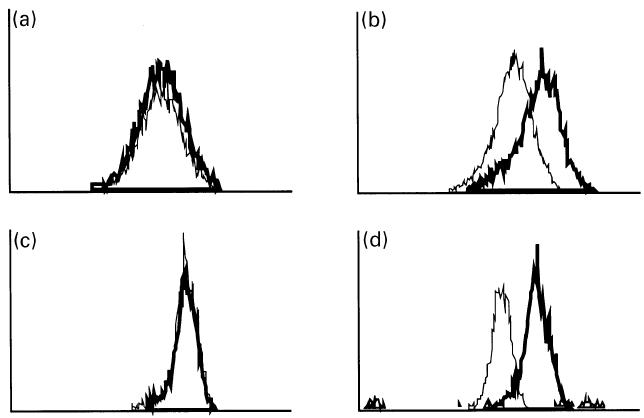
Synovial macrophage intracellular lipocortin 1. Lipocortin 1 protein was detected in cultured adjuvant arthritis (AA) synovial (a,b) and peritoneal (c,d) macrophages by permeabilization flow cytometry using a specific anti-lipocortin 1 MoAb. Data are from a single experiment representative of four experiments, and show fluorescence intensity on the abscissa (log scale) and relative cell number on the ordinate. (a) After 3 h of treatment with dexamethasone 10−7m (—), lipocortin 1 fluorescence was no different with control treatment (—). (b) After 24 h of treatment, dexamethasone was associated with increased intracellular lipocortin 1. Similar results were obtained in peritoneal macrophages studied for (c) 3 h and (d) 24 h.
The role of lipocortin 1 in glucocorticoid modulation of AA synovial macrophage NO production was next studied. Treatment of synovial macrophages with anti-lipocortin 1 MoAb alone was without effect (data not shown). Anti-lipocortin 1 MoAb in doses as low as 0·1 μg/ml was associated with significant abrogation of the inhibitory effects of dexamethasone on LPS-stimulated macrophage NO production (16·0 ± 3·0 nmol/105 cells per 24 h, P = 0·02). Significant prevention of the inhibitory effects of dexamethasone was also seen at five- and 10-fold higher doses of 1-B (0·5 μg/ml, P = 0·002; 1 μg/ml, P = 0·035), but doses <0·1 μg/ml were without effect.
Constitutive expression of iNOS protein by AA synovial macrophages was demonstrated by permeabilization flow cytometry of synovial macrophages cultured for 24 h without additional stimuli (MFI 17·3 ± 0·9 units) (Fig. 5). LPS induced a significant increase in iNOS MFI (34·1 ± 4·4, P = 0·04, cf control), which was significantly inhibited by dexamethasone 0·1 μm (13·5 ± 3·7, P = 0·03) (Fig. 5). In contrast to the effects on NO production, no effect of anti-LC-1 MoAb (0·5 μg/ml) was observed on dexamethasone inhibition of synovial macrophage intracellular iNOS protein (MFI 14·2 ± 1·7 units, NS).
Fig. 5.
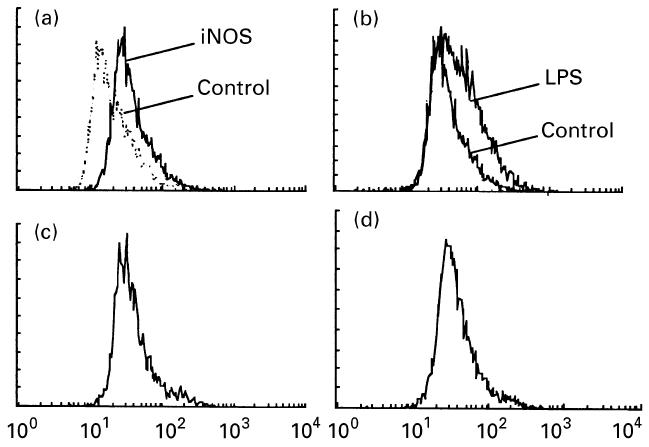
Synovial macrophage intracellular inducible nitric oxide synthase (iNOS). iNOS protein was detected in cultured adjuvant arthritis (AA) synovial macrophages by permeabilization flow cytometry using a specific anti-iNOS MoAb. Data are from a single experiment representative of six experiments, and show fluorescence intensity on the abscissa (log scale) and relative cell number on the ordinate. iNOS protein was detected in cultured AA synovial macrophages treated with lipopolysaccharide (LPS) 1 μg/ml and dexamethasone 10−7 m by permeabilization flow cytometry. (a) iNOS fluorescence was detected in unstimulated synovial macrophages (MFI P<0·05, cf negative control labelling, shown in outline). (b) iNOS expression was significantly increased by LPS (P=0·04, cf control treatment). (c) iNOS expression induced by LPS was significantly inhibited by dexamethasone (P=0·03). (d) Dexamethasone inhibition of iNOS was not reversed by anti-lipocortin 1 MoAb treatment.
DISCUSSION
RA is a complex, idiopathic disease characterized by abnormal activation of the immune system and manifested by inflammation of synovial joints. Although other cell types are important in RA, macrophages constitute a significant proportion of cells at the cartilage–pannus junction, and are capable of the production of many of the proinflammatory cytokines found in RA tissue, including IL-1, TNF-α and granulocyte-macrophage colony-stimulating factor [32, 33]. These cytokines have autocrine/paracrine effects on macrophage function, and also contribute to the activation of other cell types. The importance of the macrophage in animal models of RA has also been demonstrated by the clinical effectiveness of macrophage depletion strategies [34]. Nitric oxide is produced in sites of joint inflammation by the action of iNOS in cells such as macrophages, synovial fibroblasts, osteoblasts and chondrocytes, under the influence of endotoxin and proinflammatory cytokines such as IL-1 and TNF-α [3, 4, 7––9]. Although in vitro production of NO by fibroblast-like synoviocytes derived from human RA tissue has been reported [4], the predominant location of iNOS and source of NO in RA synovium is the macrophage [3].
Our finding of iNOS in ED-1+ macrophages in rat AA is in keeping with observations in human RA synovium, and with earlier studies in this model [5]. We have demonstrated constitutive protein expression and activity of iNOS in synovial macrophages cultured for 24 h after harvest from the joints of animals with AA, and also confirmed the endotoxin-inducibility of iNOS protein and activity in these cells. The finding that dexamethasone suppressed constitutive and endotoxin-induced NO production and iNOS protein expression by AA synovial macrophages is the first demonstration of these effects of glucocorticoids in an animal model of RA. Glucocorticoids have been previously shown to inhibit NO production in synovial fibroblasts and chondrocytes [4], but our finding represents the first demonstration of this in synovial macrophages, which are arguably the more important source of NO in arthritis.
Coexpression of lipocortin 1 and iNOS by synovial macrophages supports the concept that the inhibition of NO production may be among the anti-inflammatory actions of lipocortin 1 in the joint. Glucocorticoid induction of lipocortin 1 has been found in a range of cell types in vitro [35––38]. Our findings indicate the induction and translocation to the cell surface of lipocortin 1 in AA synovial macrophages after exposure to dexamethasone. We show that induction of cell surface lipocortin 1 occurs within 3 h of glucocorticoid treatment in vitro, whereas increases in the intracellular lipocortin 1 pool are not detected until later. The influence of anti-lipocortin 1 antibodies on the anti-inflammatory effects of glucocorticoids is consistent with the hypothesis that cell surface lipocortin 1 is important in the anti-inflammatory action of this molecule [39, 40]. The lack of correlation between effects of lipocortin 1 antagonism on NO production and on iNOS content suggests that lipocortin 1 may induce enzymatic inhibition rather than inhibition of iNOS expression. These results differ from those of Wu et al., who reported effects of lipocortin 1 on both iNOS and NO production [28]. Cell surface receptors for lipocortin 1 have been described on monocytes [41, 42], and it may be hypothesized that translocation of intracellular lipocortin 1 to these sites leads to the induction of an iNOS inhibitory mechanism. In addition, lipocortin 1 has been reported to inhibit superoxide production [43], and proinflammatory interactions between oxygen radicals and NO may therefore also be influenced by lipocortin 1 [11, 44]. Other indirect mechanisms of action of lipocortin 1 could include inhibition of IL-1 synthesis, which has been reported in mouse neutrophils [26]. The specific mechanism through which blockade of lipocortin 1 affects iNOS activity requires further investigation.
We have recently described the involvement of lipocortin 1 in endogenous and exogenous glucocorticoid inhibition of carrageenan-induced joint inflammation [25], a model in which NO is a known mediator [45]. Taken together with the current data, these results strongly suggest that lipocortin 1 has a role in glucocorticoid inhibition limitation of NO-mediated joint injury. Given that NO has recently been implicated in cytokine-induced cartilage degradation [9, 10], these findings raise the possibility that synovial lipocortin 1 effects on NO production are involved in the reported inhibitory effect of glucocorticoids on joint erosions in RA [17].
Several observations support the relevance of these findings to human disease. We have recently demonstrated adrenal glucocorticoid regulation of leucocyte lipocortin 1 in adjuvant arthritis [46], and endogenous glucocorticoids have been demonstrated to inhibit inducible NO production in mice [47]. The therapeutic effects of glucocorticoids are associated with suppression of NO production in humans with RA [16]. Macrophages have been shown to express lipocortin 1 in RA synovium [48], a distribution similar to that we have reported in human peripheral blood leucocytes [31]. Moreover, RA mononuclear leucocytes demonstrate impaired lipocortin 1 response to glucocorticoids [20], and anti-lipocortin 1 autoantibodies are associated with glucocorticoid resistance [49]. The importance of macrophages in inflammatory arthritis, the glucocorticoid-sensitive production of NO by these cells, the presence of lipocortin 1 in these same cells, and the effect of anti-lipocortin 1 MoAb on macrophage NO production, all support the hypothesis that lipocortin 1 is an important mediator of glucocorticoid anti-inflammatory effects upon synovial macrophages. The emergence of lipocortin 1 as an important mediator of the effects of therapeutic and/or endogenous glucocorticoids in arthritis has the potential to lead to the development of therapeutic strategies using recombinant lipocortin 1 or related peptides. Studies of the effects of lipocortin 1 on this and other aspects of human synovial cell function are required, and are in progress.
References
- 1.Sano H, Hla T, Maier JA, et al. In vivo cyclooxygenase expression in synovial tissues of patients with rheumatoid arthritis and osteoarthritis and rats with adjuvant and streptococcal cell wall arthritis. J Clin Invest. 1992;89:97–108. doi: 10.1172/JCI115591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santos L, Tipping PG. Attenuation of adjuvant arthritis in rats by treatment with oxygen radical scavengers. Immunol Cell Biol. 1994;72:406–14. doi: 10.1038/icb.1994.60. [DOI] [PubMed] [Google Scholar]
- 3.Sakurai H, Kohsaka H, Liu M-F, et al. Nitric oxide production and inducible nitric oxide synthase expression in inflammatory arthritides. J Clin Invest. 1995;96:2357–63. doi: 10.1172/JCI118292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grabowski PS, MacPherson H, Ralston SH. Nitric oxide production in cells derived from the human joint. Br J Rheumatol. 1996;35:207–12. doi: 10.1093/rheumatology/35.3.207. [DOI] [PubMed] [Google Scholar]
- 5.Santos LL, Morand EF, Holdsworth SR. Suppression of adjuvant arthritis and synovial macrophage inducible nitric oxide by N-iminoethyl-l-ornithine, a nitric oxide synthase inhibitor. Inflammation. 1997;21:299–311. doi: 10.1023/a:1027397816209. [DOI] [PubMed] [Google Scholar]
- 6.Moncada S, Palmer RMJ, Higgs EA. Nitric oxide: physiology, pathophysiology and pharmacology. Pharmacol Rev. 1991;43:109–42. [PubMed] [Google Scholar]
- 7.Stefanovic-Racic M, Stadler J, Georgescu HI, Evans CH. Nitric oxide synthesis and its regulation by rabbit synoviocytes. J Rheumatol. 1994;21:1892–8. [PubMed] [Google Scholar]
- 8.Ralston SH, Todd D, Helfrich M, Benjamin N, Grabowski PS. Human osteoblast-like cells produce nitric oxide and express inducible nitric oxide synthase. Endocrinology. 1994;135:330–6. doi: 10.1210/endo.135.1.7516867. [DOI] [PubMed] [Google Scholar]
- 9.Murrell GA, Jang D, Williams RJ. Nitric oxide activates metalloprotease enzymes in articular cartilage. Biochem Biophys Res Commun. 1995;206:15–21. doi: 10.1006/bbrc.1995.1003. [DOI] [PubMed] [Google Scholar]
- 10.Taskiran D, Stefanovic-Racic M, Georgescu H, Evans C. Nitric oxide mediates suppression of cartilage proteoglycan synthesis by interleukin-1. Biochem Biophys Res Commun. 1994;200:142–8. doi: 10.1006/bbrc.1994.1426. [DOI] [PubMed] [Google Scholar]
- 11.Kaur H, Halliwell B. Evidence for nitric oxide-mediated oxidative damage in chronic inflammation. Nitrotyrosine in serum and synovial fluid from rheumatoid patients. FEBS Letters. 1994;350:9–12. doi: 10.1016/0014-5793(94)00722-5. [DOI] [PubMed] [Google Scholar]
- 12.Farrell AJ, Blake DR, Palmer RM, Moncada S. Increased concentrations of nitrite in synovial fluid and serum samples suggest increased nitric oxide synthesis in rheumatic diseases. Ann Rheum Dis. 1992;51:1219–22. doi: 10.1136/ard.51.11.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morand EF, Goulding NJ. Glucocorticoids in rheumatoid arthritis—mediators and mechanisms. Br J Rheumatol. 1993;32:816–9. doi: 10.1093/rheumatology/32.9.816. [DOI] [PubMed] [Google Scholar]
- 14.Moncada S, Palmer RMJ. Inhibition of the induction of nitric oxide synthase by glucocorticoids: yet another explanation for their anti-inflammatory effects? TIPS. 1991;12:130–1. doi: 10.1016/0165-6147(91)90528-z. [DOI] [PubMed] [Google Scholar]
- 15.Hukkanen M, Hughes FJ, Buttery LD, et al. Cytokine-stimulated expression of inducible nitric oxide synthase by mouse, rat, and human osteoblast-like cells and its functional role in osteoblast metabolic activity. Endocrinology. 1995;136:5445–53. doi: 10.1210/endo.136.12.7588294. [DOI] [PubMed] [Google Scholar]
- 16.Stichtenoth DO, Fauler J, Zeidler H, Frolich JC. Urinary nitrate excretion is increased in patients with rheumatoid arthritis and reduced by prednisolone. Ann Rheum Dis. 1995;54:820–4. doi: 10.1136/ard.54.10.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirwan JR. The effect of glucocorticoids on joint destruction in rheumatoid arthritis. N Engl J Med. 1995;333:142–6. doi: 10.1056/NEJM199507203330302. [DOI] [PubMed] [Google Scholar]
- 18.Vishwanath BS, Frey FI, Bradbury M, Dallman MF, Frey BM. Adrenalectomy decreases lipocortin-1 messenger ribonucleic acid and tissue protein content in rats. Endocrinology. 1992;130:585–91. doi: 10.1210/endo.130.2.1531128. [DOI] [PubMed] [Google Scholar]
- 19.Goulding NJ, Godolphin JL, Sharland PR, et al. Anti-inflammatory lipocortin 1 production by peripheral blood leucocytes in response to hydrocortisone. Lancet. 1990;335:1416–8. doi: 10.1016/0140-6736(90)91445-g. [DOI] [PubMed] [Google Scholar]
- 20.Morand EF, Jefferiss CM, Dixey J, Mitra D, Goulding NJ. Impaired glucocorticoid induction of mononuclear leukocyte lipocortin 1 in rheumatoid arthritis. Arthritis Rheum. 1994;37:207–11. doi: 10.1002/art.1780370209. [DOI] [PubMed] [Google Scholar]
- 21.Perretti M, Flower RJ. Modulation of IL-1-induced neutrophil migration by dexamethasone and lipocortin 1. J Immunol. 1993;150:992–9. [PubMed] [Google Scholar]
- 22.Carey F, Forder R, Edge MD, et al. Lipocortin-1 fragment modifies the pyrogenic actions of cytokines in rats. Am J Physiol. 1990;259:R266–R269. doi: 10.1152/ajpregu.1990.259.2.R266. [DOI] [PubMed] [Google Scholar]
- 23.Cirino G, Peers SH, Flower RJ, Browning JL, Pepinsky RB. Human recombinant lipocortin 1 has acute local anti-inflammatory properties in the rat paw edema test. Proc Natl Acad Sci USA. 1989;86:3428–32. doi: 10.1073/pnas.86.9.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Errasfa M, Russo-Marie F. A purified lipocortin shares the anti-inflammatory effect of glucocorticosteroids in vivo in mice. Br J Pharmacol. 1989;97:1051–8. doi: 10.1111/j.1476-5381.1989.tb12561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang YH, Leech M, Hutchinson P, Holdworth SR, Morand EF. Inflammation. 1997. Anti-inflammatory effect of lipocortin 1 in experimental arthritis. in press. [DOI] [PubMed] [Google Scholar]
- 26.Perretti M, Ahluwalia A, Harris JG, Harris HJ, Wheller SK, Flower RJ. Acute inflammatory response in the mouse: exacerbation by immunoneutralization of lipocortin 1. Brit J Pharmacol. 1996;117:1146–54. doi: 10.1111/j.1476-5381.1996.tb16709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim KM, Kim DK, Park YM, Kim CK, Na DS. Annexin-I inhibits phospholipase A2 by specific interaction, not by substrate depletion. FEBS Letters. 1994;343:251–5. doi: 10.1016/0014-5793(94)80566-0. [DOI] [PubMed] [Google Scholar]
- 28.Wu C-C, Croxtall JD, Perretti M, et al. Lipocortin 1 mediates the inhibition by dexamethasone of the induction by endotoxin of nitric oxide synthase in the rat. Proc Natl Acad Sci USA. 1995;92:3473–7. doi: 10.1073/pnas.92.8.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pepinsky RB, Sinclair LK, Dougas I, Liang C-M, Lawton P, Browning JL. Monoclonal antibodies to lipocortin-1 as probes for biological function. FEBS Letters. 1990;261:247–52. doi: 10.1016/0014-5793(90)80564-y. [DOI] [PubMed] [Google Scholar]
- 30.Oswald IP, Gazzinelli RT, Sher A, James SL. IL-10 synergizes with IL-4 and transforming growth factor-beta to inhibit macrophage cytotoxic activity. J Immunol. 1992;148:3578–82. [PubMed] [Google Scholar]
- 31.Morand EF, Hutchinson P, Hargreaves A, Goulding NJ, Boyce NW, Holdsworth SR. Detection of intracellular lipocortin 1 in human leukocyte subsets. Clin Immunol Immunopathol. 1995;76:195–202. doi: 10.1006/clin.1995.1115. [DOI] [PubMed] [Google Scholar]
- 32.Alvaro-Gracia JM, Zvaifler NJ, Brown CB, Kaushansky K, Firestein GS. Cytokines in chronic inflammatory arthritis VI. Analysis of the synovial cells involved in granulocyte-macrophage colony-stimulating factor production and gene expression in rheumatoid arthritis and its regulation by IL-1 and tumour necrosis factor-α. J Immunol. 1991;146:3365–71. [PubMed] [Google Scholar]
- 33.Chu CQ, Field M, Feldman M, Maini RN. Localization of tumor necrosis factor alpha in synovial tissues and at the cartilage pannus junction in patients with rheumatoid arthritis. Arthritis Rheum. 1991;34:1125–32. doi: 10.1002/art.1780340908. [DOI] [PubMed] [Google Scholar]
- 34.Kinne RW, Schmidt CB, Buchner E, Hoppe R, Nurnberg E, Emmrich F. Treatment of rat arthritides with clodronate-containing liposomes. Scand J Rheumatol. 1995;101:91–97. doi: 10.3109/03009749509100907. [DOI] [PubMed] [Google Scholar]
- 35.Fuller PJ, Verity K. Somatostatin gene expression in the thymus gland. J Immunol. 1989;143:1015–7. [PubMed] [Google Scholar]
- 36.Suarez F, Rothhut B, Comera C, Touqui L, Marie FR, Silve C. Expression of annexin I, II, V, and VI by rat osteoblasts in primary culture: stimulation of annexin I expression by dexamethasone. J Bone Miner Res. 1993;8:1201–10. doi: 10.1002/jbmr.5650081007. [DOI] [PubMed] [Google Scholar]
- 37.Higuchi H, Katoh N, Miyamoto T, Uchida E, Yuasa A, Takahashi K. Dexamethasone-induced haptoglobin release by calf liver parenchymal cells. Am J Vet Res. 1994;55:1080–5. [PubMed] [Google Scholar]
- 38.Newman SP, Flower RJ, Croxtall JD. Dexamethasone suppression of IL-1β induced cyclooxygenase 2 expression is not mediated by lipocortin 1 in A549 cells. Biochem Biophys Res Commun. 1994;202:931–9. doi: 10.1006/bbrc.1994.2019. [DOI] [PubMed] [Google Scholar]
- 39.Goulding NJ, Guyre PM. Regulation of inflammation by lipocortin 1. Immunol Today. 1992;13:295–7. doi: 10.1016/0167-5699(92)90040-E. [DOI] [PubMed] [Google Scholar]
- 40.Goulding NJ, Guyre PM. Glucocorticoids, lipocortins and the immune response. Curr Opin Immunol. 1993;5:108–13. doi: 10.1016/0952-7915(93)90089-b. [DOI] [PubMed] [Google Scholar]
- 41.Goulding NJ, Jefferiss CM, Pan L, Rigby WF, Guyre PM. Specific binding of lipocortin 1 (annexin 1) to monocytes and neutrophils is decreased in rheumatoid arthritis. Arthritis Rheum. 1992;35:1395–7. doi: 10.1002/art.1780351126. [DOI] [PubMed] [Google Scholar]
- 42.Goulding NJ, Pan LY, Wardwell K, Guyre VC, Guyre PM. Evidence for specific annexin-I binding proteins on human monocytes. Biochem J. 1996;316:593–7. doi: 10.1042/bj3160593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maridonneau-Parini I, Errasfa M, Russo-Marie F. Inhibition of O−2 generation by dexamethasone is mimicked by lipocortin 1 in alveolar macrophages. J Clin Invest. 1989;83:1936–40. doi: 10.1172/JCI114101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Halliwell B. Oxygen radicals, nitric oxide and human inflammatory joint disease. Ann Rheum Dis. 1995;54:505–10. doi: 10.1136/ard.54.6.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei XQ, Charles IG, Smith A, et al. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995;375:408–11. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- 46.Yang YH, Hutchinson P, Leech M, Morand EF. J Rheumatol. 1997. Exacerbation of adjuvant arthritis by adrenalectomy is associated with reduced leukocyte lipocortin 1. in press. [PubMed] [Google Scholar]
- 47.Szabo C, Thiemermann C, Wu C-C, Perretti M, Vane JR. Attenuation of the induction of nitric oxide synthase by endogenous glucocorticoids accounts for endotoxin tolerance in vivo. Proc Natl Acad Sci USA. 1994;91:271–5. doi: 10.1073/pnas.91.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goulding NJ, Dixey J, Morand EF, et al. Differential expression of annexins-I, -II, -IV and -VI in synovium. Ann Rheum Dis. 1995;54:841–5. doi: 10.1136/ard.54.10.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Podgorski MR, Goulding NJ, Hall ND, Flower RJ, Maddison PJ. Autoantibodies to lipocortin 1 are associated with impaired corticosteroid responsiveness in rheumatoid arthritis. J Rheumatol. 1992;19:1668–71. [PubMed] [Google Scholar]


