Abstract
Enhancement of DNA vaccine immunogenicity is a current topic of high priority in the field of applied immunology, especially as a means of controlling HIV infection. The adjuvant effect of Ubenimex (UBX), an anti-cancer immunomodulator, on a DNA AIDS vaccine which we developed was examined in a murine model. UBX was formulated into a preparation containing DNA plasmids encoding env and rev genes of HIV-1 strain IIIB, and was inoculated intramuscularly into BALB/c mice. The sera obtained with this mixture had 23–25 times higher specific IgG titres than those obtained without the use of the adjuvant. UBX also elicited both a stronger HIV-1-specific DTH reaction, as measured by the footpad swelling test, and stronger cytotoxic T lymphocyte activity, as assayed by the 51Cr-release method, compared with responses using DNA alone. The cytokine secretion profile of restimulated immune lymphoid cells showed that UBX raised IL-2 and interferon-gamma levels and decreased IL-4 production. HIV-1-specific immunoglobulin subtype analysis demonstrated that UBX stimulated IgG2a production but suppressed synthesis of IgG1 and IgE. These results indicate that activation of the T-helper type 1 subset was induced by UBX, suggesting a mechanism of immunomodulation mediated by this agent. We conclude that UBX acts as an immunologic adjuvant for DNA vaccination against HIV-1. UBX may be a suitable adjuvant for clinical use because of its lack of antigenicity and low toxicity.
Keywords: DNA vaccine, HIV, Ubenimex
INTRODUCTION
The efficacy of using plasmid DNA-encoding microbial antigens to raise humoral and cellular immune responses against pathogens has been reported in several animal models, and this method is referred to as DNA vaccination [1–4]. In the field of AIDS vaccine development, we and others have succeeded in inducing HIV-1-specific immune responses by DNA vaccination [5, 6], and increased protection against laboratory-isolated HIV-1 challenge was recently reported in a chimpanzee model system [7]. This novel vaccination approach has therefore received much attention. DNA vaccination offers potential advantages over traditional protein-based vaccines in areas such as stability, ease of production, and induction of cell-mediated immunity [8]. To obtain stronger responses, approaches such as the use of particle bombardment by means of a gene gun system [2, 6], or application of local anaesthetics to facilitate DNA entry into muscle cells [9] have been attempted and have met with substantial success. However, most previous DNA vaccine studies employed DNA plasmids alone, and incorporation of immunologic adjuvants into vaccine formulations was not considered.
Of the various murine lymphocytes, CD4+ T cells play a central role in most immune reactions and are required for generation of cellular and humoral immune responses [10]. There has been an increase in our understanding of the CD4+ T cell, and a clear association between cytokine secretion pattern and cell function has been established [11]. T-helper type-1 (Th1) cells mediate DTH reactions and activate macrophage killing of intracellular pathogens, but these cells can also enhance production of IgG2a antibodies, which are important in protection against viral infections [12, 13]. CD8+ cytotoxic T lymphocytes (CTL) primed with Th1-type cytokines [14] are known to be involved in anti-viral mechanisms through their ability to directly recognize and lyse pathogen-infected cells [15].
Based on the above considerations, we speculated that an immunomodulating agent which could induce Th1-type responses might be useful for intensifying the potency of a vaccine, and concentrated our efforts on one promising immunomodulator, ubenimex (UBX; ((2S, 3R)-3-amino-2-hydroxy-4-phenyl-butyryl-l-leucine), a small molecular weight aminopeptidase inhibitor isolated from a culture filtrate of Streptomyces olivoreticuli [16]. UBX has been used for immunotherapy of acute leukaemia [17], and is also known to augment production of IL-2 [18] and to activate macrophages [19] via its action on the membrane aminopeptidase activity of lymphoid cells [20]. Because of these unique immunomodulatory properties, UBX is capable of acting as an immunologic adjuvant targeting Th1-type responses. Furthermore, since UBX exerts few adverse side effects and is not antigenic [21], it might prove to be a promising adjuvant candidate in strategies for developing an effective AIDS vaccine. In the present study, we demonstrate that UBX acts as an effective adjuvant for DNA vaccination against HIV-1 by elicitation of Th1-type cytokine production.
MATERIALS AND METHODS
Vaccine formulation and animal immunization
Immunogenic DNA, pCMV160IIIB and pcREV, which encode the env and rev genes of HIV-1 strain IIIB (HIV-1IIIB), respectively, featured in our previous report [5]. Although our DNA vaccine formulation was designed to elicit an env-specific immune response, the rev expression plasmid was included because a previous study [22] showed that expression of env protein is dependent on rev co-expression. UBX (Bestatin) was kindly provided by Nippon Kayaku Co., Ltd. (Tokyo, Japan). Two micrograms each of pCMV160IIIB and pcREV (hereafter referred to IIIB/REV) were diluted in sterile PBS and mixed with 10, 100 or 500 μg of UBX. BALB/c mice, aged 8–10 weeks (Japan SLC Inc., Shizuoka, Japan), were injected in the biceps femoris muscle with 100 μl of the vaccine preparation. None of the mice received a booster immunization.
ELISA
ELISA was used for titration of serum antigen-specific IgG, IgG1, IgG2a and IgE responses, and for quantification of the cytokines produced by in vitro restimulated immune lymphoid cells. Samples of blood were collected by retro-orbital puncture at 2, 4 and 8 weeks after immunization, and antibody titration was performed as follows. A gp160 protein of HIV-1IIIB (provided by courtesy of Dr B. Wahren, Department of Clinical Virology, Karolinska Institute, Stockholm, Sweden) was employed as an antigen for HIV-1IIIB. It was coated on 96-well microtitre plates (Nunc, Roskilde, Denmark), and after blocking with 3% bovine serum albumin (BSA) in PBS, serially diluted antisera was added and incubated at 37°C for 2 h. Peroxidase-conjugated goat anti-mouse IgG (Organon Teknika Corp., West Chester, PA) was used as the secondary antibody, and plates were developed with 3,3′,5,5′-tetramethylbenzidine (Dako Corp., Carpinteria, CA). Titres were expressed as the reciprocal log2 value of the final detectable dilution, which was defined as 2 s.d. above the mean optical density (OD) at 450 nm of the pre-immune samples at the same titration point. The antigen-specific IgG1, IgG2a and IgE titres were determined using sera collected 4 weeks after immunization. Horseradish peroxidase (HRP)-coupled anti-mouse IgG1 and IgG2a (Organon Teknika) or IgE (Southern Biotechnology Associates, Inc., Birmingham, AL) were used as the secondary antibodies, and results are expressed as the reciprocal log2 titre. Other conditions were the same as in the above procedure.
For quantification of IL-2, interferon-gamma (IFN-γ), and IL-4, mice were killed at 3 weeks after immunization, and freshly isolated splenic mononuclear cells were cultured in the presence of V3 peptide. This peptide, RGPGRAFVTIGK, is known as both a helper [23] and CTL [24] epitope for HIV-1IIIB. Culture media were collected at 48 h after initiating cell culture, and cell-free supernatants were stored at −80°C until assayed. Amounts of cytokines in these samples were measured with the appropriate commercial ELISA kits (Cytoscreen; Biosource, New Hampshire, MA) according to the manufacturer's instructions.
DTH response
The DTH reaction was assayed by a footpad swelling test as follows. Three weeks after immunization, mice were injected in the right footpad with 5 μg of V3 peptide. The same amount of sperm whale myoglobin peptide, ALVEADVA [25], was injected into the left footpad as a control. After 24 h, the extent of footpad swelling was assessed as the difference between the pre- and post-injection footpad thickness which were measured using a dial thickness gauge (Ozaki Seisakusho, Tokyo, Japan) and expressed in units of 10−2mm.
Determination of cytolytic activity
Three weeks after immunization, samples of splenocytes were harvested and cultured in the presence of V3 peptide. The target cells were 51Cr-sodium chromate-labelled syngeneic cell lines (P815; H-2d) pulsed with or without the same peptide. The latter were prepared to evaluate non-specific cytolytic activity. After a 5-day culture, bulk splenic mononuclear cells as effectors were cocultivated with the peptide-pulsed target cells at effector-to-target (E/T) ratios ranging from 5 to 80. Non-peptide-pulsed targets were mixed with the effectors at an E/T ratio of 80 only. Target cell lysis was measured by a gamma-ray counting of cell-free supernatants to determine the amount of 51Cr released. The percentage of chromium release was calculated as 100 × (Cr release in sample − spontaneous Cr release)/(maximum Cr release − spontaneous Cr release). Target cells incubated in the medium with or without 5% Triton X-100 were used to determine maximum or spontaneous 51Cr release, respectively.
Statistical analysis
Statistical analyses were conducted with one-way factorial analysis of variance (anova) and significance was defined at P < 0·05.
RESULTS
UBX augmented HIV-1-specific IgG production and altered the subclass profile
As Fig. 1a shows, the HIV-1-specific IgG titre was significantly enhanced when 100 or 500 μg of UBX were administered together with immunogenic DNA, and this was true of both in both 4- and 8-week samples (P < 0·01 and 0·05, respectively), whereas a 10-μg dose had no effect. The antibody titres of sera collected 2 weeks following inoculation were similar to those of 4-week samples (data not shown). In terms of antibody production, there was no statistical difference between 100- and 500-μg doses. Figure 1b shows the antigen-specific IgG1, IgG2a and IgE titres of antisera collected 4 weeks after immunization. When the immunogen was mixed with 100 or 500 μg of UBX, there was remarkable augmentation of IgG2a production which was accompanied by a significant decrease in the IgG1 and IgE titres. The relationship between the Th1–Th2 cell dichotomy and the predominant immunoglobulin subtype has been established [26], and our data suggest that a UBX dose of 100–500 μg was effective in stimulating the Th1 subset.
Fig. 1.
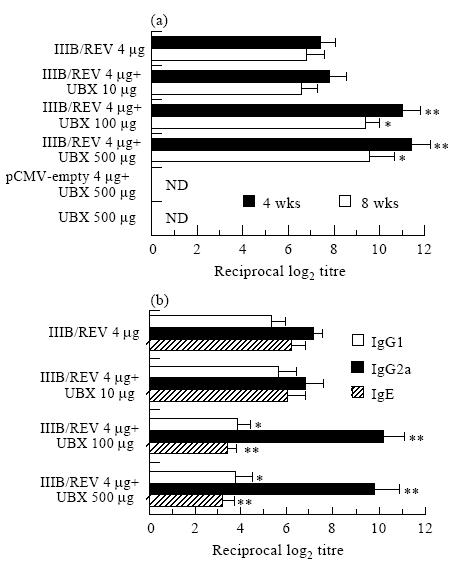
Adjuvant effect of Ubenimex (UBX) on humoral immune responses induced by DNA vaccination. The HIV-1-specific serum IgG titre (a) and IgG1, IgG2a and IgE production (b) are represented in the separate panels. Mice were intramuscularly immunized once with 2mg each of pCMV160IIIB and pcREV (total 4 μg) in a mixture formulated with the indicated doses of UBX. The HIV-1-specific IgG titre was determined in duplicate by ELISA 4 and 8 weeks following immunization. The IgG1, IgG2a and IgE responses were assayed using the 4-week sample. Results are expressed as the means±s.e.m. of four to six mice. *, ** Significant difference between the UBX-formulated and unformulated groups (P <0.05 and P <0.01, respectively). Similar results were obtained in a separate experiment. IIIB/REV, pCMV160IIIB and pcREV; ND, not detected.
Enhancement of HIV-1-specific cell-mediated immunity using UBX
The antigen-specific DTH reaction was assayed by the footpad swelling test (Table 1). As Table 1 shows, a 100- or 500-μg dose of UBX gave a stronger DTH reaction to the V3 peptide than that obtained with immunogenic DNA alone, however, pattern was similar to that of the 10 μg of UBX failed to alter the response. This is correlated to the dose-related humoral immune response, and also implies that UBX is more likely to enhance a Th1-type response. No substantial changes in responses were induced by myoglobin peptide (negative control), demonstrating that the adjuvant activity of UBX is antigen-specific.
Table 1.
Footpad swelling response induced by the DNA vaccine pCMV160IIIB and pcREV formulated with Ubenimex
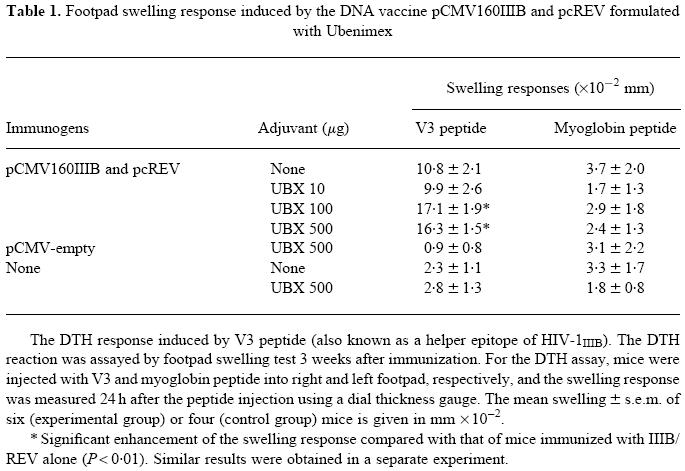
The cytolytic activity of the mononuclear cells from the spleen of an immunized animal was also examined to determine whether this adjuvant could augment specific CTL activity generated by DNA vaccination (Fig. 2). With 500 μg of UBX, specific cytolytic activity was enhanced more than two-fold compared with the group which did not receive this adjuvant, whereas a 100-μg dose was not sufficient to elicit a consistent enhancement of the CTL response. A mild increase in non-specific cytolytic activity was also observed in the UBX adjuvant groups, suggesting that antigen-unrestricted cytolytic cells such as natural killer (NK) cells were also stimulated by UBX. UBX without IIIB/REV had no effect on either the DTH or CTL response, indicating that UBX acts as an adjuvant for immunogenic DNA.
Fig. 2.
Cytolytic activity of bulk splenic mononuclear cells from BALB/c mice immunized with 2mg each of pCMV160IIIB and pcREV (total 4μg) in a mixture formulated with the indicated dose of Ubenimex (UBX). Effector spleen cells were cultured with the V3 peptide (CTL epitope of HIV-1IIIB), and syngenic cells (P815; H-2d) pulsed with or without the same peptide were used as targets. Specific cytolytic activity was titrated with an effector-to-target cell (E/T) ratio of as 5, 20 and 80, but antigen-unrestricted cytolysis was assayed at E/T ratio 80 only. Duplicated assays were performed and results are expressed as the means±s.e.m. of three or five mice comprising the control or experimental group, respectively. *, ** Significant differences between the UBX-formulated and unformulated groups (P <0.05 and P <0.01, respectively).
UBX altered cytokine secretion profile of antigen-restimulated immune lymphoid cells
Measurement of IL-2, IFN-γ and IL-4 in the culture media of antigen-restimulated bulk splenic mononuclear cells was performed by ELISA at 3 weeks after immunization. As Fig. 3 shows, a 500 -μg dose of UBX elicited maximal production of IL-2 and IFN-γ and was associated with a significant drop in IL-4 synthesis. These findings indicated that maximal activation of Th1 cells was achieved with 500 μg of UBX. A 100-μg dose was also effective in changing the cytokine profile, but Th1-type cytokine induction with this dose was less than that obtained with the 500-μg dose. When 500 μg of UBX were injected together with an empty vector, no remarkable change in cytokine profile was noted. These data indicate that a 100- or 500-μg dose of UBX augmented IL-2 and IFN-γ production and reduced IL-4 synthesis in an antigen-specific manner. An alteration of the Th1–Th2 cell dichotomy may be a basis for the adjuvanticity of UBX shown in the present study.
Fig. 3.
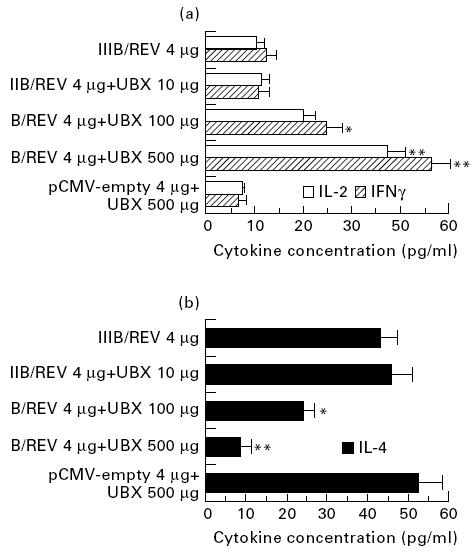
Cytokine production by restimulated splenocytes from mice inoculated with the vaccine formulated as shown. (a, b) Th1- (IL-2 and IFN-γ) and Th2-type (IL-4) cytokines, respectively. Mice were treated using the protocols specified in the legend for Fig. 2, and cells were harvested at the same time point. These cells were cultured in the presence of V3 peptide, and 48h later cell-free supernatants were collected and subjected to ELISA using the appropriate cytokine assay kits. The duplicate assays were performed and results are expressed as the means±s.e.m. of four to six mice. *, **, Significant difference between the Ubenimex (UBX)-formulated and unformulated groups (P <0.05 and P <0.01, respectively).
DISCUSSION
The present study demonstrated that UBX, an anti-cancer immunomodulator approved for clinical use, enhanced HIV-1-specific humoral and cell-mediated immune responses by DNA vaccination in a murine model. This is the first study, to our knowledge, concerning the use of UBX as an immunologic adjuvant for vaccines which target infectious diseases. Immunomodulatory effects of this compound are thought to be derived from its inhibitory action on both leucine aminopeptidase and aminopeptidase B [20]. In the case of the former enzyme, UBX directly stimulates lymphoid cells by interfering with binding of the leucine aminopeptidase onto the cell surface. As for aminopeptidase B, UBX hinders tuftsin degradation catalysed by this enzyme. This latter action indirectly facilitates the immunostimulatory effect of tuftsin on the monocyte-macrophage system. These activities have a positive effect on IL-1 and IL-2 production by Th cells [18], CTL activity against certain tumours [27] and antibody production [28].
Watanabe et al. reported that administration of IL-2 expression plasmid together with immunogenic DNA was effective in augmenting a specific DTH and antibody response [29], and our recent study [30] demonstrated that IL-12, a cytokine upstream of IL-2 and IFN-γ, also enhanced immune responses generated by the DNA AIDS vaccine through elicitation of IFN-γ. Induction of Th1-derived cell-mediated immunity is considered to be necessary for effective DNA vaccination against pathogenic viruses, including HIV [8]. It is particularly important that AIDS vaccines have the capacity to induce an HIV-1-specific CTL response, since evidence has been accumulating that a vaccine which stimulates this response may be beneficial for individuals faced with a live virus challenge. Under these conditions, these cells may eliminate or at least limit replication of the viruses [31].
We therefore attempted immunization with a combination of UBX and our DNA AIDS vaccine, and consistent enhancement of HIV-1-specific immunity was observed. Furthermore, cytokine assays revealed that UBX not only influenced IL-2 production, as described elsewhere [18], but also had an effect on IFN-γ synthesis (Fig. 3). These results suggest that UBX has potential as a vaccine adjuvant and is important in eliciting Th1-type cytokine production in subjects administered DNA vaccines. As for the mechanism associated with the UBX adjuvant activity observed here, we surmised that UBX-mediated IL-2 and IFN-γ synthesis leads to stimulation of a Th1 subset and helps to augment antigen-specific IgG2a production (Fig. 1), the DTH reaction (Table 1), and CTL activity (Fig. 3). However, it is likely that there is another mechanism involving UBX as an immunologic adjuvant, since this compound has a number of effects on the immune system [20]. HIV-1-specific CTL activity [31] and serum IgG2a production [12, 13] are reported to play central roles in protecting against HIV and other pathogens. Since the UBX-mediated DNA vaccination enhanced both a CTL and IgG2a response, it may help protect the vaccinated individual under conditions of live virus challenge.
In the CTL assay (Fig. 2), antigen-unrestricted cytolysis was also induced by UBX administration. We consider that this effect was mainly due to the use of bulk splenic mononuclear cells as effector cells. This cell population includes not only CD8+ CTL but NK cells as well, making antibody-dependent cell-mediated cytotoxicity (ADCC) possible. UBX would probably enhance ADCC, as suggested in an early study [32], by augmenting antigen-specific antibody production (Fig. 1). Another possible mechanism for this UBX-associated cytolysis is that UBX directly activates NK cells via its inhibitory action on membrane aminopeptidase. Since antigen-specific cytolysis is consistently enhanced by UBX, the non-specific immunostimulatory effect of this compound, although significant, does not mar its potential effect as a vaccine adjuvant.
This immunomodulator has been primarily used in the field of anti-cancer immunotherapy, as stated above, but a few researchers have employed UBX alone as an anti-HIV/AIDS agent and reported interesting findings. Bourinbaiar et al. [33] found that UBX had an inhibitory effect on cell-mediated and cell-free HIV infection in vitro. A double-blind clinical study of Hording et al. [34] showed UBX had no capacity for disease suppression when used to treat patients with AIDS. These two observations support the adequacy of formulating UBX into prophylactic AIDS vaccines, but also that it may be difficult to exploit the adjuvanticity of UBX in therapeutic AIDS vaccines.
Finally, UBX is advantageous as a vaccine adjuvant in another respect. Although many kinds of novel adjuvants have been exploited [35, 36], we currently have only two agents which can be used in human vaccines; these are aluminum and calcium gels, both known to stimulate a Th2-biased response [35]. UBX is a low molecular weight compound with no antigenicity and only minor side effects [21]. Avoidance of anti-adjuvant immunity and adverse reactions are important considerations when choosing adjuvants, for vaccines [35], but because the characteristics of UBX are so favourable in this respect, concerns are minimal in terms of employing this compound in vaccines for clinical use.
In conclusion, we conclude that UBX enhances HIV-1-specific humoral and cell-mediated immunity obtained by DNA vaccination. Future work will include a comparative study of several immunomodulators, the use of UBX in other animal models, and an evaluation of functional immunity in a model in which live viruses are employed in an attempt to develop a potent DNA AIDS vaccine.
Acknowledgments
The authors wish to express their gratitude to Nippon Kayaku, Co., Ltd. for providing the Ubenimex used in the present study. We also acknowledge Tamiko Kaneko, Akiko Honsho and Katsuko Niikura for their skillful technical and secretarial assistance.
References
- 1.Ulmer JB, Donnelly JJ, Parker SE, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–9. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 2.Fyna EF, Webster RG, Fuller DH, et al. DNA vaccines—protective immunizations by parenteral, mucosal, and gene-gun inoculations. Proc Natl Acad Sci USA. 1993;90:11478–82. doi: 10.1073/pnas.90.24.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiang ZQ, Spitalnik SL, Cheng J, et al. Immune response to nucleic acid vaccine to rabies virus. Virology. 1995;209:269–79. doi: 10.1006/viro.1995.1289. [DOI] [PubMed] [Google Scholar]
- 4.Davis HL, McCluskie MJ, Gerin JL, et al. DNA vaccine for hepatitis B: evidence for immunogenicity in chimpanzees and comparison with other vaccines. Proc Natl Acad Sci USA. 1996;93:7213–8. doi: 10.1073/pnas.93.14.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okuda K, Bukawa H, Hamajima K, et al. Induction of potent humoral and cell-mediated immune responses following direct injection of DNA encoding the HIV type 1 env and rev gene products. AIDS Res Hum Retroviruses. 1995;11:933–43. doi: 10.1089/aid.1995.11.933. [DOI] [PubMed] [Google Scholar]
- 6.Lu S, Santoro JC, Fuller DH, et al. Use of DNAs expressing HIV-1 Env and noninfectious HIV-1 particles to raise antibody responses in mice. Virology. 1995;209:147–54. doi: 10.1006/viro.1995.1238. [DOI] [PubMed] [Google Scholar]
- 7.Boyer JD, Ugen KE, Wang B, et al. Protection of chimpanzees from high-dose heterologous HIV-1 challenge by DNA vaccination. Nat Med. 1997;3:526–32. doi: 10.1038/nm0597-526. [DOI] [PubMed] [Google Scholar]
- 8.Hassett DE, Whitton JL. DNA immunization. Trend Microbiol. 1996;4:307–12. doi: 10.1016/0966-842x(96)10048-2. [DOI] [PubMed] [Google Scholar]
- 9.Davis HL, Michel ML, Whalen RG. Use of plasmid DNA for direct gene transfer and immunization. In: Liu MA, Hilleman MR, Kurth R, editors. DNA vaccine–new era in vaccinology. Vol. 772. New York: NY Acad Sci; 1995. pp. 21–29. [DOI] [PubMed] [Google Scholar]
- 10.Golding B, Scott DE. Vaccine strategies: targetting helper T cell responses. In: Williams JC, Goldenthal KL, Burns DA, Lewis BJJ, editors. Combined vaccine and simultaneous administration–current issues and perspectives. Vol. 754. New York: NY Acad Sci; 1995. pp. 126–37. [DOI] [PubMed] [Google Scholar]
- 11.Mossmann TR, Coffman RL. Heterogeneity of cytokine secretion patterns and function of helper T cells. Adv Immunol. 1989;46:111–6. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- 12.Ishizuka ST, Piacente P, Silva J, et al. IgG subtype is correlated with efficacy of passive protection and effector function of anti-herpes simplex virus glycoprotein D monoclonal antibodies. J Infect Dis. 1995;172:1108–11. doi: 10.1093/infdis/172.4.1108. [DOI] [PubMed] [Google Scholar]
- 13.Mahon BP, Katrak K, Nomoto A, et al. Poliovirus-specific CD4+ Th1 clones with both cytotoxic and helper activity mediate protective humoral immunity against a lethal poliovirus infection in transgenic mice expressing the human poliovirus receptor. J Exp Med. 1995;181:1285–92. doi: 10.1084/jem.181.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maraskovsky E, Chen W-F, Shortman K. IL-2 and IFN-γ are two necessary lymphokines in the development of cytolytic T cells. J Immunol. 1989;143:1210–4. [PubMed] [Google Scholar]
- 15.Ulmer JB, Donnelly JJ, Liu MA. DNA vaccines promising: A new approach to inducing protective immunity. ASM News. 1996;62:476–9. [Google Scholar]
- 16.Umezawa H, Aoyagi T, Suda H, et al. Bestatin, an inhibitor of aminopeptidase B, produced by actinomycetes. J Antibiot. 1976;29:97–104. doi: 10.7164/antibiotics.29.97. [DOI] [PubMed] [Google Scholar]
- 17.Urabe A, Mutoh Y, Mizoguchi H, et al. Ubenimex in the treatment of acute nonlymphocytic leukemia in adults. Ann Hematol. 1993;67:63–66. doi: 10.1007/BF01788128. [DOI] [PubMed] [Google Scholar]
- 18.Shibuya K, Hayashi E, Abe F, et al. Enhancement of interleukin 1 and interleukin 2 releases by ubenimex. J Antibiot. 1987;40:363–9. doi: 10.7164/antibiotics.40.363. [DOI] [PubMed] [Google Scholar]
- 19.Okamura S, Omori F, Haga K, et al. Influence of bestatin on production of granulocyte-macrophage colony-stimulating factor from human peripheral blood mononuclear cells in vitro. Acta Oncologica. 1990;29:795–7. doi: 10.3109/02841869009093002. [DOI] [PubMed] [Google Scholar]
- 20.Mathé G. Bestatin, an aminopeptidase inhibitor with a multi-pharmacological function. Biomed Pharmacother. 1991;45:49–54. doi: 10.1016/0753-3322(91)90122-a. [DOI] [PubMed] [Google Scholar]
- 21.Ito K, Handa J, Irie Y. Toxicological studies on bestatin III—chronic toxicity test and recovery study in beagle dogs. Jpn J Antibiot. 1983;36:3053–93. [PubMed] [Google Scholar]
- 22.Malim MH, Hauber J, Fenrick R, et al. Immunodeficiency virus rev transactivator modulates the expression of the viral regulatory genes. Nature. 1988;335:181–3. doi: 10.1038/335181a0. [DOI] [PubMed] [Google Scholar]
- 23.Layton GT, Harris SJ, Gearing AJ, et al. Induction of HIV-specific cytotoxic T lymphocytes in vivo with hybrid HIV-1 V3:Ty-virus-like particles. J Immunol. 1993;151:1097–107. [PubMed] [Google Scholar]
- 24.Takahashi H, Germain RN, Moss B, et al. An immunodominant class I-restricted cytotoxic T lymphocyte determinant of human immunodeficiency virus type 1 induces CD4 II-restricted help for itself. J Exp Med. 1990;171:571–6. doi: 10.1084/jem.171.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okuda K, Twining SS, David CS, et al. Genetic control of immune response to sperm whale myoglobin in mice. II. T lymphocyte proliferative response to the synthetic antigenic sites. J Immunol. 1979;123:182–8. [PubMed] [Google Scholar]
- 26.Mosmann TR, Coffman RL. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 27.Talmadge JE, Lenz BF, Pennington R, et al. Immunomodulatory and therapeutic properties of bestatin in mice. Cancer Res. 1986;46:4505–10. [PubMed] [Google Scholar]
- 28.Florentin I, Kiger N, Bruley-Rosset M, et al. Human lymphocyte differentiation. Amsterdam: North Holland Biomedical Press; 1978. Effect of seven immunomodulators on different types of immune responses in mice; p. 299. [Google Scholar]
- 29.Watanabe A, Raz E, Kohsaka H, et al. Induction of antibodies to a kappa V region by gene immunization. J Immunol. 1993;151:2871–6. [PubMed] [Google Scholar]
- 30.Tsuji T, Hamajima K, Fukushima J, et al. Enhancement of cell-mediated immunity against HIV-1 induced by coinoculation of plasmid-encoded HIV-1 antigen with plasmid expressing IL-12. J Immunol. 1997;158:4008–14. [PubMed] [Google Scholar]
- 31.Ada GL, McElrath MJ. HIV type 1 vaccine-induced cytotoxic T cell responses: Potential role in vaccine efficacy. AIDS Res Hum Retroviruses. 1997;13:205–10. doi: 10.1089/aid.1997.13.205. [DOI] [PubMed] [Google Scholar]
- 32.Abe F, Hayashi M, Uchida M, et al. Effect of Bestatin on syngenic tumor in mice. Gann. 1984;75:89–94. [PubMed] [Google Scholar]
- 33.Bourinbaiar AS, Lee HS, Krasinski K, et al. Inhibitory effect of the oral immune response modifier, bestatin, on cell- mediated and cell-free HIV infection in vitro. Biomed Pharmacother. 1994;48:55–61. doi: 10.1016/0753-3322(94)90076-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hording M, Gotzsche PC, Daih-Christensen L, et al. Double-blind trial of bestatin in HIV-positive patients. Biomed Pharmacother. 1990;44:475–8. doi: 10.1016/0753-3322(90)90208-q. [DOI] [PubMed] [Google Scholar]
- 35.Gupta RK, Siber GR. Adjuvants for human vaccines–current status, problems and future prospects. Vaccine. 1995;13:1263–76. doi: 10.1016/0264-410x(95)00011-o. [DOI] [PubMed] [Google Scholar]
- 36.Powell MF, Newman MJ. New York: Plenum Press; 1995. Vaccine design: the subunit and adjuvant approach. [Google Scholar]


