Abstract
By generating human mast cells and basophils from umbilical cord blood mononuclear cells cultured in the presence of appropriate cytokines, we investigated whether these two cultured cells could provide the cytokine and cell contact signals that are required to induce IgE synthesis in B cells. To activate cultured mast cells and basophils, cross-linking of cell surface high-affinity IgE receptor (FcεRI) was performed with specific antigen after sensitization with murine IgE. Upon FcεRI stimulation, basophils, but not mast cells, secreted significant amounts of immunoreactive IL-4 and IL-13 and expressed detectable CD40 ligand (CD40L) and a very low level of Fas ligand (FasL). These observations at the protein level were consistent with the data obtained at the gene transcriptional level, except for the faint expression of only IL-13 mRNA in mast cells. When added to normal human B cells, activated basophils induced IgE and IgG4 synthesis as well as soluble CD23 release. In contrast, neither IgE nor IgG4 synthesis could be induced by the interaction of B cells with activated mast cells, even in the presence of recombinant IL-4. The induction of IgE synthesis by activated basophils was completely abrogated by two neutralizing MoAbs against IL-4 and IL-13 and by a soluble form of CD40. This abrogation was accompanied by abolished mature Cε transcription in both cases. Addition of anti-FasL MoAb, however, did not significantly affect IgE induction mediated by activated basophils. These results demonstrate that unlike cultured mast cells, cultured basophils produce biologically active IL-4 and IL-13 and express functional CD40L after FcεRI stimulation, thereby contributing to IgE production by B cells, and suggest that relatively weak expression of FasL by cultured basophils is not involved in IgE regulation.
Keywords: mast cells, basophils, cytokines, CD40 ligand, Fas ligand, IgE production
INTRODUCTION
Mast cells and basophils play a key role in allergic inflammation by releasing various inflammatory mediators after cross-linking of the high-affinity IgE receptor (FcεRI). Both cells also participate in a form of the immunological synapse by producing several multifunctional cytokines and expressing some membrane proteins [1–9]. For example, mast cells and basophils secrete IL-3, IL-4, IL-5 and IL-13 and express CD40 ligand (CD40L) in response to immunologic or pharmacologic stimulation. Because the interaction of CD40 with CD40L in the presence of an appropriate cytokine is critical for B cell activation as well as immunoglobulin class switching [10–12], mast cells and basophils have properties similar to those of T cells with respect to cytokine production and CD40L expression. In vitro studies in human B cells have shown that IL-4 or IL-13 induce germ-line Cε transcription and direct, in conjunction with CD40–CD40L interactions, isotype switching to IgE that leads to mature Cε transcription and IgE synthesis [13–15]. In this context, mast cells and basophils can be replaced by Th2-type CD4+ T cells that take part in IgE induction. Indeed, activation of human tissue mast cells or blood basophils is shown to induce IgE synthesis in B cells [2, 7, 8].
Fas ligand (FasL), which has homology to CD40L, is a type II membrane protein that belongs to the tumour necrosis factor (TNF) family [16, 17]. Unlike CD40L, FasL induces apoptosis by binding to Fas that is expressed on various types of cells, including B cells. Several studies have shown that among CD4+and CD8+T cell subsets, Th0, Th1 and Tc1 cells express FasL upon activation, while activated Th2 and Tc2 cells do not or only weakly express it [18–21]. The latter finding supports the notion that Th2 or Tc2 cells efficiently induce IgE synthesis without causing B cell death by apoptosis. Although FasL expression has been proposed to be restricted to haematopoietic cells of the T cell and natural killer (NK) lineage, recent reports, including ours, demonstrate that other cells also express FasL upon activation [9, 22]. For example, KU812 cells, which are an immature basophilic cell line, express relatively low levels of FasL mRNA and protein in response to pharmacologic stimulation. However, there is no information on whether mature basophils and mast cells express FasL after FcεRI stimulation.
Saito et al. have established the methods for generating a large number of mature human mast cells and basophils from umbilical cord blood mononuclear cells (CBMC) by culture with appropriate cytokines [23–26]. By using such cultured human mast cells and basophils, we have investigated their capacity to provide the cytokine and cell contact signals that are required to induce IgE synthesis in B cells. Our data demonstrate that upon FcεRI stimulation, cultured basophils, but not cultured mast cells, secrete immunoreactive IL-4 and IL-13, express detectable CD40L, and display very low induction of FasL. We also show that FcεRI stimulation of cultured basophils induces B cells to synthesize IgE and IgG4, whereas the same stimulation of cultured mast cells does not. These findings indicate that the immunologic functions of cultured mast cells and basophils differ as a result of their capacity to produce Th2-type cytokines and to express CD40L.
MATERIALS AND METHODS
Reagents, antigen, and antibodies
Recombinant human stem cell factor (SCF) and IL-6 were generous gifts from Kirin Brewery Co. (Maebashi, Japan). Recombinant human IL-3 and IL-4 were obtained from Genzyme Corp. (Cambridge, MA). Prostaglandin E2 (PGE2), the 2,4-dinitrophenyl group-conjugated human serum albumin (DNP30–40-HSA), cycloheximide, and puromycin were purchased from Sigma Chemical Co. (St Louis, MO). Murine IgE specific for the DNP hapten was obtained from Seikagaku Corp. (Tokyo, Japan), and FITC-conjugated mouse anti-human CD40L MoAb (IgG1) was from Ancell (Bayport, MN). Mouse anti-human FcεRIα MoAb (CRA-1, IgG2b), which shows no competitive inhibition of the binding of IgE to FcεRI, was the same preparation as previously described [27]. Mouse anti-human FasL MoAb (clone 33, IgG1), which is specific for an 18-kD protein fragment in the extracellular region of human FasL and inhibits the binding of FasL to Fas, was purchased from Transduction Laboratories (Lexington, KY). Purified myeloma mouse IgG1 and IgG2b (Cappel, West Chester, PA) were used as isotype-matched controls. Anti-FcεRIα and anti-FasL MoAbs and control antibodies were biotinylated as described [27], and PE-conjugated streptavidin (Becton Dickinson, Mountain View, CA) was used for specific binding to biotin. A recombinant soluble form of human CD40–human IgM (soluble CD40) was kindly donated by Dr P. Lane (Basel Institute for Immunology, Basel, Switzerland) [11], and affinity-purified human IgM (Cappel) was used as a control. Two neutralizing MoAbs (rat IgG1) against human IL-4 and IL-13 and purified myeloma rat IgG1 were obtained from Pharmingen (San Diego, CA).
Cultured human mast cells
Cultured human mast cells were raised from CBMC, as previously described [24–26]. Briefly, CBMC were cultured in Media I (ImmunoBiological Labs, Gunma, Japan) supplemented with 5% fetal calf serum (FCS), 80 ng/ml SCF, 50 ng/ml IL-6, and 300 nm PGE2 in a 5% CO2, humidified atmosphere at 37°C. Cells were passaged every week and cultured for 14–16 weeks. Immunostaining analysis with the use of anti-tryptase or anti-chymase MoAb (Chemicon International, Temecula, CA) showed that 99 ± 0·4% (mean ± s.e.m., n = 5) of the cultured cells had tryptase-positive granules, while 19 ± 4·4% of the cells had chymase-positive granules. In addition, 88 ± 1·9% (n = 3) of the cells constitutively expressed FcεRIα as analysed by flow cytometry. We could obtain > 5 × 107 mast cells by culturing 5 × 107 CBMC in the presence of SCF, IL-6 and PGE2.
Cultured human basophils
Cultured human basophils were obtained as previously reported [23]. Briefly, CBMC were cultured in Media I supplemented with 5% FCS and 2 ng/ml IL-3 in a 5% CO2, humidified atmosphere at 37°C. Cells were passaged every week and cultured for 6–7 weeks. The purity of basophils in the cultured cells was 84 ± 4·2% (n = 4) as determined by May–Grünwald–Giemsa staining, and the residual cells were mainly eosinophils and macrophages. FcεRIα was constitutively expressed on 81 ± 3·4% (n = 3) of the cultured cells. We could generate > 107 basophils by culturing 5 × 107 CBMC in the presence of IL-3.
Assays for IL-4, IL-13 and histamine
Cultured human mast cells or basophils at 1 × 106cells/ml were suspended in Media I containing 5% FCS in flat-bottomed 24-well plates (Becton Dickinson), sensitized with 2 μg/ml murine IgE specific for the DNP hapten at 37°C for 12 h, washed thoroughly, and then resuspended in the same medium. The sensitized cells (each 1 × 105 cells/well) were stimulated with or without 100 ng/ml antigen (DNP–HSA) in flat-bottomed 96-well plates (Becton Dickinson) for various time periods. IL-4 and IL-13 in the cell-free supernatants were quantitatively assayed with commercially available ELISA kits (BioSource International, Camarillo, CA) according to the manufacturer's instructions. The sensitivity of each assay was 7·8 pg/ml. Histamine was determined by using an automatic histamine analyser (Tosoh, Tokyo, Japan) with double column high performance liquid chromatography [25].
Flow cytometric analysis
For single-colour analysis of CD40L and FasL expression, cultured human mast cells or basophils at 2 × 105 cells/50 μl PBS were incubated either with FITC-conjugated anti-CD40L MoAb, or with biotinylated anti-FasL MoAb and PE-conjugated streptavidin. In some experiments for two-colour analysis of CD40L expression, cells were labelled with biotinylated anti-FcεRIα MoAb, followed by incubation with both PE-conjugated streptavidin and FITC-conjugated anti-CD40L MoAb. Then, the stained cells were analysed by flow cytometry on a FACScan (Becton Dickinson) equipped with an argon ion laser running at 488 nm.
Culture conditions for immunoglobulin and soluble CD23 production
Peripheral blood mononuclear cells (PBMC) were separated from heparinized venous blood of healthy adult donors by centrifugation on a Ficoll-sodium metrizoate. B cells were enriched from PBMC that had been partially depleted of adherent cells, as previously described [27]. Highly purified B cells were further negatively isolated by biotinylated anti-human CD56 MoAb (Pharmingen) and magnetic beads coated with anti-human CD2 and anti-human CD14 MoAbs (Dynal, Oslo, Norway), followed by streptavidin-coated magnetic beads (Dynal). After magnetic beads were collected by magnetic particle concentrator (Dynal), highly purified B cells were harvested. This sorted population contained > 99% of CD20+B cells as determined by flow cytometry. Highly purified B cells (1 × 105 cells/well) were mixed in round-bottomed 96-well plates (Costar Corp., Cambridge, MA) with cultured human mast cells or basophils (each 5 × 104 cells/well) that had been stimulated for 2 h with antigen after sensitization with murine IgE. These cell mixtures were suspended in Iscove's modified Dulbecco's medium (Gibco-BRL, Grand Island, NY) supplemented with 10% FCS, 2 mml-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. They were then cultured in the absence or presence of recombinant IL-4 (200 U/ml) for 12 days in a total volume of 0·2 ml in round-bottomed 96-well plates (Costar). To estimate the passive release of preformed immunoglobulins into the culture supernatant, both cycloheximide (50 μg/ml) and puromycin (10 μg/ml) were added to some cultures. IgE and soluble CD23 in the culture supernatants were measured by solid-phase sandwich radioimmunoassays as previously described [9, 28]. IgG4 levels were also determined by a commercial ELISA kit (Zymed Labs, South San Francisco, CA). The sensitivities of these assays were 0·1 ng/ml for IgE, 2 ng/ml for IgG4, and 12·5 pg/ml for soluble CD23. Each assay was not affected by adding murine IgE at 0·005–5 μg/ml (data not shown). The net synthesis of IgE and IgG4 was calculated by subtracting the values of preformed immunoglobulins.
Reverse transcription-polymerase chain reaction
Extraction of total cellular RNA, reverse transcription (RT) to cDNA, and polymerase chain reaction (PCR) were performed as previously described [9]. Briefly, total mRNA was reverse-transcribed to cDNA and PCR performed by using the GeneAmp RNA PCR kit (Perkin-Elmer Cetus, Norwalk, CT) according to the manufacturer's instructions. IL-4 primers and G3PDH primers were obtained from Clontech Labs (Palo Alto, CA), and other primers were synthesized in our labaratory based on the known cDNA sequences. IL-4: 5′ primer, 5′-CGGCAACTTTGACCACGGACAGTGCGATA-3′; and 3′ primer, 5′-ACGTACTCTCTGGTTGGCTTTCACAGGACAG-3′; IL-13: 5′ primer, 5′-CAACGGTCATTGCTCTCACTTGCC-3′; and 3′ primer, 5′-CCTTGTGCGGGCAGAATCCGCTCA-3′; CD40L : 5′ primer, 5′-CCAGAAGATACCATTTCAACTTTAACACAG-3′; and 3′ primer, 5′-CCTGCAAGGTGACACTGTTCAGAGTTTGAG-3′; FasL: 5′ primer, 5′-CTACAGGACTGAGAAGTAAACCGTTT 3′; and 3′ primer, 5′-GAGTTCTGCCAGCTCCTTCTGTAGGTGGAA-3′; germ-line Cε: 5′ primer, 5′-ATCCACAGGCACCAAATGGACGACC-3′; and 3′ primer, 5′-GCCAGGTCC-ACCACCAGACAGGTGA-3′; mature Cε : 5′ primer, 5′-TCGA-CTTCTGGGGCCAAGGG-3′; and 3′ primer, 5′-GCCAGGTCCACCACCAGACAGGTGA-3′; G3PDH: 5′ primer, 5′-TGAAG-GTCGGAGTCAACGGATTTGGT-3′; and 3′ primer, 5′-CATGT-GGGCCATGAGGTCCACCAC-3′. PCR was expanded in a DNA thermal cycler (Nippon Genetics, Tokyo, Japan) for 20 cycles (G3PDH) or 30 cycles (except for G3PDH) under the following conditions: for pre-denaturation, 95°C for 0·5 min (IL-4, IL-13, CD40L, germ-line Cε, mature Cε, and G3PDH) or 1 min (FasL); for primer annealing, 60°C for 0·5 min (except for FasL) or 55°C for 1 min (FasL); and for primer extension, 72°C for 1 min (except for FasL) or 2 min (FasL) followed by 72°C for 2 min. The amplified product was visualized on 2% agarose gel in buffer containing ethidium bromide. PCR using above primers yields a 344-bp product (IL-4), a 263-bp product (IL-13), an 835-bp product (CD40L), a 401-bp product (FasL), a 770-bp product (germ-line Cε), a 775-bp product (mature Cε), and a 983-bp product (G3PDH).
RESULTS
IL-4 and IL-13 production by cultured human mast cells or basophils
It has been shown that tissue mast cells and blood basophils secrete several cytokines, including IL-4 and IL-13, in response to IgE-dependent activation [1, 3–8]. We therefore examined whether cultured human mast cells or basophils could secrete IL-4 and IL-13 after cross-linking of FcεRI by murine IgE and antigen. Cultured mast cells produced no detectable amounts of immunoreactive IL-4 and IL-13 after FcεRI stimulation for 1–12 h (Fig. 1a). This was not due to the failure of FcεRI-dependent activation of mast cells, because the cells released histamine in response to antigen challenge. Actually, 26 ± 4·2% (n = 3) of total cellular histamine was released at 30 min after FcεRI stimulation. On the other hand, cultured basophils that were activated through their FcεRI secreted both IL-4 and IL-13, each of which was detectable by 3 h and nearly reached a plateau at 6 h (Fig. 1b). Besides, the production of IL-13 tended to be higher compared with that of IL-4. Addition of cycloheximide (0·5 μg/ml) resulted in > 95% inhibition of both IL-4 and IL-13 production by activated basophils (data not shown), indicating that these two cytokines were synthesized after FcεRI stimulation.
Fig. 1.
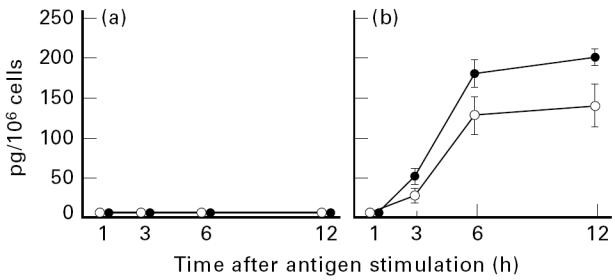
Kinetics of IgE-dependent production of IL-4 (○) and IL-13 (•) by cultured human mast cells or basophils. Mast cells (a) or basophils (b) were sensitized with murine IgE, washed, and stimulated with antigen for the indicated times. The concentrations of secreted IL-4 and IL-13 were measured by specific ELISA kits. Each point represents the mean ± s.e.m. of three or four separate experiments. Neither IL-4 nor IL-13 was detectable in the supernatants from unstimulated mast cells and basophils (data not shown).
We further analysed cytokine production by cultured mast cells or basophils at the gene transcriptional level using RT-PCR. In cultured mast cells, no expression of IL-4 mRNA was detected after FcεRI stimulation for 1–12 h, while the faint induction of IL-13 mRNA occurred within as little as 1 h of stimulation, persisted at very low levels to 3 h, and disappeared thereafter (Fig. 2a). In cultured basophils, IgE-dependent expression of mRNA for IL-4 and IL-13 was transient, beginning as early as 1 h after stimulation, peaking at 3 h, and declining thereafter (Fig. 2b). These observations at the gene transcriptional level are in keeping with the data obtained at the protein level, where cultured basophils, but not cultured mast cells, produced significant amounts of IL-4 and IL-13 after cross-linking of FcεRI.
Fig. 2.
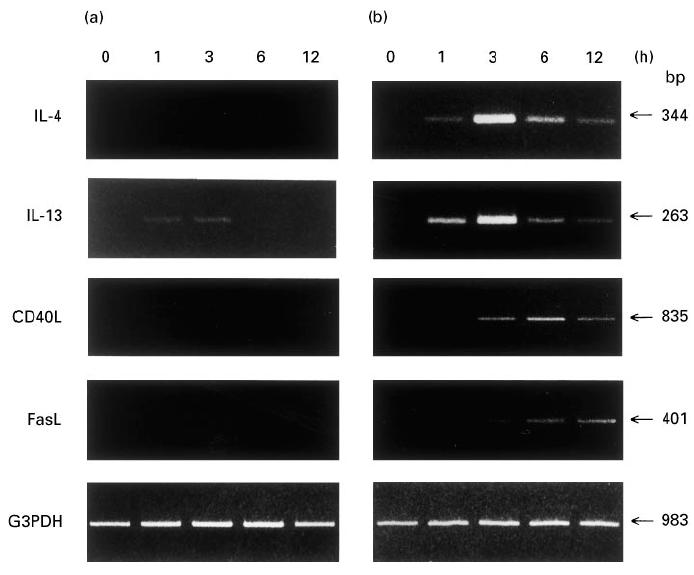
Analysis of the kinetics of IgE-dependent expression for mRNA of IL-4, IL-13, CD40L, and FasL in cultured human mast cells or basophils. Mast cells (a) or basophils (b) were stimulated with or without antigen after sensitization with murine IgE. After extraction of total cellular RNA at the indicated time points, mRNA for IL-4, IL-13, CD40L, FasL and G3PDH was amplified by reverse transcriptase-polymerase chain reaction (RT-PCR), subjected to electrophoresis, and then visualized by ethidium bromide. Similar results were obtained in two other experiments.
CD40L expression in cultured human mast cells or basophils
It has been demonstrated that in addition to T cells, mast cells and basophils, as well as eosinophils, express CD40L in reponse to immunologic or pharmacologic stimulation [2, 7–9, 29]. Accordingly, we first investigated whether cultured human mast cells could express CD40L after IgE-dependent activation. RT-PCR analysis revealed that FcεRI stimulation of cultured mast cells for 1–12 h did not induce expression of detectable CD40L mRNA (see Fig. 2a). Single-colour flow cytometric analysis also failed to detect CD40L protein on the stimulated cells (Fig. 3a). Actually, neither the FcεRI− nor the FcεRI+ cell population expressed CD40L in response to FcεRI cross-linking, as determined by two-colour flow cytometric analysis (data not shown). Next, we examined whether cultured human basophils could express CD40L upon activation. FcεRI stimulation of cultured basophils induced expression of CD40L mRNA, which occurred by 3 h, peaked at 6 h, and declined thereafter (see Fig. 2b). This is consistent with the result of single-colour flow cytometric analysis, in which about 15% of the cultured basophils were shown to express CD40L after cross-linking of FcεRI (Fig. 3b). These results demonstrate that upon FcεRI stimulation, cultured basophils, but not cultured mast cells, express CD40L mRNA and protein.
Fig. 3.
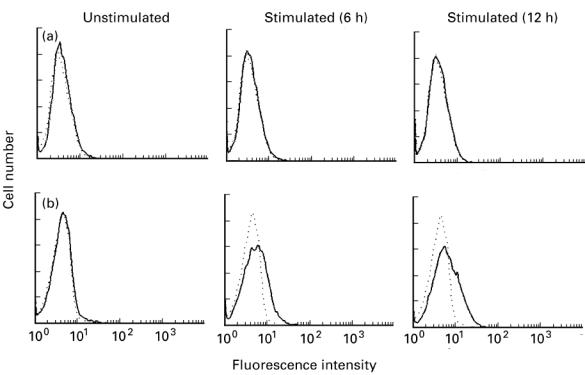
Analysis of IgE-dependent expression of CD40L on cultured human mast cells or basophils. Mast cells (a) or basophils (b) were stimulated with or without antigen after sensitization with murine IgE. Cells were harvested at the indicated times and stained with FITC-conjugated control antibody (- - -) or anti-CD40L MoAb (—). The stained cells were analysed by flow cytometry. Typical results obtained in three independent experiments are shown.
FasL expression in cultured human mast cells or basophils
We have previously shown that KU812 cells, known to be an immature basophilic cell line, express relatively low levels of FasL mRNA and protein after pharmacologic stimulation [9]. Although FasL is preferably found on cells of the T cell and NK lineage, it is not known whether mature mast cells and basophils express FasL upon activation. We therefore studied whether cultured human mast cells or basophils could express FasL in response to IgE-dependent activation. In cultured mast cells, no expression of FasL mRNA was detected at 1–12 h after FcεRI stimulation (see Fig. 2a). Single-colour flow cytometric analysis also failed to detect FasL protein on the stimulated cells (Fig. 4a). On the other hand, FcεRI stimulation of cultured basophils induced expression of a low level of FasL mRNA, which occurred by 3 h and gradually accumulated at least to 12 h (see Fig. 2b). Single-colour flow cytometric analysis showed that although about 3% of the cultured basophils expressed FasL at 6 h after FcεRI stimulation, its expression was hardly detected at 12 h after stimulation (Fig. 4b). This observation is in keeping with previous reports that membrane-type FasL on the surface of activated T cells is released in a soluble form [30, 31]. These results demonstrate that unlike cultured mast cells, cultured basophils express relatively low levels of FasL mRNA and protein in response to FcεRI cross-linking.
Fig. 4.
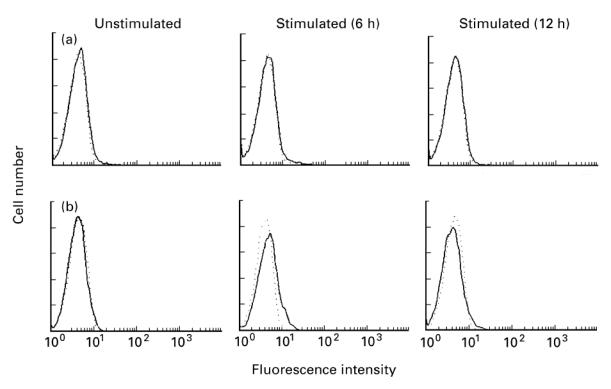
Analysis of IgE-dependent expression of FasL on cultured human mast cells or basophils. Mast cells (a) or basophils (b) were stimulated with or without antigen after sensitization with murine IgE. Cells were harvested at the indicated times and stained with biotinylated control antibody (- - -) or anti-FasL MoAb (—) followed by PE-conjugated streptavidin. The stained cells were analysed by flow cytometry. Typical results obtained in three (a) or four (b) independent experiments are shown.
Induction of IgE synthesis in B cells by cultured human mast cells or basophils
Cultured mast cells or basophils were activated through their FcεRI and added to highly purified B cells, followed by culture in the absence or presence of recombinant IL-4. After culture for 12 days, the supernatants were collected for the measurement of IgE, IgG4 and soluble CD23, and the cell pellets were saved for the analysis of germ-line and mature Cε transcripts. The results are summarized in Figs 5 and 6. FcεRI stimulation of cultured mast cells did not induce B cells to synthesize IgE and to express germ-line and mature Cε mRNA. In addition, neither IgG4 nor soluble CD23 production was enhanced when activated mast cells were added to B cells. Even in the presence of recombinant IL-4 (200 U/ml), activated mast cells had no capacity to induce IgE and IgG4 synthesis in B cells, although exogenous IL-4-dependent germ-line Cε transcription and soluble CD23 release were obviously detected. On the other hand, FcεRI stimulation of cultured basophils induced B cells to synthesize IgE and IgG4 and to release soluble CD23. Furthermore, induction of IgE synthesis in B cells by activated basophils was accompanied by both germ-line and mature Cε transcription. Addition of two neutralizing MoAbs against IL-4 and IL-13 (each 10 μg/ml) to the cultures abrogated IgE and IgG4 responses as well as soluble CD23 release. Soluble CD40 (20 μg/ml) also abolished the induction of IgE and IgG4 synthesis by activated basophils, although it only slightly decreased the release of soluble CD23. Complete inhibition of IgE synthesis by two neutralizing MoAbs against IL-4 and IL-13 or soluble CD40 was accompanied by abrogation of mature Cε transcription in each case. Treatment with anti-FasL MoAb (10 μg/ml), however, did not significantly affect the induction of IgE synthesis by activated basophils (1·5 ± 0·3 ng/ml with control MoAb versus 1·7 ± 0·5 ng/ml with anti-FasL MoAb, triplicate cultures). This observation suggests that the very weak expression of FasL by activated basophils (see Fig. 4b) was not involved in regulating IgE production by B cells. Taken together, these results demonstrate that upon FcεRI stimulation, cultured basophils, but not cultured mast cells, induce IgE and IgG4 synthesis in B cells by producing biologically active IL-4 and IL-13 and expressing functional CD40L.
Fig. 5.
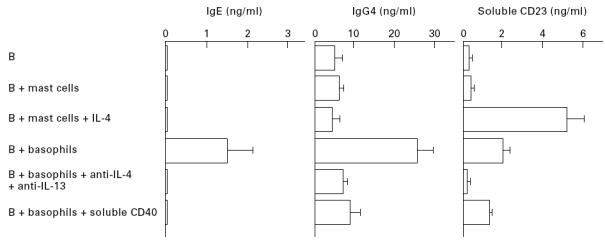
Production of IgE, IgG4 and soluble CD23 by normal B cells mixed with cultured human mast cells or basophils. Mast cells or basophils were stimulated for 2h with antigen after sensitization with murine IgE, washed, added to highly purified B cells, and then cultured for 12 days. Stimulated mast cells were incubated with B cells in the absence or presence of recombinant IL-4. Stimulated basophils were incubated with B cells in the absence or presence of two neutralizing anti-IL-4 and anti-IL-13 MoAbs or a soluble form of CD40. Supernatants were collected and measured for concentrations of IgE, IgG4 and soluble CD23. The net synthesis of IgE and IgG4 was determined, as described in Materials and Methods. Results represent the mean ± s.e.m. of triplicate cultures. One of two comparable experiments is shown. IgE production by B cells was neither induced by unstimulated basophils, nor significantly affected in the cultures containing B cells plus stimulated basophils by the addition of control antibody or IgM (data not shown).
Fig. 6.
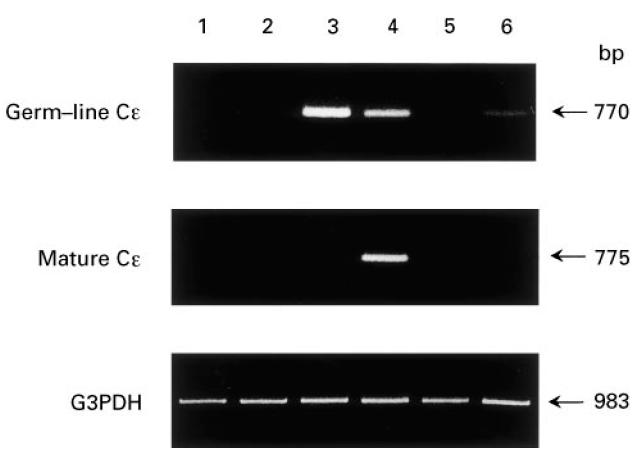
Analysis of germ-line and mature Cε mRNA expression in normal B cells mixed with cultured human mast cells or basophils. The cell pellets obtained in Fig. 5 were used for the analysis of germ-line and mature Cε transcription. Highly purified B cells were incubated with medium alone (lane 1) or with stimulated mast cells in the absence (lane 2) or presence (lane 3) of recombinant IL-4. Stimulated basophils were incubated with B cells in the absence (lane 4) or presence of two neutralizing anti-IL-4 and anti-IL-3 MoAbs (lane 5) or a soluble form of CD40 (lane 6). After extraction of total cellular RNA, mRNA for germ-line Cε, mature Cε, and G3PDH was amplified by reverse transcriptase-polymerase chain reaction (RT-PCR), subjected to electrophoresis, and then visualized by ethidium bromide. One of two comparable experiments is shown. Neither germ-line nor mature transcripts were detected in the cultures containing B cells plus unstimulated basophils (data not shown).
DISCUSSION
In this study, we demonstrate that upon FcεRI stimulation, cultured human basophils, but not cultured human mast cells, provide the cytokine and cell contact signals that are required to induce IgE synthesis in B cells. Because cultured mast cells and basophils used in the present study constitutively expressed FcεRI, cross-linking of FcεRI was performed with specific antigen after sensitization with murine IgE. FcεRI stimulation of cultured basophils induced production of significant amounts of IL-4 and IL-13 and expression of detectable CD40L, but the same stimulation of cultured mast cells did not. The latter finding is not due to the failure of IgE-mediated activation of mast cells, because the sensitized cells released histamine in response to antigen stimulation. In keeping with this view, cultured mast cells have also been shown to secrete leukotriene C4 as well as TNF-α, IL-5, and granulocyte-macrophage colony-stimulating factor (GM-CSF) after cross-linking of FcεRI by human IgE and anti-IgE antibody [24–26], thus indicating that the cells are functionally mature. The data obtained at the protein level in activated mast cells and basophils were further confirmed at the gene transcriptional level, except for the faint induction of only IL-13 mRNA in mast cells. Our finding that FcεRI stimulation of cultured mast cells failed to detect IL-4 mRNA and protein is consistent with two other reports [25, 32]. IgE-dependent expression of mRNA for IL-4, IL-13 and CD40L in cultured basophils was transient, beginning as early as 1 h (IL-4 and IL-13) or 3 h (CD40L) after stimulation, peaking at 3 h (IL-4 and IL-13) or 6 h (CD40L), and declining thereafter. In addition, secretion of IL-4 and IL-13 and expression of CD40L by cultured basophils could contribute to IgE production by B cells. Although it has been shown that upon activation tissue mast cells and blood basophils, as well as mast and basophilic cell lines, induce B cells to synthesize IgE [2, 7–9], the present results demonstrate that the immunologic functions of cultured mast cells and basophils differ as a result of their capacity to produce Th2-type cytokines and to express CD40L.
FcεRI stimulation of cultured basophils induced B cells to increase soluble CD23 release, to accumulate germ-line and mature Cε transcripts, and to synthesize IgE and IgG4. All such events were abolished by treatment with two neutralizing MoAbs against IL-4 and IL-13, indicating that cultured basophil-derived IL-4 and IL-13 were biologically active. Abrogation of mature Cε transcription by a soluble form of CD40 also revealed that CD40L expressed on activated basophils was functional and could contribute to isotype switching to IgE. On the other hand, cultured mast cells that were activated through their FcεRI were devoid of the capacity to induce IgE and IgG4 synthesis in B cells even in the presence of recombinant IL-4. This failure can be reasonably explained by the fact that cultured mast cells neither secreted IL-4 and IL-13 nor expressed CD40L after FcεRI stimulation. In vitro studies of human tissue mast cells have shown that both FcεRI and SCF stimulation may be required for sufficient IL-4 production by lung mast cells of non-atopic subjects [5], while FcεRI stimulation alone is able to induce IL-4 and IL-13 production and CD40L expression in nasal mast cells of perennial rhinitic patients [7, 8]. In such patients, the expression of β1-integrins such as very late antigen (VLA)-4 and VLA-5 is also found to be up-regulated in vivo. These findings, together with a previous report that β1-integrins and vitronectin receptor (β3-integrin) are involved in mast cell activation [33], suggest that unlike cultured mast cells that had no opportunity to adhere to fibronectin, tissue mast cells in allergic patients may be pre-activated in vivo at least through engagement of β1-integrins. It is also suggested that among mast cell subpopulations, there is functional heterogeneity with respect to cytokine production and CD40L expression.
In contrast to the CD40L/CD40 system, the FasL/Fas system plays a critical role in the induction of cell death by apoptosis. Although FasL expression is preferably found on cells of the T cell and NK lineage, B cells also express FasL upon activation [22]. In addition, our previous work showed that KU812 cells weakly synthesize FasL mRNA and protein after pharmacologic stimulation [9]. However, there has been no report studying whether mature mast cells and basophils express FasL in response to IgE-dependent activation. We found that upon FcεRI stimulation, cultured basophils expressed a relatively low level of FasL mRNA, leading to detectable surface expression, whereas no expression of FasL mRNA and protein could be induced in cultured mast cells. Of note, expression of FasL mRNA by cultured basophils was stable and lasting, because it occurred 3 h after stimulation and gradually accumulated at least to 12 h. This differs from the kinetics of CD40L mRNA expression in cultured basophils, but is similar to that of FasL mRNA expression observed in KU812 cells [9]. It is therefore likely that FasL and CD40L expression may be controlled independently in basophils. Although we did not examine whether FasL expressed on cultured basophils could induce cytolysis in Fas-expressing target cells, the observation that anti-FasL MoAb did not significantly modulate induction of IgE synthesis by activated basophils suggests that very low expression of FasL by cultured basophils could not contribute to B cell apoptosis that leads to decreased IgE production. Because treatment with hydroxamic acid inhibitors of matrix metalloproteinases has been shown to induce accumulation of membrane-type FasL by inhibiting soluble FasL release from the cell surface [31], additional experiments are required to determine whether FasL expressed on activated basophils is functional in the condition of its accumulation.
In conclusion, FcεRI stimulation of cultured human basophils induces secretion of significant amounts of IL-4 and IL-13 and expression of detectable CD40L and a very low level of FasL, but the same stimulation of cultured human mast cells fails to do so. These results explain well why cultured basophils, but not cultured mast cells, induce IgE and IgG4 synthesis in B cells after FcεRI cross-linking. With regard to the immunologic function of mast cells, further work is needed to clarify the differences between cultured and tissue mast cells.
Acknowledgments
This work was supported in part by a grant from the Japanese Ministry of Health and Welfare. We are grateful to Dr Peter Lane for kindly providing the recombinant soluble form of human CD40–human IgM.
References
- 1.Gordon JR, Burd PR, Galli SJ. Mast cells as a source of multifunctional cytokines. Immunol Today. 1990;11:458–64. doi: 10.1016/0167-5699(90)90176-a. [DOI] [PubMed] [Google Scholar]
- 2.Gauchat J-F, Henchoz S, Mazzei G, et al. Induction of human IgE synthesis by mast cells and basophils. Nature. 1993;365:340–3. doi: 10.1038/365340a0. [DOI] [PubMed] [Google Scholar]
- 3.MacGlashan D, Jr, White JM, Huang S-K, et al. Secretion of IL-4 from human basophils. The relationship between IL-4 mRNA and protein in resting and stimulated basophils. J Immunol. 1994;152:3006–16. [PubMed] [Google Scholar]
- 4.Burd PR, Thompson WC, Max EE, et al. Activated mast cells produce interleukin 13. J Exp Med. 1995;181:1373–80. doi: 10.1084/jem.181.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okayama Y, Petit-Frére C, Kassel O, et al. IgE-dependent expression of mRNA for IL-4 and IL-5 in human lung mast cells. J Immunol. 1995;155:1796–808. [PubMed] [Google Scholar]
- 6.Ochensberger B, Daepp G-C, Ribs S, et al. Human blood basophils produce interleukin-13 in response to IgE-receptor-dependent and -independent activation. Blood. 1996;88:3028–37. [PubMed] [Google Scholar]
- 7.Pawankar R, Ra C. Heterogeneity of mast cells and T cells in the nasal mucosa. J Allergy Clin Immunol. 1996;98:248–62. doi: 10.1016/s0091-6749(96)70073-8. [DOI] [PubMed] [Google Scholar]
- 8.Pawankar R, Okuda M, Yssel H, et al. Nasal mast cells in perennial allergic rhinitics exhibit increased expression of the FcεRI, CD40L, IL-4, and IL-13, and can induce IgE synthesis in B cells. J Clin Invest. 1997;99:1492–9. doi: 10.1172/JCI119311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yanagihara Y, Kajiwara K, Basaki Y, et al. Induction of human IgE synthesis in B cells by a basophilic cell line, KU812. Clin Exp Immunol. 1997;108:295–301. doi: 10.1046/j.1365-2249.1997.d01-1001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hollenbaugh D, Grosmaire LS, Kullas CD, et al. The human T cell antigen gp39, a member of the TNF gene family, is a ligand for the CD40 receptor: expression of a soluble form of gp39 with B cell-costimulatory activity. EMBO J. 1992;11:4313–21. doi: 10.1002/j.1460-2075.1992.tb05530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lane P, Traunecker A, Hubele S, et al. Activated human T cells express a ligand for the human B cell-associated antigen CD40 which participates in T cell-dependent activation of B lymphocytes. Eur J Immunol. 1992;22:2573–8. doi: 10.1002/eji.1830221016. [DOI] [PubMed] [Google Scholar]
- 12.Allen RC, Armitage RJ, Conley ME, et al. CD40 ligand gene defects responsible for X-linked hyper IgM syndrome. Science. 1993;259:990–3. doi: 10.1126/science.7679801. [DOI] [PubMed] [Google Scholar]
- 13.Shapira SK, Jabara HH, Thienes C, et al. Deletional switch recombination occurs in interleukin-4-induced isotype switching to IgE expression by human B cells. Proc Natl Acad Sci USA. 1991;147:7528–32. doi: 10.1073/pnas.88.17.7528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gascan H, Gauchat J-F, Aversa G, et al. Anti-CD40 monoclonal antibodies or CD4+ T cell clones and IL-4 induce IgG4 and IgE switching in purified human B cells via different signaling pathways. J Immunol. 1991;147:8–13. [PubMed] [Google Scholar]
- 15.Punnonen J, Aversa G, Cocks BG, et al. Interleukin-13 induces interleukin-4-independent IgG4 and IgE synthesis and CD23 expression by human B cells. Proc Natl Acad Sci USA. 1993;90:3730–4. doi: 10.1073/pnas.90.8.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith CA, Farrah T, Goodwin RG, et al. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76:959–62. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 17.Nagata S, Goldstein P. The Fas death factor. Science. 1995;267:1449–56. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 18.Ju ST, Cui H, Panka R, et al. Participation of target Fas protein in apoptosis pathway induced by CD4+ Th1 and CD8+ cytotoxic T cells. Proc Natl Acad Sci USA. 1994;91:4185–9. doi: 10.1073/pnas.91.10.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suda T, Okazaki T, Naito Y, et al. Expression of the Fas ligand in cells of T cell lineage. J Immunol. 1995;154:3806–13. [PubMed] [Google Scholar]
- 20.Yagita H, Hanabuchi S, Asano Y, et al. Fas-mediated cytotoxicity: a new immunoregulatory and pathogenic function of Th1 CD4+ T cells. Immunol Rev. 1995;146:223–39. doi: 10.1111/j.1600-065x.1995.tb00691.x. [DOI] [PubMed] [Google Scholar]
- 21.Carter LL, Dutton RW. Relative perforin- and Fas-mediated lysis in Th1 and Th2 CD8 effector populations. J Immunol. 1995;155:1028–31. [PubMed] [Google Scholar]
- 22.Hahne M, Renno T, Schroeter M, et al. Activated B cells express functional Fas ligand. Eur J Immunol. 1996;26:721–4. doi: 10.1002/eji.1830260332. [DOI] [PubMed] [Google Scholar]
- 23.Saito H, Hatake K, Dvorak AM, et al. Selective differentiation and proliferation of hematopoietic cells induced by recombinant human interleukins. Proc Natl Acad Sci USA. 1988;85:2288–92. doi: 10.1073/pnas.85.7.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saito H, Ebisawa M, Sakaguchi N, et al. Characterization of cord-blood-derived human mast cells cultured in the presence of Steel factor and interleukin-6. Int Arch Allergy Immunol. 1995;107:63–65. doi: 10.1159/000236932. [DOI] [PubMed] [Google Scholar]
- 25.Saito H, Ebisawa M, Tachimoto, et al. Selective growth of human mast cells induced by Steel factor, IL-6, and prostagladin E2 from cord blood mononuclear cells. J Immunol. 1996;157:343–50. [PubMed] [Google Scholar]
- 26.Tachimoto H, Ebisawa M, Iikura Y, et al. Activated human mast cells release factors supporting eosinophil survival in vitro. Int Arch Allergy Immunol. 1997;113:293–4. doi: 10.1159/000237578. [DOI] [PubMed] [Google Scholar]
- 27.Yanagihara Y, Kajiwara K, Ikizawa K, et al. Recombinant soluble form of the human high-affinity immunoglobulin E (IgE) receptor inhibits IgE production through its specific binding to IgE-bearing B cells. J Clin Invest. 1994;94:2162–5. doi: 10.1172/JCI117574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yanagihara Y, Kiniwa M, Kajiwara K, et al. Establishment of a sensitive radioimmunoassay for the detection of human IgE-binding factor (soluble CD23) Int Arch Allergy Immunol. 1992;98:189–99. doi: 10.1159/000236184. [DOI] [PubMed] [Google Scholar]
- 29.Gauchat J-F, Henchoz S, Fattah D, et al. CD40 ligand is functionally expressed on human eosinophils. Eur J Immunol. 1995;25:863–5. doi: 10.1002/eji.1830250335. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka M, Suda T, Takahashi T, et al. Expression of the functional soluble form of human Fas ligand in activated lymphocytes. EMBO J. 1995;14:1129–35. doi: 10.1002/j.1460-2075.1995.tb07096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kayagaki N, Kawasaki A, Ebata T, et al. Metalloproteinase-mediated release of human Fas Ligand. J Exp Med. 1995;182:1777–83. doi: 10.1084/jem.182.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toru H, Ra C, Nonoyama S, et al. Induction of the high-affinity IgE receptor (FcεRI) on human mast cells by IL-4. Int Immunol. 1996;8:1367–73. doi: 10.1093/intimm/8.9.1367. [DOI] [PubMed] [Google Scholar]
- 33.Ra C, Yasuda M, Yagita H, et al. Fibronectin receptor integrins are involved in mast cell activation. J Allergy Clin Immunol. 1994;94:625–8. doi: 10.1016/0091-6749(94)90139-2. [DOI] [PubMed] [Google Scholar]


