Abstract
Some patients develop post-kala-azar dermal leishmaniasis (PKDL) after they have been treated for the systemic infection kala-azar (visceral leishmaniasis). It has been an enigma why the parasites cause skin symptoms after the patients have been successfully treated for the systemic disease. We report here that PKDL development can be predicted before treatment of visceral leishmaniasis, and that IL-10 is involved in the pathogenesis. Before treatment of visceral leishmaniasis, Leishmania parasites were present in skin which appeared normal on all patients. However, IL-10 was detected in the keratinocytes and/or sweat glands of all patients who later developed PKDL (group 1) and not in any of the patients who did not develop PKDL (group 2). Furthermore, the levels of IL-10 in plasma as well as in peripheral blood mononuclear cell culture supernatants were higher in group 1 than in group 2.
Keywords: post-kala-azar dermal leishmaniasis, visceral leishmaniasis, kala-azar, pathogenesis, IL-10, soluble IL-2 receptor, soluble IL-4 receptor
INTRODUCTION
Post-kala-azar dermal leishmaniasis (PKDL) is an infection of the skin, which in the Sudan affects about 50% of patients treated for visceral leishmaniasis caused by Leishmania donovani[1]. Histologically the condition is characterized by inflammation and the presence of a small number of parasites in the skin [2]. It is a puzzle why the severe systemic infection characteristic of kala-azar is followed by skin symptoms after apparently successful drug treatment, but it has been suggested that failure to comply with the extended drug regimen could be a precipitating factor. The clinical outcome of Leishmania infections is critically dependent on the regulation of the immune response during the initial phase of the infections. Subclinical infections have been associated with T helper 1 (Th1)-type responses characterized by secretion of interferon-gamma (IFN-γ), IL-2 and lymphotoxin, and development of severe disease associated with T helper 2 (Th2) responses characterized by secretion of IL-4, IL-5, IL-6 and IL-10 [3].
In this study we show that Leishmania parasites were present in skin which appeared normal of all the Sudanese kala-azar patients tested. We hypothesize that in some patients the persistence of these parasites due to aberration of the local host response mediated by an over-expression of IL-10 in the skin led to development of PKDL. This hypothesis is supported by the finding that IL-10 was produced by keratinocytes or sweat glands of kala-azar patients, who up to 6 months later developed PKDL, and none of the patients who did not subsequently develop PKDL.
PATIENTS AND METHODS
Patients
Sixty-four patients with visceral leishmaniasis were recruited from villages in Eastern Sudan or hospitals in Central Sudan. The diagnosis of visceral leishmaniasis was made by the demonstration of Leishmania parasites in lymph node or bone marrow. Patients were treated with sodium stibogluconate (Pentostam; Wellcome Laboratories, Beckenham, UK) at a dose of 10 mg/kg per day for 30 days; children received 20 mg/kg per day for 30 days. After treatment the tissue in which the parasite was first demonstrated was re-examined, and found free of Leishmania in all cases. After informed consent venous blood samples were collected before initiation of treatment (day 0), 15 days after initiation of treatment, and at the end of treatment for isolation of peripheral blood mononuclear cells (PBMC) and plasma. Some randomly selected patients donated a skin biopsy at day 0. Patients were followed clinically for a period of 6–24 months. The diagnosis of PKDL was made clinically [2]. Most cases of PKDL heal spontaneously [4]; therefore PKDL treatment was only instituted if symptoms persisted >6 months. The study received ethical clearance from the Ethical Committee of the Institute of Endemic Diseases, University of Khartoum.
Detection of parasites and IL-10 in skin
Punch biopsies (4 mm) were taken from the skin of the outer right arm from patients on day 0. Biopsies were fixed in 10% formal saline, transferred to 70% ethyl alcohol, then covered by OCT and snap-frozen in liquid nitrogen. Frozen sections were cut and stained with haematoxylin and eosin (H–E). Parallel sections were stained using an anti-L. donovani mouse MoAb (LXXVIII 2E5-A8 (D2) G2D10, kindly donated by TDR/WHO) and an immunohistochemical kit (Dako Duet; Dako, Glostrup, Denmark) according to the instructions of the manufacturer. The MoAb stained L. donovani promastigotes from cultures, but did not stain L. major promastigotes.
Sections were stained for IL-10 or IFN-γ using an anti-IL-10 MoAb (clone 23738.11; R&D Systems, Abingdon, UK) or an anti-IFN-γ antibody (1598–00; Genzyme, Cambridge, MA), and the Dako Duet kit. Controls included sections obtained from a healthy Dane, three healthy Sudanese individuals and sections from patients in which incubation with the primary MoAb was omitted. All slides were evaluated without knowledge of the patient history.
Isolation of PBMC and plasma
PBMC and plasma were isolated by lymphoprep (Nyegaard, Oslo, Norway) density centrifugation. The cells were frozen by a device for gradient freezing of cells under field conditions [5], stored and transported in liquid nitrogen. The plasma was stored at −20°C.
Detection of cytokine and cytokine receptors
Plasma levels of IL-10 and soluble IL-4α receptor (sIL-4αR) were measured by Quantikine ELISA kits (R&D Systems). The sensitivities of the IL-10 assay were 2 pg/ml. According to the manufacturer the expected value of plasma from normal healthy individuals was < 7·8 pg/ml. The sensitivity of the sIL-4αR assay was 5 pg/ml, and the expected values of plasma from normal healthy individuals were 15–44 pg/ml.
Plasma levels of soluble IL-2 receptor (sIL-2R) were measured by an ELISA kit from T Cell Sciences (Cambridge, MA). The sensitivity of the ELISA assay was 24 U/ml, and the expected value of plasma from normal healthy individuals was < 913 U/ml.
IL-10 in culture supernatants was measured by an ELISA kit obtained from Immunotech (Marseilles, France). Sensitivity was 5 pg/ml.
Antigens
Crude L. donovani promastigotes and amastigote antigens were prepared as previously described [6]. The antigens were used at a final concentration in the PBMC cultures of 10 μg/ml. Purified protein derivative of tuberculin (PPD) was purchased from the State Serum Institute (Copenhagen, Denmark) and used at a final concentration in the cultures of 12 μg/ml.
In vitro production of IL-10
PBMC were thawed, washed twice in cell culture medium, and cultured in RPMI 1640 with HEPES 10 mm, l-glutamine 58·4 μg/ml, penicillin 20 U/ml, and streptomycin 20 μg/ml (all from Gibco Ltd., Paisley, UK) supplemented by 15% heat-inactivated pooled normal human serum. The cultures were incubated at 37°C in a humidified atmosphere containing 5% CO2. All experiments were performed in 96-well round-bottomed microtitre plates (Nunc, Roskilde, Denmark). Wells contained 150 μl of PBMC (0·66 × 106/ml) and 20 μl of antigen. Supernatants from the cultures were collected after 48 h and stored at −20°C until IL-10 was measured.
Statistical analysis
Data were evaluated using Sigmastat 5·0 (Jandel Scientific, San Rafael, CA) and Minitab 8 (Cleocom, Birmingham, UK) software. Sigmastat was used to test whether the data were suitable for parametric testing. If this was the case differences of sample means were evaluated by Student's t-test, and the data were presented as mean and 95% confidence interval (95% CI). If the data were not suitable for parametric testing, differences of sample medians were tested by Wilcoxon–Mann–Whitney test, and data were presented as median and 95% CI. The Fisher test was used to test intergroup differences in sex distribution, hepatomegaly and expression of IL-10 by keratinocytes. P < 0·05 was considered significant.
RESULTS
Of the 64 patients entered into the study it was possible to follow 29 for a period of 6–24 months. The patients were divided into two groups: those who developed PKDL (group 1, n = 16) and those who did not (group 2, n = 13). In 13 patients PKDL occurred after the treatment had been completed (7–180 days after), while in three patients PKDL developed during treatment. Lesions were macules, papules or nodules affecting the face, and usually the trunk and extremities. The lesions healed without treatment within a period of 30–180 days.
The characteristics of patients in group 1 and group 2 are shown in Table 1. The spleens were larger in group 1 than in group 2, and after treatment the difference was statistically significant.
Table 1.
Characteristics of the kala-azar patients who later developed post-kala-azar dermal leishmaniasis (PKDL) (group 1) and of those who did not develop PKDL (group 2)

Skin pathology
Skin biopsies were obtained from skin which appeared normal before initiation of kala-azar treatment from 10 and six patients from groups 1 and 2, respectively. In H–E-stained sections a few perivascular mononuclear inflammatory cells were seen in some sections (Fig. 1a), while in others the skin was virtually normal. Using the anti-L. donovani MoAb, parasites were seen in the dermis of all patients and in the epidermis of some. Some keratinocytes were also positive for Leishmania antigen (Fig. 1b).
Fig. 1.

Skin sections from normal appearing skin of patients suffering from visceral leishmaniasis. (a) Haematoxylin and eosin-stained section with a few perivascular mononuclear inflammatory cells. (b) Section stained with immunoperoxidase and anti-Leishmania antibodies. The keratinocytes are positive for Leishmania antigen. (c) Section stained by immunoperoxidase and anti-IL-10 antibodies. The keratinocytes contain IL-10. (d) Control section stained with immunoperoxidase in which the incubation with a primary antibody was omitted. The pigmented cells are melanocytes. (e) The section was stained as for (c). The epithelium of sweat ducts contain IL-10.
Using anti-IL-10 MoAb, IL-10 was detected in keratinocytes and/or sweat glands of all the sections from group 1 (10/10), but in none of the sections from group 2. This difference was statistically significant (P (F)<0·001). The IL-10 was mainly located in the suprabasal keratinocytes (Fig. 1c), and in the epithelium and ducts of sweat glands (Fig 1d). Occasionally cells in the perivascular infiltrates were positive for IL-10. IFN-γ was not found in any of the sections. IL-10 was not detected in the normal skin obtained from a healthy Danish and three healthy Sudanese individuals (Fig. 1e). In these sections a few cells, probably lymphocytes in the dermis, were positive for IFN-γ (data not shown).
Cytokine and cytokine receptors in plasma
The mean level of IL-10 at the initiation of treatment (Fig. 2a) was higher in group 1 than in group 2 (difference between the means (95% CI for the difference of means), 46·5 pg/ml (26·2–66·9), P (t)<0·001). During and after treatment the IL-10 plasma levels were comparable (Fig. 2b,c).
Fig. 2.
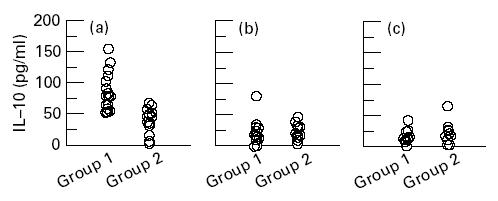
Plasma levels of IL-10 before initiation of treatment (a), and days 15 (b) and 30 (c) after initiation of treatment in patients suffering from visceral leishmaniasis. Group 1 patients developed post-kala-azar dermal leishmaniasis (PKDL) during or after treatment, group 2 patients did not develop PKDL.
The plasma levels of sIL-2R were high before the initiation of treatment (Fig. 3a), and were statistically significantly higher in group 1 than in group 2 (difference between the means (95% CI for the difference of means), 1703 U/ml (76·5–3329·8), P (t) = 0·041). After 2 weeks of treatment (Fig. 3b) the difference between groups 1 and 2 was not statistically significant (P (t) = 0·13), and after completion of treatment the levels were comparable (Fig. 3c).
Fig. 3.

Plasma levels of sIL-2R before initiation of treatment (a), and days 15 (b) and 30 (c) after initiation of treatment in patients suffering from visceral leishmaniasis. Group 1 patients developed post-kala-azar dermal leishmaniasis (PKDL) during or after treatment, group 2 patients did not develop PKDL.
The plasma sIL-4R levels were highest before treatment (median (95% CI), 121 pg/ml (l08–197)), lower 2 weeks after initiation of treatment (median (95% CI), 95 pg/ml (77–146)), and lowest after completion of treatment (median (95% CI), 87 pg/ml (74–146)). There were no statistically significant differences between the levels of plasma sIL-4R between groups 1 and 2 at any point in time (Fig. 4a,b,c, respectively).
Fig. 4.

Plasma levels of sIL-4R before initiation of treatment (a), and days 15 (b) and 30 (c) after initiation of treatment in patients suffering from visceral leishmaniasis. Group 1 patients developed post-kala-azar dermal leishmaniasis (PKDL) during or after treatment, group 2 patients did not develop PKDL.
IL-10 in cell cultures
PBMC were isolated from 14 and 10 individuals from groups 1 and 2, respectively. The IL-10 levels in unstimulated cultures (Fig. 5a) were higher in group 1 (median (95% CI), 78 pg/ml (21–128)) than in group 2 (median (95% CI), 11 pg/ml (3–46)), but the difference was not statistically significant (P (Wx) = 0·065). In the presence of amastigote antigen (Fig. 5b) the IL-10 levels were significantly higher in group 1 than in group 2 (median difference (95% CI for the difference) 26 pg/ml (7–118), (P (Wx) = 0·011). In the presence of a crude sonicate of Leishmania promastigotes (Fig. 5c) levels of IL-10 were lower than in unstimulated cultures (median difference (95%CI for the difference) 50 pg/ml (6–94), P (Wx) = 0·038, and 13 pg/ml (4–46), P (Wx) = 0·009 for groups 1 and 2, respectively). Furthermore, the levels were higher in group 1 than in group 2 (median difference and 95% CI for the difference 13 pg/ml (1–51), (P (Wx) = 0·009). After stimulation with PPD, IL-10 levels were higher in group 1 (median (95%CI), 73 pg/ml (34–266)), than in group 2 (median (95% CI), 10 pg/ml (4–96)), but the difference was not statistically significant (P (Wx) = 0·107) (data not shown).
Fig. 5.

IL-10 production in cultures of peripheral blood mononuclear cells (PBMC) isolated from patients suffering from visceral leishmaniasis, who later developed (group 1) or did not develop post-kala-azar dermal leishmaniasis (PKDL) (group 2). The PBMC were cultured in medium (a), or medium containing a sonicate of Leishmania amastigotes (b) or promastigotes (c).
DISCUSSION
The areas of Sudan where visceral leishmaniasis is endemic are difficult to reach. We were able to follow 29 of 64 kala-azar patients for a period of at least 6 months. Many patients were followed for up to 2 years and no new PKDL cases were detected after the 6-month period. Sixteen of the 29 patients (55%) developed PKDL. This figure corresponds well with results from a field-based study in Eastern Sudan, in which 56% of kala-azar patients developed PKDL [7]. We are therefore confident that our clinical classification of patients into group 1 and group 2 is accurate.
Using immunohistochemistry we could demonstrate Leishmania parasites in skin which appeared normal of all the kala-azar patients tested. Using conventional methods, Manson-Bahr [8] found parasites in the skin of 11% of kala-azar patients. Our results indicate that Sudanese L. donovani strains disseminate to the skin in most (if not all) cases of visceral leishmaniasis. The reaction to this invasion was of two types. In individuals who developed PKDL, we found production of IL-10 in keratinocytes and/or sweat glands. In patients who did not develop PKDL, IL-10 production in the skin was found in only a few patients, and in these limited to few scattered cells of discrete perivascular infiltrates. IL-10 is a powerful suppresser of macrophage activation, and one of the cytokines produced by Th2 subtype of T cells [9]. In the skin IL-10 down-regulates various inflammatory responses [10], partly through an inhibition of the production of IL-6 and tumour necrosis factor-alpha (TNF-α) by keratinocytes [11]. The latter cytokine is thought to be important for activation of the nitric oxide pathway in macrophages, which is an important parasite-killing mechanism. Production of IL-10 in the skin will thus decrease local inflammation and promote survival of the parasite. This could represent a mechanism by which the parasite survives in the skin and ensures transmission through the vector.
In further support for a role of IL-10 in the pathogenesis of PKDL, we found that plasma levels and the production of IL-10 in cultures were higher in group 1 than in group 2 patients. Indeed, all patients with plasma IL-10 levels >80 pg/ml developed PKDL. Increased plasma levels of IL-10 during kala-azar [12], and increased production of IL-10 by cells isolated from kala-azar patients [13–15], have been described previously. In vivo studies with L. major-infected mice [16] and in vitro studies in humans [13] have indicated that IL-10 plays an important role in the regulation of the immune system during visceral leishmaniasis and the bias toward type 2 immune response which occurs during the infection. Whether infection with L. donovani results in development of kala-azar or subclinical infection seems to be determined by the balance between Th1-type and Th2-type activation of the immune system. IL-10 produced by keratinocytes early in the infection may tip the balance towards a Th2-type of response, and cytokines produced by keratinocytes could therefore influence the outcome of the infection.
Group 1 patients had higher plasma levels of sIL-2R than patients of group 2. Our finding of high plasma levels of sIL-2R during kala-azar is in agreement with previous reports [17, 18]. It has earlier been suggested that incomplete treatment of kala-azar is instrumental for development of PKDL [7]. This was not the case in our patients, since they received complete supervised treatment. Our results indicate that widespread dissemination of parasites to the skin is part of the natural history of kala-azar. The parasites do not provoke marked inflammatory responses, but in some patients they induce production of IL-10 by keratinocytes. This might enable the parasites to survive in the skin of some patients. When the patients are drug-treated their immune response shifts from Th2-type towards a mixed Th1/Th2-type response [3, 19]. Some of the Th1-type cells migrate to the skin, where they lead an immunological attack on the parasites. This attack may cause skin inflammation and development of PKDL.
Acknowledgments
We thank Anne Corfitz, Jette Dalsten, Lis Christiansen and Gitte Pedersen for technical assistance. Professor Charles L. Jaffe (Hebrew University-Hadassah Medical School, Jerusalem, Israel) and Dr Farrokh Moddabar (TDR/WHO, Geneva, Switzerland) are thanked for provision of monoclonal antibodies against L. donovani. Dr Henrik Permin, Dr Seif SirElkhatim, Dr Nadia M. Nur, and the staff at Soba, Omdurman and Gedaref Hospitals are thanked for their valuable assistance at the wards. This work was supported by the Danish International Development Agency (Danida) and the Danish Biotechnology Programme.
References
- 1.Ibrahim ME, Evans DA, Theander TG, El Hassan AM, Kharazmi A. Diversity among Leishmania isolates from the Sudan: isoenzyme homogeneity of L. donovani versus heterogeneity of L. major. Trans Roy Trop Med Hyg. 1995;89:366–9. doi: 10.1016/0035-9203(95)90010-1. [DOI] [PubMed] [Google Scholar]
- 2.El Hassan AM, Ghalib HW, Zijlstra EE, ElToum IA, Satti M, Ali MS, Ali HMA. Post kala-azar dermal leishmaniasis in the Sudan: clinical features, pathology and treatment. Trans Roy Soc Trop Med Hyg. 1992;86:245–8. doi: 10.1016/0035-9203(92)90294-m. [DOI] [PubMed] [Google Scholar]
- 3.Kemp M, Theander TG, Kharazmi A. The contrasting roles of CD4+ cells in intracellular infections in human: Leishmania as an example. Immunol Today. 1996;17:13–17. doi: 10.1016/0167-5699(96)80562-7. [DOI] [PubMed] [Google Scholar]
- 4.Zijlstra EE, El Hassan AM, Ismael A. Endemic kala-azar in Eastern Sudan: post kala-azar dermal leishmaniasis. Am J Trop Med Hyg. 1995;52:299–305. doi: 10.4269/ajtmh.1995.52.299. [DOI] [PubMed] [Google Scholar]
- 5.Hviid L, Albeck G, Hansen B, Theander TG, Talbot A. A new portable device for automatic controlled-gradient cryopreservation of blood mononuclear cells. J Immunol Methods. 1993;157:135–42. doi: 10.1016/0022-1759(93)90079-m. [DOI] [PubMed] [Google Scholar]
- 6.Gaafar A, Kharazmi A, Ismail A, et al. Dichotomy of the T-cell response to Leishmania antigens in patients suffering from cutaneous leishmaniasis. Absence or scarcity of Th1 activity is associated with severe infections. Clin Exp Immunol. 1995;100:239–45. doi: 10.1111/j.1365-2249.1995.tb03660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zijlstra EE, El Hassan AM, Ismail A, Ghalib HW. Endemic kala azar in eastern Sudan: a longitudinal study on the incidence of clinical, subclinical infection and post kala-azar dermal leishmaniasis. Am J Trop Med Hyg. 1994;51:826–36. doi: 10.4269/ajtmh.1994.51.826. [DOI] [PubMed] [Google Scholar]
- 8.Manson-Bahr PEC. East African kala-azar with special reference to the pathology, prophylaxis and treatment. Trans Roy Soc Trop Med Hyg. 1959;53:123–7. doi: 10.1016/0035-9203(59)90060-4. [DOI] [PubMed] [Google Scholar]
- 9.O'Garra A, Murphy K. Role of cytokines in determining T-lymphocyte function. Curr Opin Immunol. 1994;6:458–66. doi: 10.1016/0952-7915(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 10.Berg DJ, Leach MW, Kuhn R, Rajewsky K, Muller W, Davidson NJ, Rennick D. Interleukin 10 but not interleukin 4 is a natural suppressor of cutaneous inflammatory responses. J Exp Med. 1995;182:99–108. doi: 10.1084/jem.182.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Becherel PA, Le Goff L, Ktorza S, et al. Interleukin 10 inhibits IgE-mediated nitric oxide synthase induction and cytokine synthesis in normal human keratinocytes. Eur J Immunol. 1995;25:2992–5. doi: 10.1002/eji.1830251042. [DOI] [PubMed] [Google Scholar]
- 12.Cillari E, Vitale G, Arcoleo F, et al. In vivo and in vitro cytokine profiles and mononuclear cell subsets in Sicilian patients with active visceral leishmaniasis. Cytokine. 1995;7:740–5. doi: 10.1006/cyto.1995.0088. [DOI] [PubMed] [Google Scholar]
- 13.Ghalib HW, Piuvezam MR, Sheiky Y, et al. Interleukin 10 production correlates with pathology in human Leishmania donovani infections. J Clin Invest. 1993;92:324–9. doi: 10.1172/JCI116570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karp CL, El Safi SH, Wynn TA, et al. In vivo cytokine profiles in patients with kala-azar. J Clin Invest. 1993;91:1644–8. doi: 10.1172/JCI116372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holaday BJ, Pompeu MM, Jeronimo S, Texeira MJ, de Sousa A. Potential role for interleukin 10 in the immune suppression associated with kala-azar. J Clin Invest. 1993;92:2626–32. doi: 10.1172/JCI116878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Powrie F, Menon S, Coffman RL. Interleukin-4 and interleukin 10 synergize to inhibit cell-mediated immunity in vivo. Eur J Immunol. 1993;23:2223–9. doi: 10.1002/eji.1830230926. [DOI] [PubMed] [Google Scholar]
- 17.Josimovic-Alasevic O, Feldmeier H, Zwingenberger K, Harms G, Haln H, Shrisuphanunt M, Diamantstein T. Interleukin 2 receptor in patients with localised and systemic diseases. Clin Exp Immunol. 1988;72:249–54. [PMC free article] [PubMed] [Google Scholar]
- 18.Vitale G, Reina G, Mansueto S, et al. The significance of serum soluble IL-2 receptor as a marker for active visceral leishmaniasis in Sicilian patients. Clin Exp Immunol. 1992;90:219–22. doi: 10.1111/j.1365-2249.1992.tb07932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nobors GS, Farrell JP. Successful chemotherapy in experimental leishmaniasis is influenced by the polarity of T cell response before treatment. J Infect Dis. 1996;173:979–86. doi: 10.1093/infdis/173.4.979. [DOI] [PubMed] [Google Scholar]


