Abstract
Fas antigen is constitutively expressed in the normal colon epithelium, but considerably diminished in most colorectal carcinomas. In the present study, we examine the relationship between Fas antigen expression and apoptosis using the colorectal carcinoma cell line COLO 201, on which a low grade of Fas antigen is expressed. Anti-Fas antibody had no effect on the induction of apoptosis of COLO 201. However, TNF-α and/or IFN-γ, independently and additively, up-regulated Fas antigen expression on COLO 201 and induced apoptosis in a dose-dependent manner. Both cytokines also increased the COLO 201 sensitivity to anti-Fas antibody, resulting from the down-modulation of Bcl-2 and the up-regulation of Bax. These findings indicate that cytokine(s) plus anti-Fas antibody (which mimics natural Fas ligand) are more effective in inducing apoptosis of COLO 201 than cytokine(s) alone. These findings suggest that immunotherapy in combination with cytokine(s) and lymphokine-activated killer (LAK) cells will become a more effective therapy for cancer than cytokine(s) or LAK cells alone, since the Fas ligand is expressed on activated T cells, natural killer cells and macrophages.
Keywords: apoptosis, COLO 201, tumour necrosis factor-alpha, interferon-gamma, Bcl-2, Bax
INTRODUCTION
The Fas antigen CD95, a 36-kD transmembrane protein, is a member of the nerve growth factor/ tumour necrosis factor receptor (NGF/TNF-R) superfamily that signals apoptosis [1]. Anti-Fas MoAb, which functionally mimics natural Fas ligand, induces apoptosis in sensitive cell lines [2]. Recently, it was reported that the Fas–Fas ligand system is related to physiologic T cell apoptosis, hepatitis and natural killer (NK) activity [3].
The bcl-2 gene was identified at the t(14;18) chromosomal translocation that occurs in human follicular lymphomas [4]. Bcl-2 protein, consisting of 239 amino acids [4], is located in the inner mitochondrial membrane [5] and perinuclear endoplasmic reticulum [6]. Recently, it has been shown that bcl-2 gene-transfected T cells, B cells [7], hepatocytes [8] and nerve cells [9] are resistant to apoptosis [9]. For example, the IL-2-dependent T cell line CTLL-2 dies by apoptosis after IL-2 withdrawal. However, after transfection with bcl-2, it becomes relatively IL-2-independent for survival [10, 11]. T cells expressing bcl-2 transgene show longer life-spans [12, 13]. In bcl-2-deficient mice, it has been shown that a normal number of T cells is present at birth but disappears by 6 weeks old due to fulminant lymphoid apoptosis [14]. T cells from bcl-2-deficient mice were also found to show shorter life-spans in vitro and increased sensitivity to glucocorticoids and γ-irradiation [15].
We have previously reported that exogenous TNF-α and IFN-γ up-regulate Fas antigen expression on peripheral blood mononuclear cells (PBMC) [16]. Recently, it has also been reported that IFN-γ and/or TNF-α up-regulate Fas antigen expression on colon cancer cell lines [17]. However, it is still unknown whether the up-regulation of Fas antigen expression on colon cancer cell line by cytokine(s) induces apoptosis.
Bax is an intracytoplasmic protein in the Bcl-2 family [18]. It has been reported that the heterodimer of Bcl-2 and Bax has anti-apoptotic capacity, and that the homodimer of Bax accelerates apoptosis [19]. Therefore, an increase in the homodimer of Bax (resulting from down-modulation of Bcl-2 and up-regulation of Bax) could induce apoptosis.
In the present study, we show that exogenous TNF-α and IFN-γ up-regulate Fas antigen expression on human colon adenocarcinoma, COLO 201 [20], and that these cytokines increase the sensitivity of COLO 201 to anti-Fas antibody. The sensitivity is associated with down-modulation of Bcl-2 expression and up-regulation of Bax. Therefore, COLO 201, which shows a low expression of Bcl-2, a high expression of Bax and a high expression of Fas antigen, seems to more readily undergo apoptosis when Fas antigen is stimulated by anti-Fas antibody.
MATERIALS AND METHODS
Reagents and antibodies
TNF-α and IFN-γ were purchased from Boehringer Mannheim (Indianapolis, IN) and anti-Fas antibody was obtained from AMAC Inc. (Westbrook, ME).
Cell culture
The COLO 201, a human colon adenocarcinoma cell line, was obtained from Japanese Cancer Resources Bank Cell. COLO 201 was adjusted to 1·0 × 105 cells/ml and cultured in RPMI 1640 (Gibco BRL) containing 10% fetal calf serum (FCS) with various apoptosis-inducing agents.
Fas staining
Cultured cells were washed in PBS and suspended in 50 μl staining buffer (PBS containing 1% FCS and 0·1% sodium azide), followed by the addition of 50 μg normal mouse IgG (Cappel Research Products, Durham, NC) for blocking. Samples were placed on ice for 15 min, then FITC-Fas antibody (clone UB2; MBL Inc.) or isotype-matched control antibody (Becton Dickinson Immunocytometry Systems, San Jose, CA) were added. The samples were then re-washed in staining buffer and suspended in paraformaldehyde and analysed using a FACScan (Becton Dickinson, Mountain View, CA). At least 5000 events were collected for each specimen.
Propidium iodide staining
Cultured cells were washed in PBS and suspended in 70% ethanol at 4°C for 1 h, followed by resuspension in 250 μl PBS, 500 μl RNase (1 mg/ml; Sigma Chemical Co., St Louis, MO), and 250 μl propidium iodide (PI; 100 μg/ml; Molecular Probe). The percentage of apoptotic cells was measured using the sub G0/G1 peak in PI staining.
Detection of cell surface phosphatidylserine
Cell surface phosphatidylserine was detected using the Apoptosis Detection Kit (R&D Systems, Minneapolis, MN).
Bcl-2 staining
Cell pellets were suspended in the staining buffer and fixed following surface staining with 1% paraformaldehyde in PBS, washed, and permeabilized for 10 min with saponin (Sigma) at a concentration of 0·1% (w/v) in permeabilization buffer (PB; 10 mm HEPES in PBS). After permeabilization, cells were stained for 25 min with FITC-labelled anti-human Bcl-2 antibody (Dako, Carpinteria, CA). The cells were washed with PB, resuspended in 1% paraformaldehyde PBS, and analysed using a FACScan.
Bax staining
Cell pellets were suspended in the staining buffer and fixed following surface staining with 1% paraformaldehyde in PBS, washed, and permeabilized for 10 min with saponin (Sigma) at a concentration of 0·1% (w/v) in PB. After permeabilization, the cells were stained for 25 min with anti-Bax antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Next, cells were stained with PE-labelled goat anti-rabbit IgG (Southern Biotechnology Associates, Birmingham, AL). The cells were then washed with PB, resuspended in 1% paraformaldehyde PBS, and analysed using a FACScan.
Reverse transcriptase-polymerase chain reaction detection of Fas ligand (Fas-L) mRNA expression
RNA preparation, cDNA synthesis and polymerase chain reaction (PCR) were carried out essentially as described previously [21]. Total cellular RNA was prepared using a nucleic acid extractor (TRIZOL Reagent; Life Technologies, Inc., Grand Island, NY) followed by chloroform extraction and isopropanol precipitation. cDNA was synthesized using reverse transcriptase (M-MLV Rtase in PT-PCR high (RT-PCR Kit); Toyobo, Tokyo, Japan) and Oligo(dT)20.P7 primers (RT-PCR high). PCR was performed on the cDNA using the following primers for fas-L [21] and G3PDH (RT-PCR high) with thermal cycling amplification using Takara PCR Thermal cycler MP (Takara, Otsu, Japan). The PCR products were separated on a 1·2% agarose gel (Gibco BRL) and visualized by ethidium bromide (Nakarai, Kyoto, Japan) staining.
DNA fragmentation analysis
DNA fragmentation analysis was performed as described previously [22]. Briefly, 1 × 106 cells were pelleted and resuspended in 20 μl lysis buffer (50 mm Tris–HCl pH 8·0, 10 mm EDTA containing 0·5% laurylsarcosinate, and 1·25 mg/ml proteinase K) and incubated for 1 h at 50°C. Ten microlitres of RNase A (10 mg/ml; Boehringer Mannheim, Indianapolis, IN) were added, and incubation at 50°C was carried out for 60 min, followed by the addition of 10 μl loading buffer (containing 10 mm EDTA pH 8·0, 0·25% bromophenol blue, 0·25% xylene cyanol, and 15% Ficoll 400). The sample was then heated at 70°C for 10 min before loading into the wells of a 2% agarose gel. Electrophoresis was carried out at 50 V for 3 h in Tris–borate–EDTA buffer (100 mm Tris, 83 mm boric acid, and 1 mm EDTA pH 8·3) and the gel was stained with 0·5 μg/ml ethidium bromide.
Statistical analysis
Statistical significance was assessed using the paired Wilcoxon signed rank test.
RESULTS
Augmentation of Fas antigen expression in human colon cancer cell line by TNF-α and/or IFN-γ
In the first attempt to augment Fas antigen expression on human colon adenocarcinoma COLO 201, we used flow cytometry to examine the effect of exogenous TNF-α and IFN-γ on the degree of Fas antigen expression. A low grade of Fas antigen expression was detected in cultured COLO 201 without adding cytokines (data not shown). The expression of Fas antigen increased on COLO 201 in response to exogenously added recombinant TNF-α and IFN-γ, as previously described [17] (Fig. 1). Fas antigen expression reached plateau levels on day 3 after culture with several doses of cytokines. We next analysed the dose-dependent effects of the cytokines on Fas antigen expression on COLO 201 on day 3. Both cytokines were found to augment Fas antigen expression in a dose-dependent manner (Fig. 1). The simultaneous addition of TNF-α (0·3 μg/ml) and IFN-γ (30 U/ml) also additionally augmented Fas antigen expression (Fig. 1).
Fig. 1.

Fas expression of COLO 201 by TNF-α and IFN-γ. (a) A representative pattern of Fas antigen expression on COLO 201 cultured with TNF-α. COLO 201 was cultured for 3 days with or without TNF-α. The dashed line shows isotype-matched control, and the solid line shows Fas antigen expression: without TNF-α (A), and with TNF-α at 0·01 μg/ml (B), 0·03 μg/ml (C), 0·1 μg/ml (D), 0·3 μg/ml (E) or 1·0 μg/ml (F). (b,c) Time course of percent apoptosis of COLO 201 (n =20). COLO 201 was cultured with or without TNF-α (b) or IFN-γ (c). (b) Fas expression in the absence of TNF-α (□) and the presence of 0·03 μg/ml (⋄), 0·3 μg/ml (○), 1 μg/ml (▵) TNF-α. (c) Fas expression in the absence of IFN-γ (□) and in the presence of 3 U/ml (⋄), 30 U/ml (○), 100 U/ml (Δ) IFN-γ. Means and s.d. are shown. *Significant difference at P<0·005 compared with absence of cytokine.
Correlation of Fas antigen with apoptotic cell death
The apoptotic process is characterized by morphologic changes involving chromatin condensation and by the regular fragmentation of genomic DNA into oligonucleosome fragments of 180–200 bp units [23]. However, the conventional DNA electrophoresis technique is not suitable for quantitative estimation. We therefore used a flow cytometric approach to quantify the cells undergoing apoptosis, not only after staining of nuclei with PI but also after staining the cell surface phosphatidylserine with FITC–annexin V [24]. PI staining has been shown to induce apoptosis in thymocytes [25], PBMC [26], and tumour cells [27], and is accompanied by the appearance of the sub-G0/G1 peak in the DNA histogram. When this method was applied to COLO 201, both TNF-α and IFN-γ, independently and additively, augmented the percent apoptosis of COLO 201 in a dose-dependent manner (Fig. 2a,b). There was a correlation between the percentage of apoptosis and Fas antigen expression (Fig. 4). These results suggest that COLO 201, in which Fas antigen expression was enhanced by TNF-α, IFN-γ, or both cytokines, more readily underwent apoptosis. The percentage of cells in the sub-G0/G1 peak was almost identical to the percentage expressing phosphatidylserine on the outer cell membrane (Fig. 2b). Apoptosis was confirmed by the DNA fragmentation assay (Fig. 3). Without stimulation, with only anti-Fas antibody treatment, COLO 201 showed almost intact chromosomal DNA, but after TNF-α and/or IFN-γ with or without anti-Fas antibody treatment, especially IFN-γ+ anti-Fas antibody and TNF-α+ IFN-γ+ anti-Fas antibody, COLO 201 showed many apoptotic DNA ladders of nucleosomal oligomers, i.e. DNA fragmentation.
Fig. 2.

Effect of cytokine(s) and/or anti-Fas antibody on apoptosis of COLO 201. COLO 201 was cultured for 3 days with or without cytokine(s) and anti-Fas antibody. (a) Representative data of propidium iodide (PI) staining in COLO 201: control (A), TNF-α (0·3 μg/ml) (B), IFN-γ (30 U/ml) (C), TNF-α+ IFN-γ (D), anti-Fas antibody (100 ng/ml) (E), anti-Fas antibody + TNF-α (F), anti-Fas antibody + IFN-γ (G) and anti-Fas antibody + TNF-α+ IFN-γ (H). (b) Representative data for the detection of cell surface phosphatidylserine on COLO 201: control (A), TNF-α (0·3 μg/ml) (B), IFN-γ (30 U/ml) (C), TNF-α+ IFN-γ (D), anti-Fas antibody (100 ng/ml) (E), anti-Fas antibody + TNF-α (F), anti-Fas antibody + IFN-γ (G) and anti-Fas antibody + TNF-α+ IFN-γ (H).
Fig. 4.

Effect of anti-Fas antibody on apoptosis of COLO 201 in which Fas expression was augmented by cytokine(s) (n = 8). COLO 201 was cultured for 3 days with or without cytokine(s) and anti-Fas antibody. (a) Mean ± s.d. of Fas expression of COLO 201: control, TNF-α (0·3 μg/ml), IFN-γ (30 U/ml), TNF-α + IFN-γ, anti-Fas antibody (100 ng/ml), anti-Fas antibody + TNF-α, anti-Fas antibody + IFN-γ and anti-Fas antibody + TNF-α + IFN-γ. Anti-Fas antibody had no effect on Fas antigen expression without cytokine(s), but in the condition with cytokine(s), anti-Fas antibody augmented Fas expression on COLO 201. (b) Means ± s.d. of percent apoptosis of COLO 201. Anti-Fas antibody alone has no ability to induce apoptosis in COLO 201, but anti-Fas antibody augments apoptosis of COLO 201 cultured with cytokine(s).
Fig. 3.
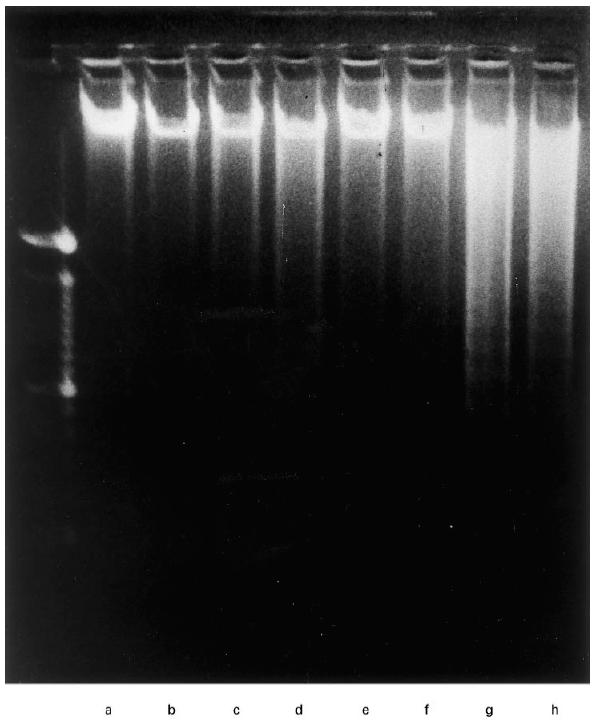
DNA fragmentation analysis of cytokine-induced apoptosis of COLO 201. DNA extracted from control (a), TNF-α (0·3 μg/ml) (b), IFN-γ (30 U/ml) (c), TNF-α+ IFN-γ (d), anti-Fas antibody (100 ng/ml) (e), anti-Fas antibody + TNF-α (f), anti-Fas antibody + IFN-γ (g) and anti-Fas antibody + TNF-α+ IFN-γ (h), was analysed by agarose gel electrophoresis, and stained with ethidium bromide.
Effect of anti-Fas antibody on apoptosis of COLO 201
The ligation of Fas antigen by a specific antibody [2, 28] or its ligand (Fas-L) [29, 30] has been shown to transduce a potent apoptotic signal in sensitive target cells in vitro. To examine the function of Fas antigen expressed on COLO 201, we cultured COLO 201 with or without anti-Fas antibody. Without cytokines, anti-Fas antibody did not induce apoptosis. However, when combined with TNF-α, IFN-γ, or both, anti-Fas antibody significantly enhanced the induction of apoptosis (Figs 2a and 4). These results suggest that TNF-α and IFN-γ enhance not only the expression of Fas antigen but also the sensitivity to stimulation by anti-Fas antibody.
Expression of Fas-L of COLO 201
Fas-L is expressed in activated NK cells and T cells, and has cytotoxic function through the death signal of Fas. It has been reported that some colorectal carcinoma cell lines express Fas-L and, theoretically, could kill Fas-expressing activated T cells and NK cells (counter attach theory [21]). Therefore, we examined the expression of fas-L mRNA in COLO 201 with or without stimulation using RT-PCR (Fig. 5). Fas-L mRNA was not expressed in COLO 201 even under the stimulation of TNF-α and IFN-γ.
Fig. 5.

Expression of mRNA of Fas ligand in COLO 201. RNA isolated from 3 h cultured control (a), TNF-α (0·3 μg/ml) (b), IFN-γ (30 U/ml) (c), TNF-α + IFN-γ (d), 6 h cultured control (e), TNF-α (0·3 μg/ml) (f), IFN-γ (30 U/ml) (g), TNF-α + IFN-γ (h), and 12 h cultured control (i), TNF-α (0·3 μg/ml) (j), IFN-γ (30 U/ml) (k), TNF-α + IFN-γ (l) were separated by agarose gel electrophoresis, and stained with ethidium bromide. Freshly isolated peripheral blood mononuclear cells (PBMC) (n), 6 h phytohaemagglutinin (PHA)-activated PBMC (m) were used as positive controls. mRNA-specific amplification product band for Fas ligand (344 bp) and G3PDH (450 bp) as internal control.
Down-modulation of Bcl-2 by TNF-α and IFN-γ
Bcl-2 is known to act as an apoptosis repressor in many experimental systems [2]. To examine how they induce apoptosis of COLO 201, and how the cytokine(s) enhance the sensitivity of COLO 201 to anti-Fas antibody, we analysed Bcl-2 expression using flow cytometry. As shown in Fig. 6, Bcl-2 expression of COLO 201 by cytokines was markedly reduced, and there was no correlation between Bcl-2 expression and the existence of anti-Fas antibody. These results indicate that Bcl-2 expression is markedly reduced by TNF-α and IFN-γ, independently and additively, and that the down-modulation of Bcl-2 augments the sensitivity to anti-Fas antibody, resulting in the induction of apoptosis.
Fig. 6.

Bcl-2 expression on COLO 201. Representative data are shown for 20 experiments. COLO 201 was cultured for 3 days with or without TNF-α (0·3 μg/ml) and/or IFN-γ (30 U/ml). Bcl-2 expression was analysed using flow cytometry as described in Materials and Methods: TNF-α (0·3 μg/ml) (b), IFN-γ (30 U/ml) (c), TNF-α + IFN-γ (d), anti-Fas antibody (100 ng/ml) (e), anti-Fas antibody + TNF-α (f), anti-Fas antibody + IFN-γ (g) and anti-Fas antibody + TNF-α + IFN-γ (h). The dashed line shows the isotype-matched control, and the solid line shows Bcl-2 expression. The signature shows the mean fluorescence intensity. Bcl-2 expression was down-modulated by cytokine(s). Anti-Fas antibody had no ability to down-modulate Bcl-2 expression with or without cytokine(s).
Expression of Bax by TNF-α and IFN-γ
Bax is an another intracytoplasmic protein in the Bcl-2 family [18]. Recently, it has been shown that the heterodimer of Bcl-2 and Bax has the capacity for anti-apoptosis, and that the homodimer of Bax accelerates apoptosis [19]. It has also been reported that increased homodimers of Bax, resulting from down-modulation of Bcl-2, induce apoptosis. Figure 6 shows the down-modulation of Bcl-2. We next examined the expression of Bax. As shown in Fig. 7, the expression of Bax in COLO 201 was augmented by cytokines; it was markedly augmented by TNF-α and/or IFN-γ. In addition, there was no correlation between Bax expression and the existence of anti-Fas antibody. These results suggest that TNF-α and IFN-γ, independently and additively, markedly suppress Bcl-2 expression and augment Bax expression, resulting in increased Bax homodimers, and that the Bax homodimers augment the sensitivity to anti-Fas antibody, resulting in the induction of apoptosis.
Fig. 7.

Augmented Bax expression on COLO 201. COLO 201 was cultured for 3 days with or without TNF-α (0·3 μg/ml) and/or IFN-γ (30 U/ml). The Bax experiment was analysed using flow cytometry as described in Materials and Methods. This figure shows the expression of Bax: control (a), TNF-α (0·3 μg/ml) (b), IFN-γ (30 U/ml) (c), TNF-α + IFN-γ (d), anti-Fas antibody (100 ng/ml) (e), anti-Fas antibody + TNF-α (f), anti-Fas antibody + IFN-γ (g) and anti-Fas antibody + TNF-α + IFN-γ (h). The dashed line shows the isotype-matched control and the solid line shows Bax expression. The signature shows the mean fluorescence intensity. Bax expression was up-regulated by cytokine(s). Anti-Fas antibody had no ability to up-regulate Bax expression with or without cytokine(s).
DISCUSSION
In the present study we have examined the in vitro effects of TNF-α and/or IFN-γ on apoptosis of the colorectal adenocarcinoma cell line COLO 201, and have demonstrated that only approximately 2% of COLO 201 expresses Fas antigen, and that anti-Fas antibody has no effect on the apoptosis of COLO 201. However, we have found that TNF-α, IFN-γ, or both cytokines, augment not only Fas antigen expression, but also the sensitivity to anti-Fas antibody; COLO 201 therefore undergoes marked apoptosis. Delineation of the mechanisms behind TNF-α- and IFN-γ-inducing apoptosis revealed that it is closely associated with Bcl-2 down-regulation.
Recently it has been reported that Fas antigen is constitutively expressed in the normal colon epithelium, but that the Fas antigen density is considerably diminished in most colorectal carcinomas [17]. TNF-α and IFN-γ have also been shown to up-regulate the expression of Fas antigen on colorectal carcinoma cell lines [17]. However, it has not been clarified whether the Fas antigen stimulation induced by TNF-α and IFN-γ causes apoptosis. Fas antigen is well known to be associated with cell apoptosis, and its stimulation induces apoptosis [2], although some leukaemic cell lines proliferate when their Fas antigen is stimulated [31]. It has also been reported that anti-Fas antibody does not induce apoptosis of PBMC from normal donors, while anti-Fas antibody induces significant apoptosis of PBMC from HIV+ patients [32]. These data suggest that, under some conditions, signals from Fas antigen are not easy to transduce. Some proteins downstream of Fas antigen disturb the transduction of signals from Fas antigen. Several proteins have been reported to be candidates as the obstacles (Bcl-2, Bcl-xL and Mcl-1). Bcl-2 has been shown to block apoptosis in many experiments. For example, the over-expression of Bcl-2 has been shown to result in the inhibition of anti-Fas antibody- and TNF-α-mediated apoptosis [33]. In the present study, we found that Bcl-2 protein is expressed in COLO 201 under several conditions. When COLO 201 was cultured with TNF-α, IFN-γ or both cytokines, Bcl-2 protein of COLO 201 was down-modulated and the number of apoptotic cells increased. To ascertain the association of Bcl-2 with apoptosis of COLO 201, we examined the expression of Bax. Bax is another intracytoplasmic protein in the Bcl-2 family [18], and the heterodimer of Bcl-2 and Bax has the capacity for anti-apoptosis, while the homodimer of Bax accelerates apoptosis [19]. It is conceivable that increased homodimers of Bax, resulting from the down-modulation of Bcl-2, induce apoptosis. The expression of Bax protein in COLO 201 was, indeed, augmented by TNF-α, IFN-γ or both cytokines. These results suggest that TNF-α, IFN-γ or both cytokines have the ability to down-modulate Bcl-2 expression and augment Bax expression, resulting in increased Bax homodimers; Bax homodimers induce apoptosis of COLO 201 and augment the sensitivity to anti-Fas antibody stimulation, due not only to the up-regulation of Fas antigen but also to the easy transduction of Fas antigen stimulation. Therefore, it seems that the combination of cytokine(s) and anti-Fas antibody has a more profound effect on apoptosis of COLO 201 than independent administration.
Clinical trials of combination or individual cytokine(s) therapy are now being carried out, and we have found some reports of cytokine therapy for cancer patients: for example, the combination IFN-α + IL-2 for metastatic cancer [34], IFN-γ + IL-2 for metastatic melanoma [35] or metastatic renal cancer [36] and TNF-α + IFN-γ [37]. However, the results of these therapies are still controversial. The variety of responses to these therapies might be due to the individual characteristics of the hosts and the tumours. It has been reported that, in late stage cancer patients, the production of transforming growth factor-beta (TGF-β), which suppresses host immune function, increases [38], and that some colorectal carcinoma cell lines express non-functional Fas [21]. However, in our experience, COLO 201 expresses functional Fas when stimulated by TNF-α and/or IFN-γ. Actually, in some clinical cases cytokine therapies are effective. Our data provide an experimental basis for cytokine therapies for cancer.
In conclusion, TNF-α, IFN-γ or both cytokines up-regulate Fas antigen expression and the sensitivity of the colorectal adenocarcinoma COLO 201, which results in apoptosis. The administration of anti-Fas antibody had no effect on COLO 201 cultured in the control medium, but cytokine plus anti-Fas antibody was more effective in inducing COLO 201 apoptosis than the cytokine(s) alone. In the apoptotic cells, Bcl-2 expression decreased markedly, while Bax expression increased, suggesting that apoptosis of colon 201 can be attributed to down-modulation of Bcl-2 and up-regulation of Bax.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research of Japan 09877051.
References
- 1.Ito N, Yonehara S, Ishii A, et al. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991;66:233–43. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- 2.Yonehara S, Ishii A, Yonehara M. A cell-killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J Exp Med. 1989;169:1747–56. doi: 10.1084/jem.169.5.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montel AH, Bochan MR, Goebel WS, Brahmi Z. Fas-mediated cytotoxicity remains in perforin and granzyme B antisense transfectants of a human NK-like cell line. Cell Immunol. 1995;165:312–7. doi: 10.1006/cimm.1995.1219. [DOI] [PubMed] [Google Scholar]
- 4.Tsujimoto Y, Croce CM. Analysis of structure, transcripts, and protein products of bcl-2, the gene involved in human follicular lymphoma. Proc Natl Acad Sci USA. 1986;83:5214–8. doi: 10.1073/pnas.83.14.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hockenbery D, Nunez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an innermitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334–6. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- 6.Chen-Levy Z, Nouese J, Cleary ML. The bcl-2 candidate proto-oncogene product is a 24-kilodalton integral-membrane protein highly expressed in lymphoid cell lines and lymphomas carrying the t(14;18) translocation. Mol Cell Biol. 1989;9:701–10. doi: 10.1128/mcb.9.2.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonnell TJ, Denne N, Platt FM, Nunez G, Jaeger U, McKearn JP, Korsmeyer SJ. Bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989;57:79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- 8.Lacronique V, Mignon A, Fabre M, et al. Bcl-2 protects from lethal hepatic apoptosis induced by an anti-Fas antibody in mice. Nature Med. 1996;2:80–86. doi: 10.1038/nm0196-80. [DOI] [PubMed] [Google Scholar]
- 9.Johnson EM, Jr, Deckwerth TL. Molecular mechanisms of developmental neuronal death. Annu Rev Neurosci. 1993;16:31–46. doi: 10.1146/annurev.ne.16.030193.000335. [DOI] [PubMed] [Google Scholar]
- 10.Broome HE, Dargan CM, Bessent EF, Krajewski S, Reed JC. Apoptosis and Bcl-2 expression in cultured murine splenic T cells. Immunology. 1995;84:375–82. [PMC free article] [PubMed] [Google Scholar]
- 11.Deng G, Podack ER. Suppression of apoptosis in a cytotoxic T-cell line by interleukin 2-mediated gene transcription and deregulated expression of the protooncogene bcl-2. Proc Natl Acad Sci USA. 1993;90:2189–93. doi: 10.1073/pnas.90.6.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Straser A, Harris AW, Cory S. bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991;67:889–99. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- 13.Katsumata M, Siegel RM, Louie DC, Miyashita T, Tsujimoto Y, Nowell PC, Greene MI, Reed JC. Differential effects of Bcl-2 on T cell and B cells in transgenic mice. Proc Natl Acad Sci USA. 1992;89:11376–80. doi: 10.1073/pnas.89.23.11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993;75:229–40. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- 15.Nakayama KI, Nakayama K, Negishi I, et al. Disappearance of the lymphoid system in bcl-2 homozygous mutant chimeric mice. Science. 1993;261:1584–8. doi: 10.1126/science.8372353. [DOI] [PubMed] [Google Scholar]
- 16.Oyaizu N, Thomas WM, Than S, Hu R, Kalyanaraman VS, Pahwa S. Cross-linking of CD4 molecules upregulates Fas antigen expression in lymphocytes by inducing interferon-γ and tumor necrosis factor-α secretion. Blood. 1994;84:2622–31. [PubMed] [Google Scholar]
- 17.Moller P, Koretz K, Leithauser F, Bruderlein S, Henne C, Quentmeier A, Krammer PH. Expression of APO-1 (CD95), a member of the NGF/TNF receptor superfamily, in normal and neoplastic colon epithelium. Int J Cancer. 1994;57:371–7. doi: 10.1002/ijc.2910570314. [DOI] [PubMed] [Google Scholar]
- 18.Oltvai Z, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–19. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 19.Yin X-M, Olitvai ZN, Korsmeyer SJ. BH1 and BH2 domains of Bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax. Nature. 1994;369:321–3. doi: 10.1038/369321a0. [DOI] [PubMed] [Google Scholar]
- 20.Semple TU, Quinn LA, Woods LK, Moore GE. Tumor and lymphoid cell lines from a patient with carcinoma of the colon for cytotoxicity model. Cancer Res. 1978;38:1345–55. [PubMed] [Google Scholar]
- 21.O'Connell J, O'Sullivan CO, Collins JK, Shanaha F. The Fas counterattack: Fas-mediated T cell killing by colon cancer expression Fas ligand. J Exp Med. 1996;184:1075–82. doi: 10.1084/jem.184.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gauthier ER, Piche L, Lemieux G, Lemieux R. Role of bcl-XL in the control of apoptosis in the murine melanoma cells. Cancer Res. 1996;56:1451–6. [PubMed] [Google Scholar]
- 23.Smith CA, Williams GT, Kingston R, Jenkinson EJ, Owen JT. Antibodies to CD3/T-cell receptor complex induce death by apoptosis in immature T cells in thymic cultures. Nature. 1989;337:181–4. doi: 10.1038/337181a0. [DOI] [PubMed] [Google Scholar]
- 24.Adachi Y, Oyaizu N, Than S, McCloskey TW, Pahwa S. IL-2 rescues in vivo lymphocyte apoptosis in patients with HIV infection. Correlation with its ability to block culture-induced down-modulation of Bcl-2. J Immunol. 1996;157:4184–93. [PubMed] [Google Scholar]
- 25.Nicoletti I, Migliorati G, Pagliacci MC, Riccardi C. A rapid simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–9. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 26.Oyaizu N, McCloskey TW, Than S, Hu R, Kalyanaraman VS, Pahwa S. Cross-linking of CD4 molecules upregulates Fas antigen expression in lymphocytes by inducing interferon-γ and tumor necrosis factor-α secretion. Blood. 1994;84:2622–31. [PubMed] [Google Scholar]
- 27.Oyaizu N, Than S, McCloskey TW, Pahwa S. Requirement of p56lck in T-cell receptor/CD3-mediated apoptosis and Fas-ligand induction in Jurkat cells. BBRC. 1995;213:994–1001. doi: 10.1006/bbrc.1995.2227. [DOI] [PubMed] [Google Scholar]
- 28.Trauth BC, Klas C, Peter AM, Matzuki S, Moller P, Falk W, Debatin KM, Krammer PH. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989;245:301–4. doi: 10.1126/science.2787530. [DOI] [PubMed] [Google Scholar]
- 29.Suda T, Takahashi T, Golstein P, Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993;75:1169–78. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- 30.Alderson MR, Tough TW, Davis-Smith T, et al. Fas ligand mediates activation-induced cell death in human T lymphocytes. J Exp Med. 1995;181:71–77. doi: 10.1084/jem.181.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mapara MY, Bargou R, Zugck C, Dohner H, Ustaoglu F, Jonker RR, Krammer PH, Dorken B. Apo-1 mediated apoptosis or proliferation in human chronic B lymphocytic leukemia: correlation with bcl-2 oncogene expression. Eur J Immunol. 1993;23:702–8. doi: 10.1002/eji.1830230320. [DOI] [PubMed] [Google Scholar]
- 32.Katsikis PD, Wunderlich ES, Smith CA, Herzenberg LA. Fas antigen stimulation induces marked apoptosis of T lymphocytes in human immunodeficiency virus-infected individuals. J Exp Med. 1995;181:2029–46. doi: 10.1084/jem.181.6.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Itoh N, Tsujimoto Y, Nagata S. Effect of bcl-2 on Fas antigen-mediated cell death. J Immunol. 1993;151:621–7. [PubMed] [Google Scholar]
- 34.Marincola FM, White DE, Wise AP, Rosenberg SA. Combination therapy with interferon Alfa-2a and interleukin-2 for the treatment of metastatic cancer. J Clin Oncol. 1995;13:1110–22. doi: 10.1200/JCO.1995.13.5.1110. [DOI] [PubMed] [Google Scholar]
- 35.Kim CJ, Taubenberger JK, Simonis TB, White DE, Rosenberg SA, Marincola FM. Combination therapy with interferon-gamma and interleukin-2 for treatment of metastatic melanoma. J Immunother Emphasis Tumor Immunol. 1996;19:50–58. doi: 10.1097/00002371-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Mani S, Todd MB, Katz K, Poo WJ. Prognostic factors for survival in patients with metastatic renal cancer treated with biological response modifiers. J Urol. 1995;154:41–42. [PubMed] [Google Scholar]
- 37.Sciller JH, Witt PL, Storer B, et al. Clinical and biologic effect of combination therapy with gamma-interferon and tumor necrosis factor. Cancer. 1992;69:562–71. doi: 10.1002/1097-0142(19920115)69:2<562::aid-cncr2820690247>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 38.Li XF, Takiuchi H, Zou JP, et al. Transforming growth factor-β-mediated immunosuppression in the tumor bearing state: enhanced production of TGF-β and a progressive increase in TGF-β susceptibility of anti-tumor CD4+ T cell function. Jpn J Cancer Res. 1993;84:315–25. doi: 10.1111/j.1349-7006.1993.tb02873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


