Abstract
Hydrophobic interaction chromatography and two-dimensional electrophoresis were used to isolate and characterize mouse SAA, and to study the in vivo effect of separate or combined administrations of cytokines, dexamethasone (DEX) and LPS on mouse SAA. Four SAA spots containing partial amino acid sequence in accordance with mouse apoSAA1 and apoSAA2/SAASJL/J pI 5.9 were demonstrated in serum. One of these proteins represents a previously undescribed, acidic acute-phase mouse SAA protein. Both DEX and interferon-gamma (IFN-γ) proved to be capable of increasing SAA serum levels. In contrast to what has been shown in previous in vivo studies, administration of IL-6 did increase the SAA levels to nearly the same magnitude as IL-1, and the effect of IL-6 and LPS on SAA production was not significantly altered by the addition of DEX. Irrespective of the inflammatory stimuli that was administered, a non-selective production of SAA1 and SAA2 was observed in most groups, including the group that received IL-6. The results illustrate that data obtained about mouse SAA are highly dependent on which models, isolation and identification methods are used.
Keywords: cytokines, dexamethasone, mouse, serum amyloid A, two-dimensional electrophoresis
INTRODUCTION
SAA proteins constitute a superfamily of mainly 12-kD acute-phase proteins [1]. In the mouse, four SAA genes (SAA1, SAA2, SAA3, SAA4) have been characterized [2–4] The mouse SAA prototype consists of 103 amino acids, and in serum, six isotypes—SAA1, SAA2, SAA3, SAA4, SAACE/J pI 6.15, SAASJL/J pI 5·9—have been identified [5–9]. SAA4, previously referred to as SAA5 [4], is the constitutive variant, and the remaining isotypes comprise the acute-phase mouse SAA proteins [1].
Based on immunological cross-reactivity, sequence homology and kinetic studies, SAA is believed to be the precursor of protein amyloid A (AA), the major protein component of secondary amyloid deposits [10]. The latter is a serious complication of some chronic inflammatory diseases, and in various species it has been speculated whether AA is derived specifically from either SAA1 or SAA2, or from both (reviewed in [11]).
The hepatic synthesis of acute-phase proteins is modulated by cytokines, which mediate protective and destructive events of inflammation [12]. The effects of cytokines on SAA production, and their interaction with glucocorticosteroids during the acute-phase response, have been studied largely in hepatic cell lines, analysing SAA mRNA and/or the total protein concentration [13–17]. In these studies it has been shown that IL-1 and IL-6 act in synergism [15–17], and that glucocorticoids potentiate the effect of cytokines on SAA production [14, 18]. In mouse plasma, and prior to the present study, the above mentioned aspects have been investigated by using separate administrations of cytokines, combined with ELISA techniques for quantification of total SAA [18–21]. In recent years, SAA has been isolated by a combination of hydrophobic interaction chromatography and two-dimensional electrophoresis with immobilized pH gradients (HIC/2-D IPG) [22]. 2-D IPG is currently the most powerful tool to resolve proteins [23], and a number of novel SAA variants have been recognized by this technique [24].
The aims of the present study were to characterize mouse SAA isotypes isolated by a small scale HIC/2-D IPG [25], and to use this method to study the in vivo effect of separate or combined administrations of dexamethasone (DEX), LPS and various cytokines on SAA production, as judged from the distribution of individual isotypes, and the total amount of SAA in serum.
MATERIALS AND METHODS
Materials
Recombinant mouse cytokines were obtained from Sigma (St Louis, MO). DEX was obtained from Merck, Sharp & Dome (Haarlem, The Netherlands), and LPS (Escherichia coli serotype 026:B6) was purchased from Difco Labs (UK).
Methods
Induction of inflammation.
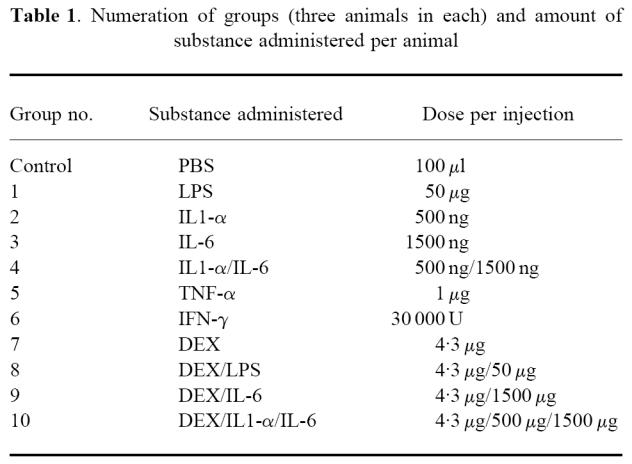
Animal experiments were conducted in accordance with the appropriate standards for the regulatory agencies in Norway. Ten to fourteen-week-old C57Bl/6 mice (Charles River Labs, Sultzfeld, Germany) of both gender, weighing ≈ 30 g, were used. Animals received i.p. injections of cytokines, DEX and LPS, and were divided into groups according to Table 1. IL-1 and tumour necrosis factor (TNF) dosages were based on a previous study [21], and DEX was administered according to high dosage in humans (0·14 mg/kg). IL-6 and interferon (IFN) doses were chosen on the basis of a pilot study, in which lower doses (500 ng/10 000 U) did not yield a satisfactory SAA response. Cytokines were diluted in PBS, to a total injection volume of 100–150 μl per animal. Control animals received 100 μl PBS. Animals were killed by i.p. injections of thiopentobarbital 24 h after the injections.
Table 1.
Numeration of groups (three animals in each) and amount of substance administered per animal
Small scale HIC and 2-D IPG.
For the quantification studies, small scale HIC and 2-D IPG were performed as described in a recent report [25]. Pooled serum (250 μl) from three animals in each group was subjected to HIC, and 100 μl HIC eluate (corresponding to 50 μl serum) were loaded onto Immobiline DryStrips pH 4–7 (18 cm). Because of haemolysis, Immobiline DryStrip (18 cm) pH 3–9 was used for group 4. Excel Gels gradient 8–18 were silver-stained according to the modification of Heukeshoven and Dernick (Multiphor II manual; Pharmacia, Uppsala, Sweden).
Identification of SAA.
In order to identify the SAA isotypes, small scale HIC and 2-D IPG were used in combination with semidry blotting and amino acid sequence studies [25]. Serum (960 μl) from LPS-stimulated mice was subjected to HIC, and the eluate was distributed to a total of eight Dry Strips (pH 4–7), loading 240 μl on each. Spots of interest were excised from the PVDF membranes, and material from a total of eight blots from each spot of interest was subjected to N-terminal amino acid and cyanogen bromide (CNBr) cleavage studies [25].
In two spots, co-migration of proteins was found. To ensure that this was not caused by non-optimal isoelectric focusing conditions, N-terminal analysis was also performed on material separated in the pH 3–10 gradient, and on the area of the PVDF membranes which was located between the spots of interest (when separated in the pH 4–7 gradient). Sequence searches were performed with the FASTA and TagIdent programs, which can be accessed through SwissProt (http://expasy.hcuge.ch/).
Image acquisition and data analysis.
Silver-stained 2-D gels were scanned by a Molecular Dynamics (Switzerland) laser densitometer, and image computer analysis was carried out using the MelView Tool in the Melanie II 2-D software package (BioRad, Switzerland) [26]. All images were located in the pH 4–7 and 14–94-kD range. In addition to the quantification studies, MelView was also used to match the gels of all groups with that of the control group.
Quantification.
MelView automatically calculates spot volumes by integrating the optical density values within the spot areas. For each group of animals, absolute figures for SAA were obtained by dividing the spot volumes of the SAA spots of interest (spots 1, 2 and 3) with the total volume comprised by all spots in the image, and multiplying by 100. The volume comprised by all spots in the image thus represented an internal reference for 100% proteins in each gel.
Total SAA levels were obtained by a summation of the absolute figures obtained for SAA in spot 1, spot 2 and spot 3. Amounts of individual isotypes were expressed as their relative percentage contribution to the total SAA, when the latter constituted 100%.
RESULTS
No animals died after the injections.
2–D electrophoresis
2–D gels from groups 1–10 contained four spots of interest (spots 1–4), which were not present in the control group (Fig. 1a–d). The spots were located in the 14 kD and pH 6·7–5·9 range, and were present in varying amounts. Another spot (spot 5) was observed between spots 3 and 4, but with a lower molecular mass than these spots (Fig. 1c). A group of one to three minor spots was located in close proximity to the basic side of spot 3. For simplification, this group of spots was designated spot 6 (Fig. 1a–c). An inhomogeneous staining was observed for spots 1–3 in groups 2 and 10.
Fig. 1.
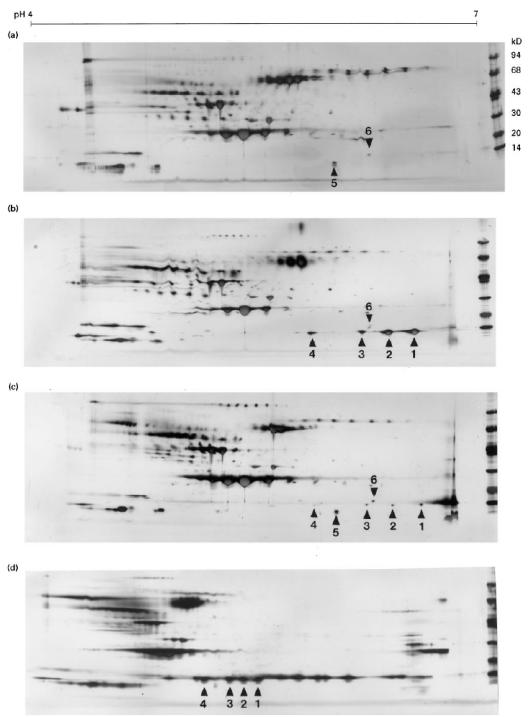
Selected, silver-stained two-dimensional electrophoresic (2-D) gels containing hydrophobic interaction chromatography (HIC) extracted proteins from acute-phase mouse serum. Immobiline DryStrips (18 cm) pH 4–7 (a–c) and 3–9 (d), ExcelGel SDS 8–18 and low molecular weight markers (Pharmacia) were used. Spots of interest are indicated by arrowheads and numbers. The sequence found in spot 5 was partially in accordance with mouse apoA2, and the protein in spot 6 could not be revealed by the presented techniques. The relative focusing positions and partial amino acid sequences of spots 1–3 are in accordance with SAA1 (spot 1), SAA2 (spot 2) and SAASJL/J pI 5.9 (spot 3). Spot 4 represents an SAA2- or SAASJL/J pI 5.9-like protein. In spots 2 and 3 small amounts of SAA1-like sequence could also be detected. (a) Control group. (b) Group 1. (c) Group 6. (d) Group 4. In the latter group, the five spots which are located on the basic side of the SAA spots (corresponding to pH 7–8) probably represent haemoglobin [32].
Amino acid sequence analysis
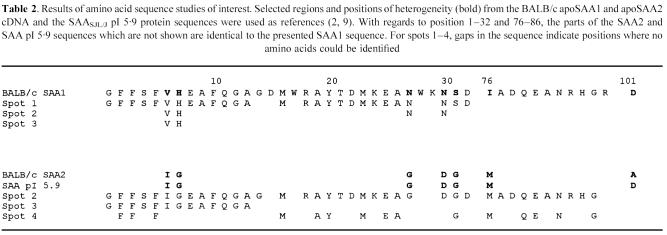
Direct N-terminal analysis and CNBr-cleavage studies of spots 1–4 revealed 13–38 positions of the murine SAA sequence (Table 2). Spots 2 and 3 contained also Val and His in positions 6 and 7, and in spot 2 Asn was also present in positions 27 and 30 (Table 2). The yield of Ile and Val in position 6 was 150:30 and 80:16 pmol, giving a ratio of 83 (Ile):17 (Val)% in both spots 2 and 3. Position 101 in the latter spots could not be obtained by the presented techniques. Sequence search on Val6-His7 did not reveal any other murine proteins than SAA1 containing these data. Thus, the presented sequence data were partially in accordance with mouse SAA1 (spots 1, 2 and 3), and SAA2 or SAASJL/J pI 5·9 (spots 2, 3 and 4) (Table 2) [2, 9]. For the quantification, and also based on differences in electrophoretic mobilities, the major SAA variants that were identified in spots 1–3 were referred to as SAA1 (spot 1), SAA2 (spot 2) and SAA pI 5·9 (spot 3).
Table 2.
Results of amino acid sequence studies of interest. Selected regions and positions of heterogeneity (bold) from the BALB/c apoSAA1 and apoSAA2 cDNA and the SAASJL/J pI 5.9 protein sequences were used as references (2, 9). With regards to position 1–32 and 76–86, the parts of the SAA2 and SAA pI 5.9 sequences which are not shown are identical to the presented SAA1 sequence. For spots 1–4, gaps in the sequence indicate positions where no amino acids could be identified
Analysis of spot 5 yielded the N-terminal sequence -Leu2-Val3-Lys/Pro4-(Arg5)-Gln6-Ala7, which is partially in accordance with mouse apoA2. Due to either insufficient amounts of proteins and/or N-terminal blockage, no information could be obtained from spot 6.
Quantification
A variable increase of total SAA levels was seen for all groups (Fig. 2a) Disregarding the groups in which SAA was inhomogeneously stained, the highest increase was seen in group 4, and the lowest in group 6. The difference in total SAA between groups 1 and 8, and for groups 3 and 9, was 5%.
Fig. 2.
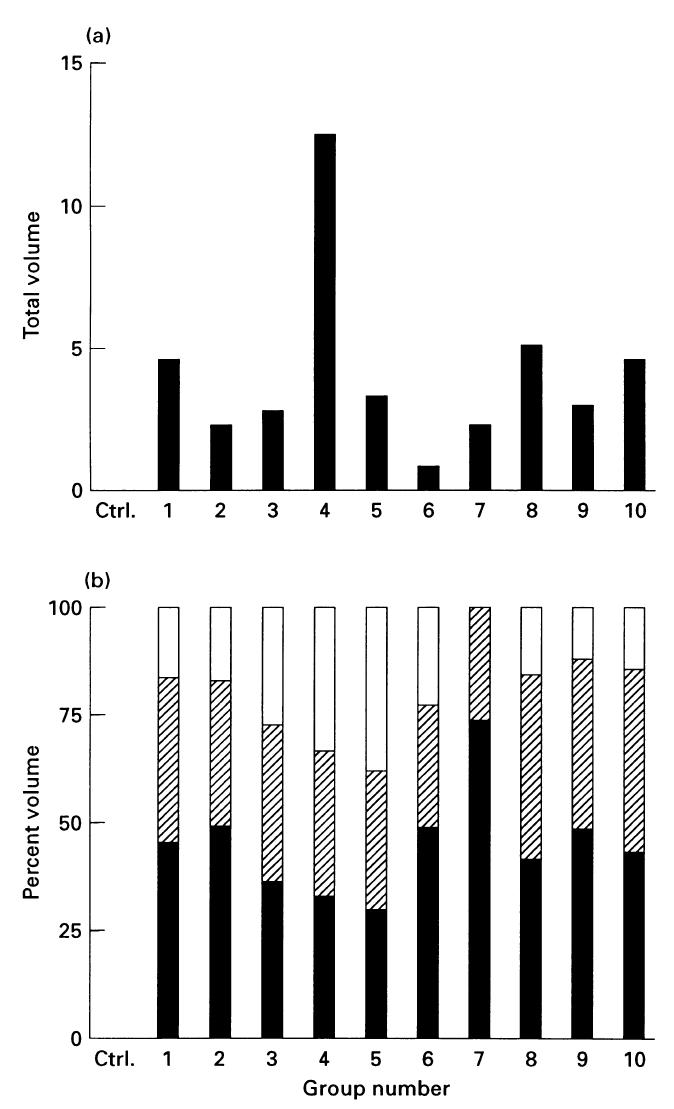
Relative SAA levels in groups 1–10. (a) Total SAA. (b) Percentage contribution of each isotype to the total SAA. ▪, SAA1;, SAA2; □, SAA pI 5.9.
SAA1 and SAA2 comprised, respectively, 30–73% and 27–43% of the total SAA (Fig. 2b), corresponding to 43 (SAA1) and 16 (SAA2)% variation. In eight out of 10 groups, however, the difference between SAA1 and SAA2 was < 15%. In five of these groups (groups 3, 4, 5, 8 and 10) the relative ratios between SAA1 and SAA2 were ≈ 1:1, and in the three remaining groups (groups 1, 2 and 9) the ratio ranged from 1:0·7 to 1:0·85.
Except for group 7, where SAA pI 5·9 could not be detected, this isotype comprised 12–23% (groups 1, 2 and 8–10) or 23–38% (groups 3–6) of the total SAA, corresponding to 11% and 5% variation. In six groups (groups 1, 2, 6 and 8–10), SAA pI 5·9 contributed with the lowest percentage volume of all isotypes (Fig. 2b).
DISCUSSION
The presence of 13–37% of the total murine SAA protein sequence was demonstrated in spots 1, 2, 3 and 4. The presented sequence data were partially in accordance with apoSAA1 (spots 1, 2 and 3) and apoSAA2 or SAASJL/J pI 5·9 (spots 2, 3 and 4) [2, 8, 9].
Considering charge differences, SAA1 (predicted pI 6·07) is more basic than both SAA2 (predicted pI 5·9) and SAASJL/J pI 5·9. The latter proteins differ only by one amino acid substitution, namely alanine (SAA2)—a neutral amino acid—and aspartic acid (SAA pI 5·9) in position 101 [9]. Consequently, SAA pI 5·9 will focus even more acidic than SAA2.
Thus, the major SAA proteins that were identified in spots 1–3 were regarded to represent SAA1 (spot 1)-, SAA2 (spot 2)- and SAACE/J pI 5·9 (spot 3)-like proteins. For the quantification, they were designated SAA1 (spot 1), SAA2 (spot 2) and SAA pI 5·9 (spot 3). Spot 4 represents a previously undescribed SAA2- or SAASJL/J pI 5·9-like protein.
The presence of Val-His6–7 and Asn27–30 in spots 2 and 3 was regarded to represent small amounts of previously undescribed, co-migrating SAA1-like proteins, with similar mass and charge properties to the major SAA variants that were identified in these spots. As the co-migration occurred irrespective of which pH gradient was used, and no proteins could be detected in the area between these spots, the latter phenomenon was considered to be unrelated to incomplete separation in the first dimension.
The presented SAA variants might all represent unmodified or modified protein products of defined or yet uncharacterized murine SAA genes. However, the major SAA variants identified in spots 1 and 2 were regarded most probably as products of the SAA1 and SAA2 genes [2]. With regard to the major SAA variant in spot 3—and also the minor, co-migrating SAA variants in spots 2 and 3—no conclusions could be drawn as to whether these are also derived from the SAA1/SAA2 genes, or from yet undefined genes. As the observed charge heterogeneity was seen also in individual samples, it was thus considered unrelated to genetic variations within pooled samples. With regard to post-translational modifications of SAA, this has been described only for human SAA [27, 28].
In murine amyloid deposits, the presence of AA, derived from both SAA1 and SAA2, and also SAA2, has been demonstrated [11, 29]. Taking into account the extensive sequence homology that exists between SAA2 and SAA pI 5·9, the presented SAA2/SAA pI 5·9-like proteins in spots 2, 3 and 4, and also the major and minor SAA1-like proteins in spots 1, 2 and 3 might all be the source of AA and SAA in murine amyloid deposits.
Considering the amino acid sequence results, the presented study introduces a previously undescribed mouse SAA isoelectric focusing (IEF) pattern. However, a visually similar IEF pattern to that presented has been demonstrated in BALB/c and SJL/J mice [7, 9, 21]. As identified by one-dimensional carrier ampholyte-based IEF techniques and immunological detection, the latter pattern consists of four SAA variants, defined as SAA1/SAA pI 6·45, SAA2/SAA pI 6·3, SAA pI 6·15 and SAA pI 5·9. Because the identities of these IEF variants have not been confirmed by amino acid sequence analysis, they might represent the same proteins as the major SAA proteins that were identified in spots 1–4. With regard to the major SAA sequences that were identified in spots 1–3, however, the SAACE/J pI 6·15 sequence could be excluded, because it has Leu in position 11 [8].
A variable increase in total SAA response was found in all groups, including those that received DEX and IFN.
For the groups that received IL-1 and IL-1/IL-6/DEX, the quantification was complicated by an inhomogeneous staining of SAA. This is a well known phenomenon in relation to silver staining, and is due to excessive amounts of proteins [30]. The presented ratio of SAA induced by, respectively, IL-6 and IL-1 was 1:0·8. In a previous in vivo study of B6D2F1 mice, this ratio was 1:600 [20]. Even if the presented total SAA levels induced by IL-1 and IL-6 were not strictly comparable, it is unlikely that the difference between these figures is of the same magnitude as in the latter report.
For the groups that received LPS and TNF, the ratio between total SAA levels was 1:1·4, respectively. For the groups that received IL-6 and IL-6/IL-1, this ratio was 1:4·5. The latter increase is in accordance with a previously described synergism between lL-1 and IL-6 in Hep3B cells [15–17]. In an in vivo study on CE/J mice, and in three in vitro studies on the human hepatoma cell line Hep3B, the corresponding differences in the amounts of induced SAA were, respectively, 1:0·1 (LPS:TNF) [21] and 1:3 [17], 1:10 [16] and 1:20 [15] (IL-6:IL-6 + IL-1).
The effects of IL-6 or LPS on SAA production were not significantly altered by the addition of DEX. This is in contrast to previous studies, where either a much larger increase (66%), but also a considerable decrease (60%), was demonstrated in human hepatocytes [14] and in serum from CD-1 mice [18].
The above results confirm that the total SAA response is related to characteristics of the primary stimuli. In the present study, however, generally smaller variations were observed than have been demonstrated before. This divergence might be explained by different properties and dosages of the primary stimuli, or kinetics and genetic factors in the host, the latter implying different cell lines and mouse strains.
Largely equal proportions of SAA1 and SAA2 were induced by all inflammatory stimuli that were administered, including IL-6. This is in contrast to a previous report on SAA isotypes in the human hepatoma cell line HuH7, in which IL-6 was capable of inducing only SAA1 [31].
The relative contribution of SAA pI 5·9 showed a larger variation than SAA1 or SAA2, and in six out of nine groups this isotype was present in the smallest amounts of all isotypes. The latter is in accordance with a previous study on BALB/c mice, where the protein ratio of SAA1:SAA2:two minor acidic SAAs was 1:1:0·1 [7].
The diverging isotype ratios of SAA pI 5·9 might reflect that this protein reaches its maximum concentration at different times from SAA1 and SAA2. Disregarding kinetic aspects, the results also suggest that the proportions of SAA1 and SAA2 that are produced are decided by a common regulatory mechanism, which can be initiated by a variety of exogenously administered inflammatory stimuli.
The above mentioned results illustrate that the presented type of animal experiments are needed, in order to obtain balanced information about the ultimate effects of substances which are used to mimic the acute-phase response. In future, application of the presented data and methodology might yield further insight into the complexity of the acute-phase response and the mechanisms involved in amyloid formation.
Acknowledgments
Equipment needed for the image evaluation was provided by the Swiss 2-D PAGE team, Clinical Chemistry Department, University Hospital Geneva, Switzerland.
References
- 1.Whitehead AS, de Beer MC, Steel DM, Rits M, Lelias JM, Lane WS, de Beer FC. Identification of novel members of the serum amyloid A protein superfamily as constitutive apolipoproteins of high density lipoprotein. J Biol Chem. 1991;267:3862–7. [PubMed] [Google Scholar]
- 2.Lowell CA, Potters DA, Stearman RS, Morrow JF. Structure of the murine serum amyloid A gene family. Gene conversion. J Biol Chem. 1986;261:8442–52. [PubMed] [Google Scholar]
- 3.de Beer MC, Kindy MS, Lanes WS, de Beer FC. Mouse serum amyloid A protein (SAA5) structure and expression. J Biol Chem. 1994;269:4661–7. [PubMed] [Google Scholar]
- 4.Sipe JD. Serum amyloid A protein classification: a preliminary report of a subcommittee of the International Society of Amyloidosis. Amyloid: Int J Exp Clin Invest. 1995;2:69–70. [Google Scholar]
- 5.Hoffmann JS, Ericsson LH, Eriksen E, Walsh KA, Benditt EPJ. Murine tissue amyloid protein AA. Exp Med. 1984;159:641–6. doi: 10.1084/jem.159.2.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meek RL, Eriksen N, Benditt EP. Murine serum amyloid A-3 is a high density apolipoprotein and is secreted by macrophages. Proc Natl Acad Sci USA. 1992;89:7949–52. doi: 10.1073/pnas.89.17.7949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Beer MC, Beach CM, Shedlofsky SI, de Beer FC. Identification of a novel serum amyloid A protein in Balb/c mice. Biochem J. 1991;280:45–49. doi: 10.1042/bj2800045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Beer MC, de Beer FC, McCubbin WD, Kay CM, Kindy MS. Structural prerequisites for serum amyloid A fibril formation. J Biol Chem. 1993;268:20606–12. [PubMed] [Google Scholar]
- 9.de Beer MC, de Beer FC, Beach CM, Carreras I, Sipe JD. Complete amino acid sequence and mRNA analysis of a new isoform. Biochem J. 1992;283:673–8. doi: 10.1042/bj2830673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Husebekk A, Skogen B, Husby G, Marhaug G. Transformation of amyloid precursor SAA to protein AA and incorporation in amyloid fibrils in vivo. Scand J Immunol. 1984;21:283–7. doi: 10.1111/j.1365-3083.1985.tb01431.x. [DOI] [PubMed] [Google Scholar]
- 11.Bell AW, Chan SL, Marcantonio D, Ali-Khan Z. Both murine SAA1 and SAA2 yield AA amyloid in alveolar hydatid cyst-infected mice. Scand J Immunol. 1996;43:173–80. doi: 10.1046/j.1365-3083.1996.d01-26.x. [DOI] [PubMed] [Google Scholar]
- 12.Duff GW. Cytokines and acute phase proteins in rheumatoid arthritis. Scand J Rheumatol. 1994;23(Suppl. 100):9–19. doi: 10.3109/03009749409095197. [DOI] [PubMed] [Google Scholar]
- 13.Ramadori G, Van Damme J, Rieder H, Meyer zum Buschenfelde KH. Interleukin 6, the third mediator of acute-phase reaction, modulates hepatic protein synthesis in human and mouse. Comparison with interleukin 1β and tumour necrosis factor-α. Eur J Immunol. 1988;18:1259–64. doi: 10.1002/eji.1830180817. [DOI] [PubMed] [Google Scholar]
- 14.Castell JV, Gomez-Lechon MJ, David M, Hirano T, Kishimoto T, Heinrich PC. Recombinant human interleukin-6 (IL-6/BSF-2/HSF) regulates the synthesis of acute phase proteins in human hepatocytes. FEB. 1988;232:347–50. doi: 10.1016/0014-5793(88)80766-x. [DOI] [PubMed] [Google Scholar]
- 15.Ganapathi MK, Rzewnicki D, Samols D, Jiang S-H, Kushner I. Effect of combinations of cytokines and hormones on synthesis of serum amyloid A and C-reactive protein in Hep 3B cells. J Immunol. 1991;147:1261–5. [PubMed] [Google Scholar]
- 16.Rokita H, Loose L, Bartle LM, Sipe JD. Synergism of interleukin 1 and interleukin 6 induces serum amyloid A production while depressing fibrinogen: a quantitative analysis. J Rheumatol. 1994;21:400–5. [PubMed] [Google Scholar]
- 17.Conti P, Bartle L, Barbacane RC, Reale M, Placido FC, Sipe JD. Synergistic activation of serum amyloid A (SAA) by IL-6 and IL-1 in combination on human HEP3B hepatoma cell lin%, role of PGE2 and IL–1 receptor antagonist. Immunol Invest. 1995;24:523–35. doi: 10.3109/08820139509066848. [DOI] [PubMed] [Google Scholar]
- 18.Ghezzi P, Sipe JD. Dexamethasone modulation of LPS, IL-1, and TNF stimulated serum amyloid A synthesis in mice. Lymph Res. 1987;2:157–64. [PubMed] [Google Scholar]
- 19.Sipe JD, Vogel SN, Douches S, Neta R. Tumour necrosis factor/Cachectin is a less potent inducer of serum amyloid A protein than interleukin 1. Lymph Res. 1987;6:93–101. [PubMed] [Google Scholar]
- 20.Neta R, Vogel S, Sipe JD, Wong GG, Nordan RP. Comparison of in vivo effects of human recombinant IL-1 and human recombinant IL-6 in mice. Lymph Res. 1988;7:403–12. [PubMed] [Google Scholar]
- 21.Sipe JD, Carreras I, Gonnerman WA, Cathcart ES, de Beer MC, de Beer FC. Characterization of the inbred CE/J mouse strain as amyloid resistant. Am J Pathol. 1993;143:1480–5. [PMC free article] [PubMed] [Google Scholar]
- 22.Foyn Bruun C, Sletten K, Husby G, Marhaug G. Characterization and quantification of mink serum amyloid A protein (SAA) using two-dimensional electrophoresis with immobilized pH gradient. Electrophoresis. 1993;14:1372–4. doi: 10.1002/elps.11501401211. [DOI] [PubMed] [Google Scholar]
- 23.Wilkins MR, Sanchez JC, Williams KL, Hochstrasser DF. Current challenges and future applications for protein maps and post-translational vector maps in proteome projects. Electrophoresis. 1996;17:830–8. doi: 10.1002/elps.1150170504. [DOI] [PubMed] [Google Scholar]
- 24.Foyn Bruun C, Nordstoga K, Sletten K, Husby G, Marhaug G. Serum amyloid A protein in humans and four animal species: a comparison by two-dimensional electrophoresis. Comp Biochem Physiol. 1995;112B:227–34. doi: 10.1016/0305-0491(95)00074-7. [DOI] [PubMed] [Google Scholar]
- 25.Foyn Bruun C, Mehlum A, Marhaug G, Sletten K. Isolation of serum amyloid A protein by small scale hydrophobic interaction chromatography and two-dimensional electrophoresis. J Chromatogr. 1996;685:360–3. doi: 10.1016/s0378-4347(96)00194-6. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez JC, Appel RD, Golaz O, Pasquali C, Ravier F, Bairoch A, Hochstrasser DF. Inside SWISS-2DPAGE data base. Electrophoresis. 1995;16:1131–51. doi: 10.1002/elps.11501601190. [DOI] [PubMed] [Google Scholar]
- 27.Ducret A, Foyn Bruun C, Bures EJ, Marhaug G, Husby G, Aebersold R. Characterization of human serum amyloid A protein isoforms separated by liquid chromatography/electrospray ionization tandem mass spectrometry. Electrophoresis. 1996;17:866–76. doi: 10.1002/elps.1150170508. [DOI] [PubMed] [Google Scholar]
- 28.Whitehead AS, de Beer MC, Steel DM, Rits M, Lelias JM, Lane WS, de Beer FC. Identification of novel members of the serum amyloid A protein superfamily as constitutive apolipoproteins of high density lipoprotein. J Biol Chem. 1992;267:3862–7. [PubMed] [Google Scholar]
- 29.Yakar S, Kaplan B, Livneh A, Martin B, Miura K, Ali-Khan Z, Shtrasburg Pras M. Direct evidence for SAA deposition in tissues during murine amyloidogenesis. Scand J Immunol. 1994;40:653–8. doi: 10.1111/j.1365-3083.1994.tb03519.x. [DOI] [PubMed] [Google Scholar]
- 30.Rabilloud T. A comparison between low background silver diamine and silver nitrate protein stains. Electrophoresis. 1992;13:429–39. doi: 10.1002/elps.1150130190. [DOI] [PubMed] [Google Scholar]
- 31.Raynes JG, Eagling S, McAdam Kpwj. Acute-phase protein synthesis in human hepatoma cells: differential regulation of serum amyloid A (SAA) and haptoglobin by interleukin-1 and interleukin-6. Clin Exp Immunol. 1991;83:448–91. doi: 10.1111/j.1365-2249.1991.tb05666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whitney JB, Cobb RR, Popp RA, O'Rourke TW. Detection of neutral amino acid substitutions in proteins. Proc Natl Acad Sci USA. 1985;82:7646–50. doi: 10.1073/pnas.82.22.7646. [DOI] [PMC free article] [PubMed] [Google Scholar]