Abstract
AD is associated with a bias of the T helper cells to show increased IL-4 and reduced interferon-gamma (IFN-γ) production. The production of IFN-γ and IL-4 and the development of Th cells into either high IFN-γ or high IL-4 producers is strongly influenced by factors produced by antigen-presenting cells (APC), like IL-12 and prostaglandin E2 (PGE2). IL-12 selectively enhances IFN-γ production and favours the development of IFN-γ-producing Th cells, whereas PGE2 selectively inhibits IFN-γ production by Th cells. The aim of this study was to test whether the increased IL-4/IFN-γ production ratio by Th cells in AD can be explained by an increased PGE2/IL-12 production ratio by the APC. Monocytes were used as APC source. PGE2 and IL-12 production by lipopolysaccharide (LPS)-stimulated monocytes from 12 AD patients and 12 non-atopic controls was determined using two complementary experimental systems, whole blood cultures and purified monocytes. In addition, we determined IL-6 production as a measure of monocyte activation, and IL-10 production because IL-12 production by monocytes is highly influenced by endogenously produced IL-10. The monocytes from AD patients showed normal production levels of IL-6 and IL-10, a two-fold, but non-significant decrease in IL-12 production, and a significantly (three-fold) higher PGE2 production than those from non-atopic controls. Here we show for the first time that enhanced PGE2 production by monocytes in AD is not accompanied by a general rise in cytokine production. We conclude that AD is indeed associated with an increased PGE2/IL-12 production ratio by monocytes.
Keywords: atopic dermatitis, PGE2, IL-12, monocytes
INTRODUCTION
Various studies indicate that AD is associated with the occurrence of Th cells showing a high IL-4 (and IL-5) to interferon-gamma (IFN-γ) production ratio. IL-4 production is enhanced and IFN-γ production decreased in mitogen-stimulated peripheral blood T cells from AD patients [1–4] and IL-4-producing allergen-specific Th cells (Th2) can be cloned from the skin and blood of these patients [5,6]. Reduced IFN-γ production by mononuclear cells of neonates even precedes the development of atopy [7], indicating that the reduced IFN-γ-producing capacity of the T cells is a cause and not a consequence of the development of atopy. The IL-4 to IFN-γ production ratio and the development of naive Th cells into either high IFN-γ or high IL-4-producing memory Th cells is highly influenced by factors produced by antigen-presenting cells (APC), such as monocytes and dendritic cells. Important factors in this respect are IL-12 and prostaglandin E2(PGE2). IL-12 selectively enhances IFN-γ production and favours the development of IFN-γ-producing Th cells [8,9]. PGE2 selectively inhibits IFN-γ production [10,11] and is reported to favour the development of IL-4- and IL-5-producing T cells [12]. Previously we provided evidence that the production ratio between IL-12 and PGE2 by APC during T cell activation is highly predictive for the level of IFN-γ production by Th cells [13].
The aim of this study was to test whether the increased IL-4/IFN-γ ratio by T cells in AD is indeed associated with an increased PGE2/IL-12 production ratio by APC. Monocytes were used as a source of APC. Results of others using peripheral blood mononuclear cell (PBMC) cultures indicated that PGE2 production by monocytes of AD patients is increased compared with that of non-atopic individuals [4,14]. From those studies it is, however, still unclear whether the enhanced PGE2 production by monocytes from atopic persons is selective, and not the result of a higher activation state.
In this study, PGE2 and IL-12 production by unstimulated and lipopolysaccharide (LPS)-stimulated monocytes from AD patients and non-atopic controls were determined using two complementary experimental systems, whole blood cultures and isolated monocytes. At first, the production of PGE2 and IL-12 was determined in purified monocyte cultures. However, with these cultures a comparison of IL-12 production between patients and controls proved impossible, because IL-12 production was below detection level for most donors. Since the monocyte isolation procedure is known to selectively hamper the ability of monocytes to produce IL-12 [15,16], we changed to whole blood cultures for the determination of IL-12 production. The production of IL-6 and IL-10 was determined in both systems. IL-6 production was used as a measure of monocyte activation. IL-10 production was determined, because IL-12 production by monocytes is highly influenced by endogenously produced IL-10 [17]. The use of whole blood cultures for the determination of monocyte IL-12, IL-6 and IL-10 production was justified by the argument that monocytes are the major producers of these three cytokines in LPS-stimulated whole blood cultures [15,17–19]. PGE2 production was only determined in purified monocyte cultures, because granulocytes can produce large amounts of PGE2 [20,21] and because plasma interferes with the PGE2 assay, complicating an accurate determination of PGE2 production in whole blood cultures. Using the two systems, we found a significantly enhanced production of PGE2, which was not accompanied by a rise in IL-12, IL-10 or IL-6 production by monocytes from AD patients compared with controls.
MATERIALS AND METHODS
Selection of patients and control donors
The control group consisted of non-atopic, healthy persons without any symptoms or history of atopy. Their serum IgE titre was < 50 U/ml and their serum showed no positive RAST for either food or inhaled antigens.
The patients suffered from exacerbations on a background of chronic moderate to severe AD according to the criteria of Hanifin & Rajka [22] and had a marked elevation of serum IgE (> 1000 U/ml). None of the patients was receiving oral steroids in the 6 weeks preceding blood collection.
The donors in both groups were aged between 18 and 50 years. Only persons without overt clinical symptoms of bacterial, viral or parasitic infections were included. All persons gave their informed consent to participate in the study.
Antibodies and reagents
LPS from Escherichia coli 0111:B4 Westphal was obtained from Difco (Detroit, MI). Endotoxin-low fetal calf serum (FCS) was a kind gift from Bodinco BV (Alkmaar, The Netherlands).
rhIFN-γ (specific activity 107 U/mg) was a kind gift of Dr P. v.d. Meide (TNO, Rijswijk, The Netherlands), and rhIL-10 (sp. act. 5 × 105 U/mg) was obtained from Pharmingen (San Diego, CA). The IL-12 p70-specific MoAb 20C2 and human rIL-12 (sp. act. 17 × 107 U/mg) were kindly provided by Dr M. K. Gately (Hoffmann-LaRoche, Nutley, NJ). The hybridomas producing the IL-12 p40-specific MoAbs C8.6 and C11.79 were kindly provided by Dr G. Trinchieri (The Wistar Institute, Philadelphia, PA).
Whole blood cultures
Whole blood was obtained from the selected donors by venepuncture in sodium heparin-containing blood collecting tubes (VT100H tubes; Venoject; Terumo Europe, Leuven, Belgium). The blood was diluted five-fold in endotoxin-free Iscove's modified Dulbecco's medium (IMDM; BioWhittaker, Walkersville, MD) supplemented with 0.1% FCS and 30 U/ml of sodium heparin (Leo Pharmaceutical Products B.V., Weesp, The Netherlands). Diluted whole blood was cultured in triplicate in 96-well flat-bottomed tissue culture plates (Costar, Cambridge, MA). The period between blood collection and start of the culture was between 1 and 2 h, because prolonged standing of the blood selectively hampers subsequent IL-12 production. Supernatants were harvested after 20 h of culture and stored at −20°C until determination of cytokine concentrations.
Monocyte cultures
Monocytes were purified from whole blood in two density centrifugation steps as described previously [17]. In short, mononuclear cells were isolated using Ficoll–Hypaque and monocytes using Percoll. After the isolation procedure monocyte purity was 85–90% as assessed by CD14 staining and FACS analysis. Monocytes were allowed to adhere to the culture plate for 1 h, subsequently washed and, after overnight culture in endotoxin-free IMDM with 10% FCS, the medium was replaced by fresh medium containing the indicated stimulatory agents.
Cytokine and PGE2 determinations
The IL-12 p70 ELISA, based on the MoAbs 20C2 and biotinylated C8.6, and the IL-12 p40 ELISA, based on the MoAbs C11.79 and biotinylated C8.6, were performed as described [15,17]. The p70 ELISA used in this study is highly specific for IL-12 p70, as indicated by our unpublished finding that 30 ng/ml p40 was still not detectable in this assay. Furthermore, a good correlation was found between IL-12 concentration measured in a MoAb-capture bioassay and in this p70 ELISA [23,17], indicating that the p70 ELISA detects biologically active IL-12. The IL-10 ELISA was performed as described [24], using the IL-10-specific MoAb JES3-9D7, the biotinylated IL-10-specific MoAb JES-12G8 and the IL-10 standard from Pharmingen. The IL-6 ELISA was performed using an IL-6 compact kit (Central Laboratory of the Red Cross Blood Transfusion Service, CLB, Amsterdam, The Netherlands). Interference of plasma and heparin in the cytokine ELISAs was prevented by the use of a high-performance ELISA buffer developed at the CLB. Detection levels of the assays were 2 pg/ml for IL-12, 20 pg/ml for p40, 10 pg/ml for IL-10 and 10 pg/ml for IL-6.
PGE2 concentrations were determined using the Enzyme Inhibition Assay from Boehringer (Mannheim, Germany), which has a detection limit of 0.2 nm.
Statistical analysis
All data comparisons were performed by calculating the two-sided P values using the Mann–Whitney U-test. Two-sided P values < 0.05 were considered significant.
RESULTS
IL-12, IL-10 and IL-6 production in whole blood cultures
Whole blood samples obtained from 10 AD patients (five men, five women) and 12 non-atopic individuals (six men, six women) were diluted five-fold and stimulated with increasing concentrations of LPS. Production of IL-12 (p70 and p40), IL-10 and IL-6 was determined in 20-h culture supernatants. Unstimulated cultures showed no or very low cytokine production. Stimulation with LPS induced IL-6, IL-10 and IL-12 production, which increased with increasing LPS concentration. LPS-induced production of IL-12 p70, the bioactive dimer, tended to be decreased in cultures from AD patients compared with controls (Fig. 1a), but due to the large inter-donor variation the difference was not significant (P > 0.159 at all LPS concentrations). Production of p40, the non-bioactive subunit of IL-12 that is produced in excess, also tended to be decreased (Fig. 1b) non-significantly (P > 0.180 at all LPS concentrations) in the patients. IL-10 and IL-6 production (Fig. 1) was similar (P > 0.346 and > 0.417, respectively), indicating that the activation level of the monocytes in both groups was similar. Statistical calculations showed that, assuming no change in the observed difference and s.d., at least 50 individuals in each group would be necessary to determine whether the reduction in IL-12 production was significant.
Fig. 1.
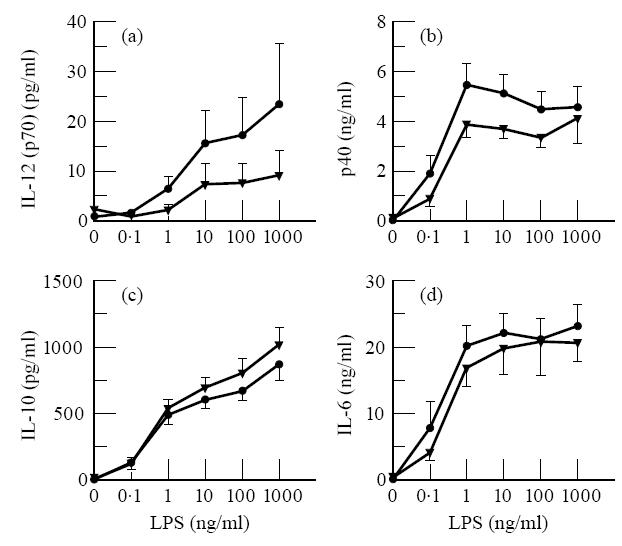
No enhancement in IL-12, IL-10 or IL-6 production in whole blood cultures from AD patients compared with those from non-atopic persons. Five-fold diluted whole blood was stimulated with the indicated concentrations of lipopolysaccharide (LPS). After 20 h culture supernatants were collected. Results are the mean cytokine concentrations ± s.e.m. in the culture supernatants from 10 different AD (five men, five women) and 12 different non-atopic control donors (six men, six women).
Sensitivity of monocytes to IFN-γ
IFN-γ is known to enhance IL-12 production strongly, to inhibit IL-10 production, and to affect IL-6 production only slightly [17,25]. To determine whether there is a difference in sensitivity for IFN-γ between AD patients and non-atopic persons, we tested the effect of IFN-γ on the production of IL-12 (p70 and p40), IL-10 and IL-6 in LPS-stimulated whole blood cultures from both groups. The results show (Fig. 2) that IL-12 p70 and p40 production by monocytes from AD patients and that from non-atopic persons, as observed in whole blood cultures stimulated with 10 ng/ml LPS and IFN-γ, was similar. The increase in IL-12 p70 production (expressed as mean increase ± s.e.m., calculated from the individual increase factors) was 49-fold ± 11 for patients and 39-fold ± 14 for controls, which was not significantly different (P > 0.314). The effect of IFN-γ on LPS-induced IL-10 and IL-6 production in cultures from AD patients was also similar to that in cultures from control donors (Fig. 2), indicating that monocytes from AD patients have a normal sensitivity to IFN-γ.
Fig. 2.
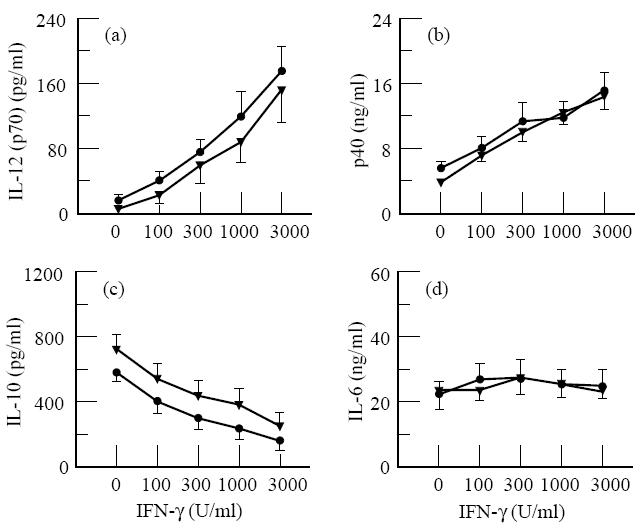
IFN-γ affects lipopolysaccharide (LPS)-induced IL-12 p70 and p40 production in whole blood cultures from AD patients and non-atopic persons similarly. Five-fold diluted whole blood was stimulated with 10 ng/ml LPS and the indicated concentration of IFN-γ. After 20 h culture supernatants were collected. Results are the mean cytokine concentration ± s.e.m. in the culture supernatants from 10 different AD (five men, five women) and 12 different non-atopic control donors (six men, six women).
PGE2 and cytokine production by monocytes
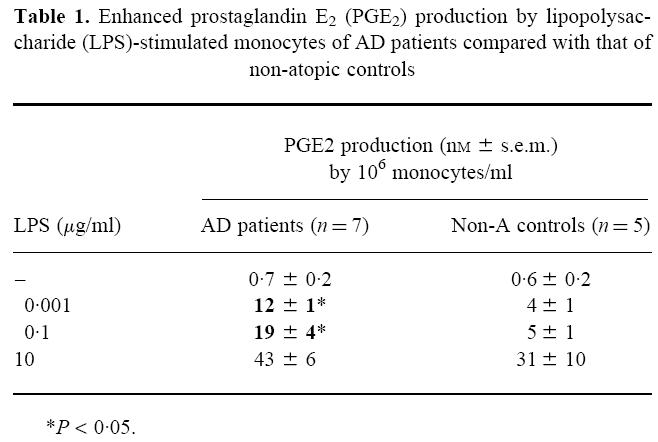
In initial experiments, purified monocytes where stimulated with varying doses of LPS, and PGE2 production was determined in 24 h culture supernatants. Unstimulated monocytes produced low levels of PGE2 (< 1 nm), and upon LPS stimulation the production increased strongly (Table 1). Production of PGE2 by monocytes of AD patients (n = 7, three men, four women) proved to be enhanced compared with that in the control group (n = 5, three men, two women). However, whether the difference was significant depended on the LPS concentration (Table 1). Stimulation of the monocytes with 1–100 ng/ml LPS resulted in a three to four-fold higher PGE2 production in the AD group (P < 0.05), but in unstimulated cultures or cultures stimulated with an abnormally high LPS concentration (10 μg/ml) PGE2 production was not significantly different from the control group. The enhanced PGE2 production at the intermediate, more physiological LPS concentrations could not be explained by a higher expression of CD14, the receptor for LPS, on the monocytes from AD patients, since expression levels were similar (mean fluorescence ± s.e.m.: 789 ± 79 for patients versus 687 ± 135 for controls).
Table 1.
Enhanced prostaglandin E2(PGE2) production by lipopolysaccharide (LPS)-stimulated monocytes of AD patients compared with that of non-atopic controls
In subsequent experiments we determined whether the enhanced PGE2 production was accompanied by an increased production of IL-10 and IL-6. To this aim, monocytes from 12 AD patients (six men, six women) and 10 non-atopic donors (five men, five women) were isolated and either stimulated with 100 ng/ml LPS or cultured without a stimulatory agent. With these larger donor groups, we again observed a three-fold enhancement of PGE2 production by LPS-stimulated monocytes from AD patients. Unstimulated monocytes from atopic and non-atopic donors produced low amounts of PGE2, IL-10 and IL-6, similar to that by monocytes from the control group. In contrast to the enhanced PGE2 production, LPS-stimulated monocytes from AD patients produced normal levels of IL-10 and IL-6 (Fig. 3), suggesting that the activation state of monocytes from both groups was the same.
Fig. 3.
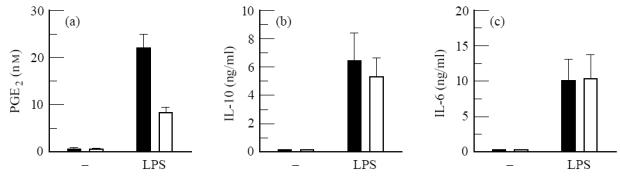
Selectively enhanced prostaglandin E2(PGE2) production by lipopolysaccharide (LPS)-stimulated purified monocytes from AD patients compared with non-atopic control persons. Monocytes (106/ml) were either cultured in medium alone (−) or stimulated with 100 ng/ml LPS. After 24 h culture supernatants were collected. Results are the mean concentration ± s.e.m. in the culture supernatants from 12 different AD (six men, six women; ▪) and 10 non-atopic control donors (five men, five women; □).
DISCUSSION
In this study we show that PGE2 production by LPS-stimulated monocytes of AD patients was enhanced, whereas concomitant production of IL-6 and IL-10 was normal, indicating that the activation state of the monocytes was normal. IL-12 production was either normal or decreased in AD, but larger groups of patients and controls are needed to resolve this issue. Nonetheless, our results clearly show that, in agreement with an increased IL-4/IFN-γ production ratio by the Th cells, the PGE2/IL-12 production ratio by activated monocytes was enhanced in AD.
The question whether atopic disease is associated with a decrease in IL-12 production is still controversial. In a collaborative study [26] we found that IL-12 production in whole blood cultures stimulated with fixed Staphylococcus aureus strain Cowan was reduced for allergic asthma patients compared with controls, while in parallel cultures stimulated with fixed E. coli no significant difference between both groups was observed. Using a different system, superantigen TSST-1-stimulated PBMC, Lester et al. [27] found unaltered IL-12 production by AD patients compared with that from non-atopic controls. These findings indicate that the mode of stimulation is critical for the detection of significant differences in IL-12 production.
Our finding that IL-10 production by LPS-stimulated and unstimulated monocytes from AD patients is normal contrasts with the results of Ohmen et al. [28], who reported enhanced IL-10 production in unstimulated monocytes from AD patients. The contrasting results may be explained by differences in patient selection and in experimental procedures.
Our finding that monocytes from AD patients produce significantly more PGE2 is in agreement with previous studies reporting enhanced PGE2 production by leucocyte cultures [29] and by monocytes in PBMC cultures [4,14]. However, from those studies it was unclear whether the enhanced PGE2 production by monocytes from atopic persons was selective and not the result of a higher activation state. The selective enhancement in PGE2 production without a concomitant rise in IL-10 production, reported here, strongly suggests that PGE2 is largely responsible for the decrease in IFN-γ production by stimulated T cells in PBMC from AD patients observed by many others [1–4,27]. Furthermore, PGE2 may exert its effect on IFN-γ production by T cells not only directly, but also indirectly, by inhibiting IL-12 production. However, in spite of the fact that PGE2 and other cAMP-up-regulating agents are known to inhibit IL-12 production by monocytes [15], the effect of enhanced PGE2 production on IL-12 production is expected to be small, since complete inhibition of PGE2 production by indomethacin resulted only in a 1.1–1.5-fold enhancement of IL-12 production (T.vdP.K. and A.S., unpublished results). In these in vitro experiments endogenously produced PGE2 is apparently too late in reaching sufficient concentrations to inhibit IL-12 production substantially. In vivo, however, enhanced PGE2 levels in the microenvironment of the IL-12-producing cells may still suppress IL-12 production.
The difference we found in PGE2 production by monocytes from AD patients and those of non-atopic controls was dependent on the stimulatory LPS concentration. The difference was observed only in the range from 1 to 100 ng/ml LPS, but not at higher concentrations. Therefore, the report that PGE2 production by LPS-stimulated monocytes from AD patients is normal, whereas IL-1 production is decreased, may be explained by the high LPS concentration (1 μg/ml) used in those experiments [30].
Our finding that only LPS-stimulated monocytes show a significantly enhanced PGE2 production in AD suggests an increased activity of cyclooxygenase 2 (COX-2), a key enzyme in LPS-induced PGE2 production [31,32]. Chan et al. [4] reported an enhanced PGE2 production by resting PBMC, suggesting involvement of COX-1, which is constitutively expressed. However, the results of Jakob et al. [14], who found enhanced PGE2 production by concanavalin A (Con A)-stimulated PBMC from AD patients and not by resting PBMC, also suggest involvement of COX-2. Furthermore, corticosteroids, which are the most potent drugs available for the treatment of AD, selectively inhibit COX-2 without affecting COX-1 activity [33]. The report that monocytes from newborns have normal COX-1 and COX-2 protein levels, but produce less PGE2 than those of adults due to altered fatty acid content and reduced COX activity [34], suggests a special protective mechanism against overproduction of PGE2 in early infancy and raises the question whether monocytes from atopic newborns are different in this respect.
In this study, we determined the PGE2/IL-12 balance during the active phase of the disease. Although it is still unclear whether the disturbed PGE2/IL-12 balance was present during the development of allergy and contributed to that development, it is, however, likely to contribute to the chronic character of the disease.
Acknowledgments
A.S. was supported by a grant from the Netherlands Asthma Foundation. The authors wish to thank Dr S. O. Stapel for providing the IgE and RAST data for the non-atopic control persons, and M. van de Brug for technical assistance.
References
- 1.Reinhold U, Wehrmann W, Kukel S, Kreysel HW. Evidence that defective interferon-gamma production in atopic dermatitis patients is due to intrinsic abnormalities. Clin Exp Immunol. 1990;79:374–9. doi: 10.1111/j.1365-2249.1990.tb08098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rousset F, Robert J, Andary M, et al. Shifts in interleukin-4 and interferon-gamma production by T cells of patients with elevated serum IgE levels and the modulatory effects of these lymphokines on spontaneous IgE synthesis. J Allergy Clin Immunol. 1991;87:58–69. doi: 10.1016/0091-6749(91)90213-8. [DOI] [PubMed] [Google Scholar]
- 3.Jujo K, Renz H, Abe J, Gelfand EW, Leung DYM. Decreased interferon gamma and increased interleukin-4 production in atopic dermatitis promotes IgE synthesis. J Allergy Clin Immunol. 1992;90:323–31. doi: 10.1016/s0091-6749(05)80010-7. [DOI] [PubMed] [Google Scholar]
- 4.Chan SC, Kim J-W, Henderson WR, Jr, Hanifin JM. Altered prostaglandin E2 regulation of cytokine production in atopic dermatitis. J Immunol. 1993;151:3345–52. [PubMed] [Google Scholar]
- 5.Van der Heijden FL, Wierenga EA, Bos JD, Kapsenberg ML. High frequency of IL-4-producing CD4+ allergen-specific T lymphocytes in atopic dermatitis lesional skin. J Invest Dermatol. 1991;97:389–94. doi: 10.1111/1523-1747.ep12480966. [DOI] [PubMed] [Google Scholar]
- 6.Wierenga EA, Snoek M, Jansen HM, Bos JD, van Lier RA, Kapsenberg ML. Human atopen-specific types 1 and 2 T helper cell clones. J Immunol. 1991;147:2942–9. [PubMed] [Google Scholar]
- 7.Tang MLK, Kemp AS, Thorburn J, Hill DJ. Reduced interferon-gammma secretion in neonates and subsequent atopy. Lancet. 1994;344:983–5. doi: 10.1016/s0140-6736(94)91641-1. [DOI] [PubMed] [Google Scholar]
- 8.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–76. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 9.Hsieh C-S, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–9. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 10.Snijdewint FGM, Kalinski P, Wierenga EA, Bos JD, Kapsenberg ML. Prostaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytes. J Immunol. 1993;150:5321–9. [PubMed] [Google Scholar]
- 11.Hilkens CMU, Vermeulen H, Van Neerven RJJ, Snijdewint FGM, Wierenga EA, Kapsenberg ML. Differential modulation of T helper type 1 (Th1) and T helper type 2 (Th2) cytokine secretion by prostaglandin E2 critically depends on interleukin-2. Eur J Immunol. 1995;25:59–63. doi: 10.1002/eji.1830250112. [DOI] [PubMed] [Google Scholar]
- 12.Katamura K, Shintaku N, Yamauchi Y, et al. Prostaglandin E2 at priming of naive CD4+ T cells inhibits acquisition of ability to produce IFN-gamma and IL-2, but not IL-4 and IL-5. J Immunol. 1995;155:4604–12. [PubMed] [Google Scholar]
- 13.Hilkens CMU, Snijders A, Vermeulen H, Van der Meide PH, Wierenga EA, Kapsenberg ML. Accessory cell-derived IL-12 and prostaglandin E2 determine the IFN-gamma level of activated human CD4+ T cells. J Immunol. 1996;156:1722–7. [PubMed] [Google Scholar]
- 14.Jakob T, Huspith N, Latchman E, Rycroft R, Brostoff J. Depressed lymphocyte transformation and the role of prostaglandins in atopic dermatitis. Clin Exp Immunol. 1990;79:380–4. doi: 10.1111/j.1365-2249.1990.tb08099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van der Pouw Kraan Tctm, Boeije LCM, Smeenk RJT, Wijdenes J, Aarden LA. Prostaglandin-E2 is a potent inhibitor of human interleukin 12 production. J Exp Med. 1995;181:775–9. doi: 10.1084/jem.181.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van der Pouw Kraan Tctm, Boeije LCM, Snijders A, Smeenk RJT, Wijdenes J, Aarden LA. Regulation of IL-12 production by human monocytes and the influence of prostaglandin E2. Ann NY Acad Sci. 1996;795:147–57. doi: 10.1111/j.1749-6632.1996.tb52663.x. [DOI] [PubMed] [Google Scholar]
- 17.Snijders A, Hilkens CMU, Van der Pouw Kraan Tctm, Engel M, Aarden LA, Kapsenberg ML. Regulation of bioactive IL-12 production in LPS-stimulated human monocytes is determined by the expression of the p35 subunit. J Immunol. 1996;156:1207–12. [PubMed] [Google Scholar]
- 18.Wang P, Wu P, Anthes JC, Siegel MI, Egan RW, Billah MM. Interleukin-10 inhibits interleukin-8 production in human neutrophils. Blood. 1994;83:2678–83. [PubMed] [Google Scholar]
- 19.Moore KW, O'Garra A, De Waal Malefyt R, Vieira P, Mosmann TR. Interleukin-10. Annu Rev Immunol. 1993;11:165–90. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 20.Saareks V, Riutta A, Mucha I, Alanko J, Vapaatalo H. Nicotine and cotinine modulate eicosanoid production in human leukocytes and platelet rich plasma. Eur J Pharmacol. 1993;248:345–9. doi: 10.1016/0926-6917(93)90012-f. [DOI] [PubMed] [Google Scholar]
- 21.Walker TS, Hoover CS. Rickettsial effects on leukotiene and prostaglandin secretion by mouse polymorphonuclear leukocytes. Infect Immun. 1991;59:351–6. doi: 10.1128/iai.59.1.351-356.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Dermatol Venereol. 1980;92:44–48. [Google Scholar]
- 23.Jansen PM, Van der Pouw Kraan Tctm, De Jong IW, et al. Release of interleukin-12 in experimental Escherichia coli septic shock in baboons: relation to plasma levels of interleukin-10 and interferon-gamma. Blood. 1996;87:5144–51. [PubMed] [Google Scholar]
- 24.Van der Poll T, Jansen J, Levi M, Ten Cate H, Ten Cate JW, Van Deventer SJH. Regulation of interleukin 10 release by tumor necrosis factor in humans and chimpanzees. J Exp Med. 1994;180:1985–8. doi: 10.1084/jem.180.5.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kubin M, Chow JM, Trinchieri G. Differential regulation of interleukin-12 (IL-12), tumor necrosis factor alpha, and IL-1 beta production in human myeloid leukemia cell lines and peripheral blood mononuclear cells. Blood. 1994;83:1847–55. [PubMed] [Google Scholar]
- 26.Van der Pouw Kraan Tctm, Boeije LCM, de Groot ER, et al. Reduced production of interleukin 12 (IL-12) and IL-12-dependant IFN-gamma release by allergic asthma patients. J Immunol. 1997;158:5560–5. [PubMed] [Google Scholar]
- 27.Lester MR, Hofer MF, Gately M, Trumble A, Leung DY. Down-regulating effects of IL-4 and IL-10 on the IFN-gamma response in atopic dermatitis. J Immunol. 1995;154:6174–81. [PubMed] [Google Scholar]
- 28.Ohmen JD, Hanifin JM, Nickoloff BJ, et al. Overexpression of IL-10 in atopic dermatitis: contrasting cytokine patterns with delayed-type hypersensitivity reactions. J Immunol. 1995;154:1956–63. [PubMed] [Google Scholar]
- 29.Ruzicka T, Ring J. Enhanced releasability of prostaglandin E2 and leukotrienes B4 and C4 from leukocytes of patients with atopic eczema. Acta Dermatol Venereol. 1987;67:469–75. [PubMed] [Google Scholar]
- 30.Jakob T, Neuber K, Ring J. Decreased monocyte interleukin-1β production in atopic eczema. Br J Dermatol. 1995;132:384–90. doi: 10.1111/j.1365-2133.1995.tb08671.x. [DOI] [PubMed] [Google Scholar]
- 31.Hla T, Neilson K. Human cyclooxygenase-2 cDNA. Proc Natl Acad Sci USA. 1992;89:7384–8. doi: 10.1073/pnas.89.16.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mertz PM, DeWitt DL, Stetler-Stevenson WG, Wahl LM. Interleukin 10 suppression of monocyte prostaglandin H synthase-2. Mechanism of inhibition of prostaglandin-dependent matrix metalloproteinase production. J Biol Chem. 1994;269:21322–9. [PubMed] [Google Scholar]
- 33.Lee SH, Soyoola E, Chanmugam P, et al. Selective expression of mitogen-inducible cyclooxygenase in macrophages stimulated with lipopolysaccharide. J Biol Chem. 1992;267:25934–8. [PubMed] [Google Scholar]
- 34.Murphy FJ, Reen DJ. Diminished production of prostaglandin E2 by monocytes of newborns is due to altered fatty acid membrane content and reduced cyclooxygenase activity. J Immunol. 1996;157:3116–21. [PubMed] [Google Scholar]


