Abstract
Increased proportions of circulating antigen-primed CD45RO+ TCR γδ cells have been found in untreated CoD patients. As certain immunological features are now found in both CoD and healthy persons carrying the HLA DQ2 heterodimer, we sought to establish whether healthy members of the families of CoD patients who are positive for HLA DQ2 and also have increased densities of TCR γδ intraepithelial lymphocytes (IEL) in their small bowel mucosa have elevated levels of circulating TCR γδ memory cells. Peripheral blood T cells were analysed by flow cytometry in 22 patients with CoD and 16 healthy family members. Untreated CoD patients had higher percentages of circulating CD45RO+ TCR γδ cells and CD45RO+ Vδ1+ cells than healthy family members. On the other hand, the amount of circulating Vδ1+ lymphocytes was lower in patients with CoD compared with healthy family members. In contrast, no differences were found between HLA DQ2+ and HLA DQ2− healthy family members in respect of circulating TCR γδ cell subsets. The change in circulating TCR γδ cell subsets found in patients with CoD is thus a consequence of an ongoing immunological process which diminishes on a gluten-free diet rather than a phenomenon directly caused by DQ2. These changes in peripheral blood are not found in healthy individuals who have the same HLA alleles DQA1*0501 and DQB1*0201 encoding the HLA DQ2 and who also have increased densities of TCR γδ IEL in their otherwise normal jejunal mucosa.
Keywords: coeliac disease, T lymphocytes, TCR γδ cells, HLA DQ2
INTRODUCTION
CoD is defined as a permanent intolerance of the small intestine mucosa to gluten, where clinical and histological improvement is seen after withdrawal of gluten from the diet. The pathogenesis of CoD is thought to involve a cell-mediated mucosal immune response in a genetically susceptible individual [1,2]. The HLA alleles DQA1*0501 and DQB1*0201 encoding the HLA DQ2 heterodimer confer genetic susceptibility to CoD; DQ2 is carried by ≈ 90% of patients [3,4].
CoD is heterogeneous in its clinical presentation and it is known that there is also a latent form of the disease, in which typical gluten-sensitive enteropathy develops later in an individual who has previously had normal jejunal histology while eating normal gluten-containing food [5–8]. Increased densities of the total intraepithelial lymphocyte (IEL) count [9,10] and particularly T cell receptor (TCR) γδ IEL have been reported in both untreated and treated CoD and also in latent CoD [11–15], and it is especially the increase in Vδ1-Jδ1 phenotype that accounts for this phenomenon [11,16]. In addition, in the coeliac mucosal lesion T cells in the lamina propria have been shown to be activated (CD45RO+, IL-2R+) [17,18], and the same applies also to genetically susceptible healthy individuals [19].
We have recently reported elevated levels of antigen-primed CD45RO+ lymphocytes in the peripheral blood of CoD patients, especially in the population of TCR γδ cells [20]. A similar finding was reported in patients with Sjögren's syndrome (SS): patients with HLA DQ2, but without CoD, had higher percentages of circulating CD45RO+ TCR γδ cells than those without DQ2 [21]. Since increased densities of TCR γδ+ IEL can be found in the infiltrative prestage of coeliac lesion at the time when the mucosal morphology is still normal, we hypothesized that the elevation of circulating CD45RO+ TCR γδ cells could also be one of the immunological hallmarks typical for CoD latency in individuals positive for DQ2. In the present study we sought to establish whether DQ2+ healthy members of the families of CoD patients also have elevated levels of circulating TCR γδ‘memory cells’, being a peripheral blood indicator of CoD latency.
PATIENTS AND METHODS
Subjects
We studied 12 CoD patients (age 16–53 years, mean 29.9 years) who had been on a gluten-free diet for 7–19 years. At the time of diagnosis all had villous atrophy with crypt hyperplasia compatible with CoD, and the jejunal mucosa had recovered on a gluten-free diet. At the time of sample taking all patients were negative for serum IgA class R1-type reticulin and endomysial antibodies. All these CoD patients had DQA1*0501 and DQB1*0201 alleles [22].
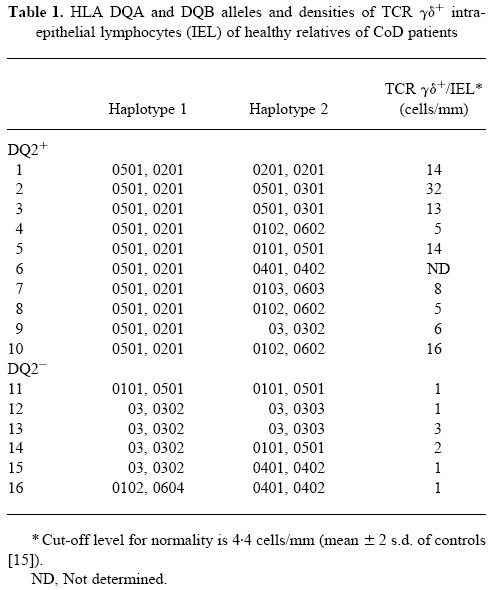
We also studied 16 healthy first-degree relatives of CoD patients. These healthy relatives had been biopsied after their family member had been found to have CoD. All these relatives had normal small bowel mucosa, although nine of them had increased densities of TCR γδ IEL [15]. After a further follow-up of 7 years all these family members were negative for serum IgA class R1-type reticulin and endomysial antibodies while consuming a normal gluten-containing diet. The family members were HLA-typed [22]. Two groups of family members were selected: group ‘DQ2+’ (10 relatives, 17–54 years, mean 41.3 years) had HLA DQA1*0501 and DQB1*0201 alleles and an increased density of TCR γδ+ IEL, while group ‘DQ2−’ (six relatives, 21–47 years, mean 31.8 years) had other DQ alleles and a low density of TCR γδ+ IEL (Table 1).
Table 1.
HLA DQA and DQB alleles and densities of TCR γδ+ intraepithelial lymphocytes (IEL) of healthy relatives of CoD patients
Ten adult patients with newly diagnosed untreated CoD were also studied (18–73 years, mean 40.2 years).
Samples
Ethylenediamine tetraacetic acid (EDTA) whole blood samples were taken from all the CoD patients and healthy first-degree relatives for lymphocyte phenotypic analysis and lymphocyte counts.
Lymphocyte counts
Lymphocyte counts were derived from an automated cell counter (Technicon H1, Tarrytown, NY).
Lymphocyte phenotypic analysis by flow cytometry
Peripheral blood lymphocyte subsets were analysed by two- or three-colour direct immunofluorescence flow cytometry with a FACScan (Becton Dickinson, Oxford, UK) using a lysed whole blood technique. All blood samples were analysed within 24 h of collection.
Monoclonal antibodies
The following commercial MoAbs were used: anti-Leu-4 (anti-CD3, peridin chlorophyll protein (PerCP)-conjugated), Pan-TCR αβ (anti-TCR αβ, FITC-conjugated), TCR δ1 (anti-TCR γδ, FITC-conjugated), δTCS1 (anti-Vδ1-Jδ1, FITC-conjugated), anti-Leu-45RO (anti-CD45RO, PE-conjugated). Pan-TCR αβ, TCRδ1 and δTCS1 were purchased from T Cell Diagnostics (Cambridge, MA) and anti-Leu-4 and anti-Leu-45RO from Becton Dickinson (San Jose, CA).
Labelling procedure and flow cytometry
The labelling procedure and protocol of flow cytometry were identical to that described earlier [20]. Briefly, 100 μl of EDTA blood were first incubated with a mixture of MoAbs, erythrocytes were lysed by addition of lysing solution and incubated a further 10 min. After centrifugation and washing in PBS, the cells were resuspended in sheath fluid containing 1% paraformaldehyde and kept at 4°C until analysis. In flow cytometry the lymphocytes were identified using Simultest LeucoGATE (CD45/CD14) from Becton Dickinson. The instrument set-up, fluorescence compensations and subtraction of unspecific labelling were as previously described [20]. Lymphocytes were counted and analysed with the LYSYS II software (Becton Dickinson Immunocytometry Systems, San Jose, CA). Absolute numbers of lymphocyte subsets were derived with the help of the results of an automated cell counter (Technicon H1) and expressed as cells/μl.
Statistical analysis
Non-parametric tests were used in the statistical analysis because part of the data was not normally distributed and the study groups were relatively small. The lymphocyte subsets were analysed using Kruskal–Wallis one-way analysis of variance, and if significant the groups were compared with Mann–Whitney U-test (two-tailed).
The study protocol was approved by the ethical committee of Tampere University Hospital.
RESULTS
Lymphocyte counts
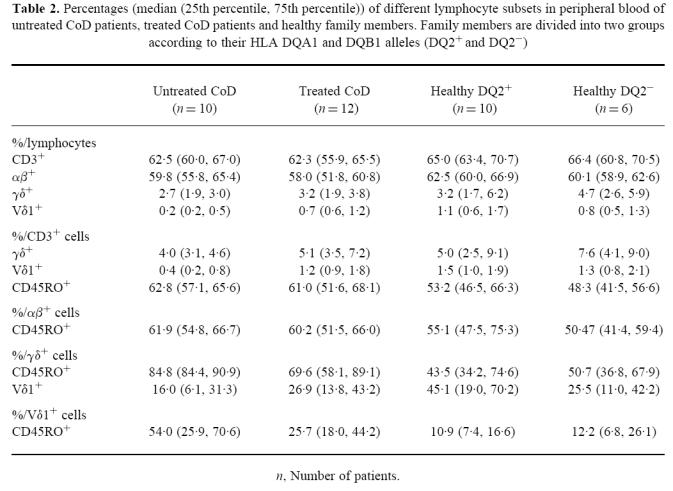
Lymphocyte counts of peripheral blood did not differ statistically between the study groups (median (25th, 75th percentiles)) 1.7 (1.3, 1.9) × 103/μl in untreated CoD, 2.0 (1.7, 2.4) × 103/μl in treated CoD patients, 2.3 (1.8, 2.6) × 103/μl in DQ2+ family members and 2.5 (1.9, 2.7) × 103/μl in DQ2− family members. The percentages of different T lymphocyte subsets including TCR αβ+ or γδ+ lymphocytes are shown in Table 2.
Table 2.
Percentages (median (25th percentile, 75th percentile)) of different lymphocyte subsets in peripheral blood of untreated CoD patients, treated CoD patients and healthy family members. Family members are divided into two groups according to their HLA DQA1 and DQB1 alleles (DQ2+ and DQ2−)
Percentages and absolute numbers of circulating Vδ1+ lymphocytes
It will be seen from Fig. 1a that DQ2+ and DQ2− family members did not differ from each other in percentages of Vδ1+ lymphocytes. In contrast, there was a statistically significant difference between the four groups (P = 0.031) and the difference was greatest between the group of untreated CoD patients and DQ2+ family members (P = 0.011). When the absolute numbers of Vδ1+ lymphocytes were compared (Fig. 1b), again, there was no difference between the DQ2+ and DQ2− groups of healthy family members (24.6 (14.1, 42.3)/μl in the DQ2+ group and 16.2 (7.9, 34.3)/μl in DQ2−), but there was a statistically significant difference between the four groups (P = 0.006). The group of untreated CoD patients, differed from both groups of healthy family members (3.5 (2.5, 9.4)/μl in untreated CoD, versus DQ2+ group P = 0.003 and versus DQ2− group P = 0.027). The absolute count of Vδ1+ lymphocytes in treated CoD patients was 14.0 (9.1, 26.1)/μl.
Fig. 1.
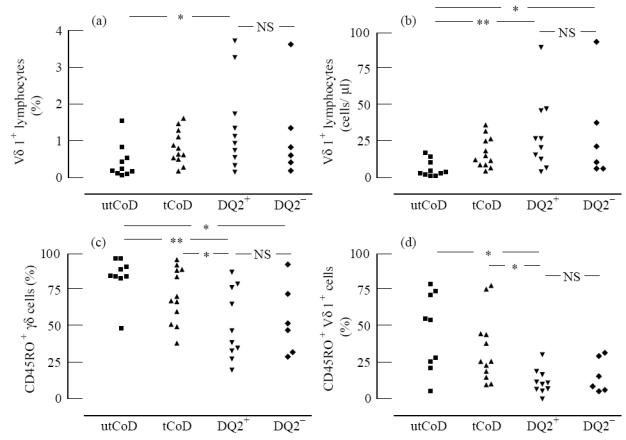
Frequency of Vδ1+ lymphocytes (a), the absolute counts of Vδ1+ lymphocytes (b), frequency of CD45RO expression on TCR γδ cells (c) and frequency of CD45RO expression on Vδ1+ lymphocytes (d) in peripheral blood of untreated CoD (utCoD), treated CoD (tCoD) and healthy family members who are either positive (DQ2+) or negative (DQ2−) for the HLA DQ2 heterodimer. In Kruskal–Wallis one-way analysis of variance P = 0.031 in (a), P = 0.006 in (b), P = 0.010 in (c) and P = 0.014 in (d). Intergroup statistics are shown: *P < 0.05; **P < 0.01; NS, not significant.
Circulating CD45RO+ T cells
As Fig. 1c,d shows, the percentages of CD45RO+ γδ+ lymphocytes and CD45RO+ Vδ1+ lymphocytes differed between the four groups (P = 0.010 and P = 0.014, respectively). The percentage of CD45RO+ γδ+ lymphocytes was higher in untreated CoD patients than those in healthy groups of family members (versus DQ2+ group P = 0.003 and versus DQ2− group P = 0.034). Treated CoD patients also differed from the DQ2+ group (P = 0.035). Percentages of CD45RO+ Vδ1+ cells in both untreated and treated CoD patients differed from the DQ2+ group (P = 0.012 and P = 0.011, respectively). Instead, the percentages of CD45RO+ (primed) T lymphocytes (CD3+), αβ+, γδ+ or Vδ1+ cells were not statistically significantly different between the two groups of healthy family members carrying different HLA (DQ2+ or DQ2−), as shown in Table 2.
DISCUSSION
It is accepted that CoD has a large spectrum of clinical as well as histological features, ranging from active CoD to silent and latent CoD and even to potential CoD, where individuals have several immunological features typical of CoD (increased IEL counts, increased densities of TCR γδ IEL, the ‘coeliac-like intestinal antibody’ pattern, HLA DQ2+, serum reticulin/endomysial antibody positivity) with normal villous architecture in their small bowel mucosa [8,23,24]. There would thus appear to be no strict border between CoD and healthy individuals carrying HLA DQ2 and other immunological features typical of CoD.
In the present study we found elevated percentages of circulating antigen-primed (CD45RO+) TCR γδ cells in active CoD together with some elevation also in treated patients compared with healthy controls, as previously reported [20]. This elevation is due to the increased expression of CD45RO on Vδ1+ TCR γδ cells. Another finding was a decrease in the amount of circulating Vδ1+ cells in CoD patients.
However, between the two groups of healthy family members either carrying (DQ2+) or not carrying (DQ2−) the HLA DQ2 heterodimer and where the former group was also known to have elevated densities of TCR γδ cells in their otherwise normal small bowel mucosa, we found no differences in the percentages of circulating CD45RO+ T lymphocytes. This is opposite to our finding in patients with SS, where HLA DQ2+ individuals had higher percentages of circulating CD45RO+ TCR γδ cells than HLA DQ2−individuals even if their jejunal mucosa showed normal morphology [21].
Vδ1+ cells are the pronounced subpopulation of intraepithelial TCR γδ cells in the small bowel mucosa of CoD patients [11,16]. There is an increase in CD45RO expression especially in TCR αβ cells of the lamina propria in CoD, but also the percentage of intraepithelial Vδ1+ cells expressing CD45RO is markedly higher than that of their counterparts in normal peripheral blood [17,25]. It is thus possible to speculate that a proportion of these cells as well as a proportion of CD45RO+ lymphocytes also in the peripheral blood of CoD patients may be originally activated because of gluten. The changes in circulating TCR γδ populations might function as a marker for immunological activity even though the significance of these cells in coeliac lesion is still unknown.
In conclusion, it seems that the elevation of circulating antigen-primed TCR γδ or Vδ1+ cells, whether gluten-induced or not, cannot be found in healthy first-degree relatives of CoD patients who have the HLA alleles DQA1*0501 and DQB1*0201 and also an increased density of TCR γδ IEL in their otherwise normal jejunal mucosa. Rather, we suggest that an ongoing autoimmune process such as CoD [20,26–28] or SS [21] is a prerequisite for the emergence of these changes.
Acknowledgments
The Coeliac Disease Study Project is supported by the Medical Research Council, the Academy of Finland, the Sigrid Juselius Foundation, the Päivikki and Sakari Sohlberg Foundation, the Medical Research Fund of Tampere University Hospital, the Yrjö Jahnsson Foundation and the Emil Aaltonen Foundation.
References
- 1.Trejdosiewicz L, Howdle P. T-cell responses and cellular immunity in coeliac disease. Baillieres Clin Gastroenterol. 1995;9:251–72. doi: 10.1016/0950-3528(95)90031-4. [DOI] [PubMed] [Google Scholar]
- 2.Cerf-Bensussan N, Cerf M, Guy-Grand D. Gut intraepithelial lymphocytes and gastrointestinal diseases. Curr Opin Gastroenterol. 1993;9:953–61. [Google Scholar]
- 3.Sollid L, Markussen G, Ek J, Gjerde H, Vartdal F, Thorsby E. Evidence for a primary association of celiac disease to a particular HLA-DQ α/β heterodimer. J Exp Med. 1989;169:345–50. doi: 10.1084/jem.169.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sollid LM, Thorsby E. HLA susceptibility genes in celiac disease: genetic mapping and role in pathogenesis. Gastroenterol. 1993;105:910–22. doi: 10.1016/0016-5085(93)90912-v. [DOI] [PubMed] [Google Scholar]
- 5.Mäki M, Holm K, Koskimies S, Hällström O, Visakorpi J. Normal small bowel biopsy followed by coeliac disease. Arch Dis Child. 1990;65:1137–41. doi: 10.1136/adc.65.10.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Troncone R. Latent coeliac disease in Italy. Acta Pediatr. 1995;84:1252–7. doi: 10.1111/j.1651-2227.1995.tb13543.x. [DOI] [PubMed] [Google Scholar]
- 7.Corazza GR, Andreani ML, Biagi F, Bonvicini F, Bernardi M, Gasbarrini G. Clinical, pathological, and antibody pattern of latent celiac disease: report of three adult cases. Am J Gastroenterol. 1996;91:2203–7. [PubMed] [Google Scholar]
- 8.Ferguson A, Arranz E, O'Mahony S. Clinical and pathological spectrum of coeliac disease—active, silent, latent, potential. Gut. 1993;34:150–1. doi: 10.1136/gut.34.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferguson A, Murray D. Quantitation of intraepithelial lymphocytes in human jejunum. Gut. 1971;12:988–94. doi: 10.1136/gut.12.12.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marsh MN, Hinde J. Morphometric analysis of small intestinal mucosa III. The quantitation of crypt epithelial volumes and lymphoid cell infiltrates, with reference to celiac sprue mucosae. Virchows Arch [Pathol Anat] 1986;409:11–22. doi: 10.1007/BF00705403. [DOI] [PubMed] [Google Scholar]
- 11.Spencer J, Isaacson PG, Diss TC, MacDonald TT. Expression of disulfide-linked and non-disulfide-linked forms of the T cell receptor γ/δ heterodimer in human intestinal intraepithelial lymphocytes. Eur J Immunol. 1989;19:1335–8. doi: 10.1002/eji.1830190728. [DOI] [PubMed] [Google Scholar]
- 12.Sturgess R, Kontakou M, Nelufer J, Hung T, Ciclitira PJ. γ/δ T-cell receptor expression in the jejunal epithelium of patients with dermatitis herpetiformis and coeliac disease. Clin Exp Dermatol. 1993;18:318–21. doi: 10.1111/j.1365-2230.1993.tb02206.x. [DOI] [PubMed] [Google Scholar]
- 13.Savilahti E, Arato A, Verkasalo M. Intestinal γ/δ receptor-bearing T lymphocytes in celiac disease and inflammatory bowel diseases in children. Constant increase in celiac disease. Pediatr Res. 1990;28:579–81. doi: 10.1203/00006450-199012000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Mäki M, Holm K, Collin P, Savilahti E. Increase in γ/δ T cell receptor bearing lymphocytes in normal small bowel mucosa in latent coeliac disease. Gut. 1991;32:1412–4. doi: 10.1136/gut.32.11.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holm K, Mäki M, Savilahti E, Lipsanen V, Laippala P, Koskimies S. Intraepithelial γδ T-cell-receptor lymphocytes and genetic susceptibility to coeliac disease. Lancet. 1992;339:1500–3. doi: 10.1016/0140-6736(92)91262-7. [DOI] [PubMed] [Google Scholar]
- 16.Halstensen TS, Scott H, Brandtzaeg P. Intraepithelial T cells of the TCRγ/δ+ CD8− and Vδ1/Jδ1+ phenotypes are increased in coeliac disease. Scand J Immunol. 1989;30:665–72. doi: 10.1111/j.1365-3083.1989.tb02474.x. [DOI] [PubMed] [Google Scholar]
- 17.Halstensen TS, Farstad IN, Scott H, Fausa O, Brandtzaeg P. Intraepithelial TcRα/β+ lymphocytes express CD45RO more often than the TcRγ/δ counterparts in coeliac disease. Immunol. 1990;71:460–6. [PMC free article] [PubMed] [Google Scholar]
- 18.Halstensen T, Brandtzaeg P. Activated T lymphocytes in the celiac lesion: non-proliferative activation (CD25) of CD4+α/β cells in the lamina propria but proliferation (Ki-67) of α/β and γ/δ cells in the epithelium. Eur J Immunol. 1993;23:505–10. doi: 10.1002/eji.1830230231. [DOI] [PubMed] [Google Scholar]
- 19.Holm K, Savilahti E, Koskimies S, Lipsanen V, Mäki M. Immunohistochemical changes in the jejunum in first degree relatives of patients with coeliac disease and the coeliac disease marker DQ genes. HLA class II antigen expression, interleukin-2-receptor positive cells and dividing crypt cells. Gut. 1994;35:55–60. doi: 10.1136/gut.35.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kerttula TO, Hällström O, Mäki M. Phenotypical characterization of peripheral blood T cells in patients with coeliac disease: elevation of antigen-primed CD45RO+ T lymphocytes. Immunol. 1995;86:104–9. [PMC free article] [PubMed] [Google Scholar]
- 21.Kerttula TO, Collin P, Polvi A, Korpela M, Partanen J, Mäki M. Distinct immunologic features of Finnish Sjögren's syndrome patients with HLA alleles DRB1*0301, DQA1*0501, and DQB1*0201. Alterations in circulating T cell receptor γ/δ subsets. Arthritis Rheum. 1996;39:1733–9. doi: 10.1002/art.1780391017. [DOI] [PubMed] [Google Scholar]
- 22.Polvi A, Eland C, Koskimies S, Mäki M, Partanen J. HLA DQ and DP in Finnish families with celiac disease. Eur J Immunogen. 1996;23:221–34. doi: 10.1111/j.1744-313x.1996.tb00117.x. [DOI] [PubMed] [Google Scholar]
- 23.Marsh MN. Gluten, major histocompatibility complex, and the small intestine. Gastroenterol. 1992;102:330–54. [PubMed] [Google Scholar]
- 24.Mäki M. The humoral immune system in coeliac disease. Baillieres Clin Gastroenterol. 1995;9:231–49. doi: 10.1016/0950-3528(95)90030-6. [DOI] [PubMed] [Google Scholar]
- 25.Miyawaki T, Kasahara Y, Taga K, Yachie A, Taniguchi N. Differential expression of CD45RO (UCHL1) and its functional relevance in two subpopulations of circulating TcR-γ/δ+ lymphocytes. J Exp Med. 1990;171:1833–8. doi: 10.1084/jem.171.5.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piccarelli A, Maiuri L, Frate A, Greco M, Auricchio S, Londei M. Production of antiendomysial antibodies after in-vitro gliadin challenge of small intestine biopsy samples from patients with coeliac disease. Lancet. 1996;348:1065–7. doi: 10.1016/S0140-6736(96)03060-7. [DOI] [PubMed] [Google Scholar]
- 27.Mäki M. Coeliac disease and autoimmunity due to unmasking of cryptic epitopes? Lancet. 1996;348:1046–7. doi: 10.1016/S0140-6736(05)64411-X. [DOI] [PubMed] [Google Scholar]
- 28.Dieterich W, Ehnis T, Bauer M, Donner P, Volta U, Riecken EO, Schuppan D. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med. 1997;3:797–801. doi: 10.1038/nm0797-797. [DOI] [PubMed] [Google Scholar]


