Abstract
Since IL-8 and MCP-1 are chemoattractant proteins that participate in the recruitment of inflammatory cells into the arthritic joint, we examined the effects of tenidap, a new anti-inflammatory drug of the oxindole family, on IL-8 and MCP-1 expression in the joints of rabbits with acute antigen arthritis. The model was induced by injecting 5 mg/ml ovalbumin into the knees of 20 preimmunized rabbits. Animals were randomized into two groups: treated with tenidap (15 mg/kg per 12 h), or untreated. The effect of tenidap treatment was evaluated on chemokine production in synovial membranes of rabbits with arthritis and in cultured monocytic and synovial cells (SC). By immunoperoxidase staining, chemokines were localized in the synovial tissue. Chemokine messenger RNA levels in the synovial membranes and in cultured cells were analysed by reverse transcription-polymerase chain reaction (RT-PCR). At the end of the study, tenidap significantly reduced neutrophil infiltration into the joint cavity (27 ± 4 × 106 cells/ml versus 45 ± 6 × 106 cells/ml in untreated; P < 0.05), and synovial effusion (134 ± 15 μl versus 236 ± 19 μl in untreated; P < 0.005). Untreated rabbits showed synovial membrane up-regulation in mRNA expression of IL-8 and MCP-1 (11- and seven-fold versus healthy rabbits, respectively) that was markedly decreased by tenidap (two- and three-fold versus healthy rabbits, respectively). IL-8 and MCP-1 were localized in the synovial tissue in a perivascular pattern and areas of the interstitium and lining, mostly coinciding with cell infiltration. Tenidap also reduced the accumulation of IL-8 and MCP-1 proteins. In cultured synovial and monocytic cells, tumour necrosis factor-alpha (TNF-α) elicited an increase in gene expression of IL-8 (four- and nine-fold, respectively) and MCP-1 (nine- and four-fold, respectively) that was significantly reversed in both cell types by 10 μm tenidap. These results suggest that the beneficial effect of tenidap in acute antigen arthritis could be related to the down-regulation in gene expression and synthesis of IL-8 and MCP-1, two key chemokines involved in the recruitment of inflammatory cells.
Keywords: arthritis, chemokines, tenidap
INTRODUCTION
Rheumatoid arthritis (RA) is characterized by proliferation of resident synovial cells (SC) and accumulation of leucocytes in the synovial membrane and fluid (SF) [1]. Both SC and mononuclear cells contribute, by the production of various cytokines and tissue-degrading enzymes, to the amplification of the inflammatory response and joint destruction [2].
Polymorphonuclear and mononuclear cells are recruited into the inflammatory site by the local production of chemokines [3]. Based on structural analysis, the chemokine superfamily can be divided into two groups: the C-X-C and the C-C subfamilies [4]. IL-8 belongs to the first group and acts mainly on neutrophils [5], whereas MCP-1 belongs to the second group and acts on mononuclear cells with varying degrees of specificity [6]. The presence of several chemokines in the SF and synovial membrane of experimental and human arthritis has been reported [7–9]. Fibroblast-like and macrophage-like cells of the synovial membrane, together with the infiltrating leucocytes, are the major source of these mediators in the rheumatoid synovial pannus [10,11].
Among the cytokines implicated in the pathogenesis of RA, tumour necrosis factor-alpha (TNF-α) is considered one of the most powerful mediators of inflammation [12]. In particular, TNF-α levels are elevated in the SF of RA patients [13]. In addition, TNF-α transgenic mice develop chronic inflammatory arthritis [14]. Recent studies have demonstrated clinical improvement of RA following treatment with MoAb to TNF-α [15]. In vitro, TNF-α stimulates the production of chemokines by cultured human SC [16].
Tenidap is the first representative of a new chemical family of anti-arthritic agents, the oxindoles. Tenidap sodium ((Z)-5-chloro-2,3 dihydro-3-(hydroxy-2-thienyl methylene)-2-oxo-1H-indole-1-carboxamide, sodium salt) is a novel drug which combines cyclooxygenase- and 5-lipoxygenase-inhibitory activity in vitro [17]. The ability to modulate cytokine synthesis [18], the protection of cartilage integrity [19], the inhibition of the release of activated neutrophil collagenase [20], as well as anti-inflammatory and analgesic activities are several properties of tenidap that account for its beneficial effects in rheumatic diseases.
In this study we show that in a model of acute arthritis in rabbits there was increased synovial IL-8 and MCP-1 gene expression and proteins coinciding with an augmentation in the influx of leucocytes. The administration of tenidap prevented the increase of IL-8 and MCP-1 expression and reduced the accumulation of leucocytes. In cultured SC and macrophages, tenidap down-regulated TNF-α-induced IL-8 and MCP-1 gene expression. The current experiments afford an additional pathogenic mechanism supporting the known beneficial effect of tenidap in the treatment of RA.
MATERIALS AND METHODS
Experimental design
Studies were conducted in white adult New Zealand rabbits with initial weights of 2.5–3 kg (obtained from B&K Universal, Madrid, Spain). Acute antigen arthritis was induced with minimal modifications according to a previously described technique [21] (Fig. 1). The animals were randomly divided into two experimental groups: in group I (n = 10) rabbits did not receive any treatment; in group II (n = 9) animals received tenidap (15 mg/kg per 12 h) (Pfizer, Central Research, Groton, CT) from 24 h before the intra-articular injection of ovalbumin (OVA) to 12 h after disease induction. Medication was given in capsules by oral gavage to unanaesthetized animals by an atraumatic procedure. A parallel group of healthy animals, matched for age and weight, was studied as controls. Synovial membranes were removed to obtain a sample for histological studies and RNA extraction. SF from rabbit knees was carefully collected by direct arthrotomy, the fluid was mixed 1:1 with PBS, and the number of cells was determined. In order to avoid cellular aggregation, the samples were incubated with hyaluronidase (Boehringer, Mannheim, Germany) for 1 h at 37°C. All animal studies were performed according to institutional and federal guidelines.
Fig. 1.

Schedule of experimental protocol for the induction of acute antigen-induced arthritis. Acute antigen-induced arthritis was induced in rabbits. Twenty-four hours before the intra-articular injection of ovalbumin (OVA) into the joint cavity, animals were randomly distributed into two groups: tenidap-treated (15 mg/kg per 12 h); and untreated. Animals were killed 24 h after the intra-articular injection. FCA, Freund's complete adjuvant.
Pharmacokinetic studies
After early dose-range studies, tenidap was administered to rabbits at a dosage designed to achieve therapeutic blood concentrations in humans with RA.
Plasma tenidap concentrations were determined 8 h after the morning administration by high performance liquid chromatography (HPLC). Acetonitrile (100 μl) containing the internal standard (CP-66 993; 5 μg/ml) was added to 50 μl of plasma. The supernatant was mixed with an equal volume of Tris buffer pH 7.4. Separations were performed using a 2-cm LC-Si Supelco precolumn and a 3.9 × 150-mm 4-μm C18 Waters Novapak column (Waters, Milford, MA) with the detector set at 360 nm. The mobile phase consisted of 0.025 m Tris buffer pH 8.2/methanol (45:55, v/v), adjusted to an apparent pH of 6.3 with phosphoric acid. The lower limit of quantification in the assay of plasma tenidap was 0.5 μg/ml.
Immunohistochemistry
After SF collection, the infrapatellar synovial tissue was carefully dissected. The material for microscopic studies was fixed in 4% paraformaldehyde in 0.005 m Tris–HCl buffer pH 7.2 for 4–16 h at room temperature, dehydrated in graded ethanols and embedded in paraffin wax. Paraffin-embedded synovial membranes were sectioned in 7 μm thick pieces, dewaxed and rehydrated. IL-8 and MCP-1 were detected with a monoclonal mouse anti-human IL-8 and a polyclonal goat anti-human MCP-1 antibody, respectively (Immunogenex Corp., Los Angeles, CA). Endogenous peroxidase activity was quenched by incubating the sections in 1% hydrogenous peroxide in methanol for 30 min. Non-specific antibody binding was blocked by incubation of the tissue section for 10 min in suppressor serum, consisting of 3% second antibody host serum, 5% rabbit serum, and 10% bovine serum albumin (BSA) in PBS, pH 7. Anti-IL-8 (50 μg/ml in 2% sheep serum, 5% rabbit serum, and 4% BSA in PBS) and anti-MCP-1 (70 μg/ml in 2% horse serum, 5% rabbit serum, and 4% BSA in PBS) antibodies were applied overnight. As secondary antibodies, a sheep anti-mouse horseradish peroxidase (HRP)-conjugated IgG (The Binding Site, Birmingham, UK) for IL-8 detection, and a donkey anti-goat IgG peroxidase-labelled (The Binding Site), both diluted 1:200 in 4% BSA–PBS, were applied for 30 min, and the sections stained for 5 min at room temperature with 0.05% 3,3′-diaminobenzidine tetrahydrochloride (Dako, Glostrup, Denmark) and 0.01% hydrogen peroxide in PBS. Finally, sections were counterstained with haematoxylin, dehydrated, and mounted in DPX. To assess non-immune unspecific binding, each sample had its own negative control being similarly processed but for the addition of the first antibody. The score was the mean of the two evaluations, with interobserver difference being never > 1 point. Briefly, the scoring was conducted as follows: grade 0 was the staining of healthy tissue, i.e. no signal or minimal binding at the lining layer; grade I was given for discontinuous or weak staining of the lining and scanty patches of positivity coinciding with infiltrates; grade II applied to cases of strong perivascular staining, positive infiltrates and wide areas of binding at the lining layer; grade III represented a generalized staining throughout the tissue.
Cell cultures
Synovial cells
SC were isolated from synovial tissue from healthy New Zealand rabbits (2–2.5 kg) according to a method previously described [22]. SC were cultured in RPMI 1640 medium (Gibco BRL, Paisley, UK) at pH 7.4, supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mm glutamine (Gibco) in the presence of 10% fetal calf serum (FCS; Gibco), plated on Petri dishes (Costar, Cambridge, MA). SC were characterized by phase contrast microscopy, negative staining for factor VIII antigen (Biomeda, Foster City, CA) and anti-CD68 antibody (Dako), excluding endothelial contamination, and confirming the presence of an unique fibroblast-like population in the cultures.
U937 cell line
U937 cells (a human monocytic cell line) were obtained from the American Type Culture Collection (Rockville, MD) (1593-CRL) and were cultured in RPMI 1640 medium (Gibco) at pH 7.4, supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mm glutamine (Gibco) in the presence of 10% FCS (Gibco).
RNA extraction, reverse transcription and semiquantitative polymerase chain reaction analysis
SC and U937 cells were growth-arrested by incubation in 0.5% FCS medium for 48 h, and then incubated with the corresponding stimuli. Frozen synovial membranes were pulverized in a metallic chamber. RNA was obtained by the acid guanidine-thiocyanate-phenol-chloroform method [23] and quantified by absorbance at 260 nm in duplicate. One microgram of RNA from each animal was reverse transcribed to single-stranded cDNA by incubation with 20 μl of reverse transcription mixture (5 mmol/l MgCl2, 10 mmol/l Tris–HCl pH 8.8, 50 mmol/l KCl, 0.1% Triton X-100, 1 mmol/l dNTPs mixture, 20 U rRNAsin (ribonuclease inhibitor), 15 U AMV reverse transcriptase and 50 ng oligo dT) at 42°C for 30 min following the manufacturer's instructions (Promega, Madison, WI). Polymerase chain reaction (PCR) was conducted in the presence of α-32P-dCTP for 25, 30, 35 and 40 cycles in the same conditions as for IL-8, MCP-1 and glyceraldehyde 3′-phosphate dehydrogenase (G3PDH) used as internal control (1 min at 54°C to allow annealing of the primers, 3 min at 72°C for primer extension and 1 min at 94°C to denature the double-stranded DNA). The following primers were used for rabbit MCP-1 [24]: (sense) 5′-TGTGCTTGCCCAGCCAGATG-3′ and (antisense) 5′-GTGTCTGCATTTTCTTGTCC-3′, that yielded a product of 230 bp; for IL-8 [25]: (sense) 5′-AACCTTCCTGCTGTCTCTGA-3′ and (antisense) 5′-TCTGCACCCACTTTTTCCTTG-3′, that yielded products of 226 bp; for G3PDH [26]: (sense) 5′-AATGCATCCTGCACCACCAA-3′ and (antisense) 5′-ATACTGTTACTTATACCGATG-3′, that yielded a product of 515 bp. All amplifications were done for 20, 30, 35 and 40 cycles in order to establish the linearity of the reaction. In all experiments, the presence of possible contaminants was checked by control reactions in which the amplification was carried out in complete reaction mixture lacking template DNA or with RNA samples from reverse transcriptase (RT) reactions done in the absence of AMV reverse transcriptase. The DNA products from the PCR reactions were analysed on a 4% polyacrylamide/urea gel in Tris-borate EDTA (TBE) buffer (45 mmol/l Tris–HCl, 45 mmol/l boric acid, 1 mmol/l EDTA). The polyacrylamide gels were dried, exposed to x-ray films and scanned using the IQ densitometer.
Statistical analysis
Statistical analysis was performed using Student's t-test for unpaired samples. P < 0.05 was considered statistically significant. Values are expressed as mean ± s.e.m.
RESULTS
Development of experimental arthritis
SF volume from non-treated rabbits showed definite signs of a marked inflammatory reaction, with a large SF effusion (236 ± 19 μl) and infiltration of polymorphonuclear neutrophils (PMN) (45 ± 6 × 106). By contrast, tenidap treatment resulted in a diminution of the inflammatory signs in SF. In relation to untreated rabbits, treatment with tenidap significantly reduced the SF volume (134 ± 15 μl, P < 0.005) (Fig. 2a) and the SF cellular count (27 ± 4 × 106 PMN, P < 0.05) (Fig. 2b).
Fig. 2.
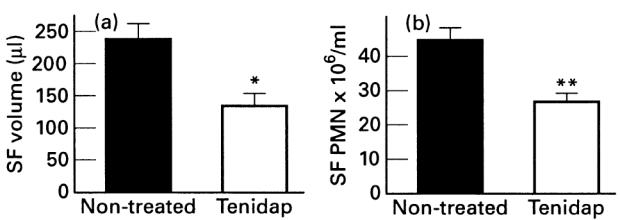
Synovial fluid (SF) volume and leucocyte concentration. SF was drawn on the day of sacrifice and SF volume (a) and cellular count (b) were determined. Data are expressed as the mean ± s.e.m. (n = 8–10 per group). *P < 0.005; **P < 0.05 versus untreated rabbits.
At the time of sacrifice (8 h after last administration of tenidap), plasma and SF levels of tenidap were 23 ± 19 μg/ml and 13 ± 3 μg/ml (mean ± s.e.m.), similar to those considered therapeutic in humans, according to the recommended dosage for RA. In addition, there was a good correlation between both parameters (r = 0.80, P < 0.02).
IL-8 and MCP-1 gene expression in arthritis synovial membrane
We assessed IL-8 and MCP-1 mRNA levels in each individual synovial membrane employing a semiquantitative RT-PCR method. As shown in Fig. 3, IL-8 and MCP-1 mRNA expression in the synovial membrane of normal rabbits was low, while they were significantly up-regulated in untreated arthritis animals (11- and seven-fold versus healthy rabbits, respectively; P < 0.005). In the tenidap-treated group, IL-8 and MCP-1 mRNA levels were significantly lower than in the untreated group (two- and three-fold versus healthy animals, respectively; P < 0.05) (Fig. 3).
Fig. 3.
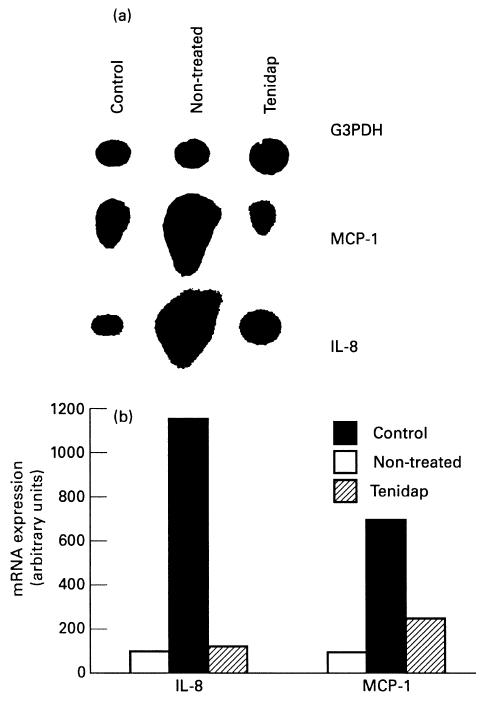
IL-8 and MCP-1 gene expression in arthritic synovial membrane. Total RNA was isolated from joint tissues, the mRNA reversed transcribed and amplified using chemokine-specific primers. Glyceraldehyde 3′-phosphate dehydrogenase (G3DPH) primers were used as an internal control.
Immunolocalization of IL-8 and MCP-1
The histological pattern of joint lesions appeared to be correlated with the influx of leucocytes into the synovial membrane and the SF. In healthy control animals both chemokines were almost absent, except for occasional binding of the antibodies to the lining synovial cells. By contrast, in untreated animals the inflamed synovial membranes had a similar distribution of both peptides (Fig. 4) that followed areas of neutrophil infiltration, while endothelial and lining cells were commonly positive. As shown in Figs 5C and 6C, healthy controls showed a light staining with the anti-IL-8 (0.3 ± 0.1) and MCP-1 (0.4 ± 0.2) antibodies. Untreated rabbits had an increase in IL-8 (2.5 ± 0.3) and MCP-1 staining (1.8 ± 0.3), mainly related to the lining cells and vascular walls (Figs 5B and 6B) (P < 0.0005 and < 0.05 versus healthy rabbits, respectively). By contrast, tenidap treatment was associated with a diminution in IL-8 and MCP-1 staining (1.3 ± 0.4 and 1 ± 0.2, respectively, P < 0.05) (Figs 5A and 6A). No staining was observed in the negative controls included in each experiment (not shown).
Fig. 4.
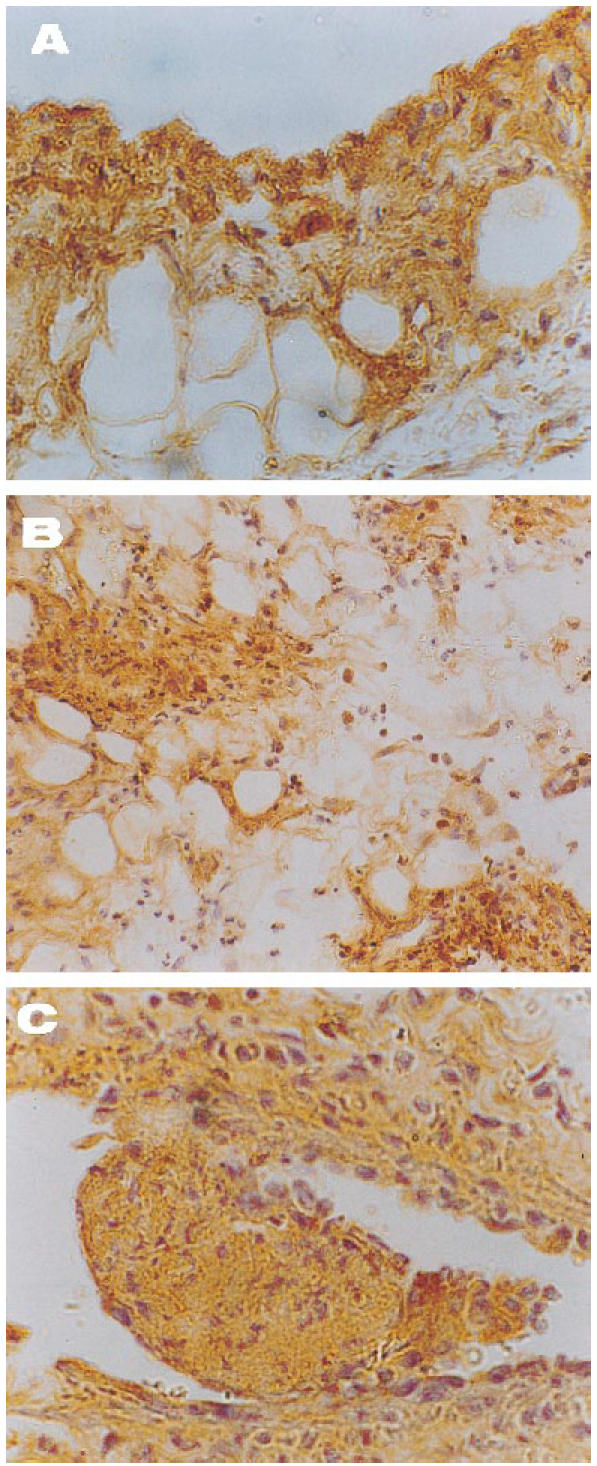
Pattern of localization of IL-8 and MCP-1 in synovial tissues of rabbits with acute antigen arthritis. Both anti-chemokine antibodies displayed a similar distribution in the injured tissue, preferentially staining the lining layer, areas of neutrophil infiltration and endothelium. The figure shows representative findings of untreated specimens employing immunoperoxidase techniques. (A) Binding of anti-IL-8 antibody to synovial lining (×400). (B) The strong IL-8 staining of the interstitium is limited to granulocyte accumules (×200). (C) Organized thromboses surrounded by vasculitic damage are typical of this experimental disease. Note the striking positivity for MCP-1 within the thrombosis and around the vessel wall (×400).
Fig. 5.
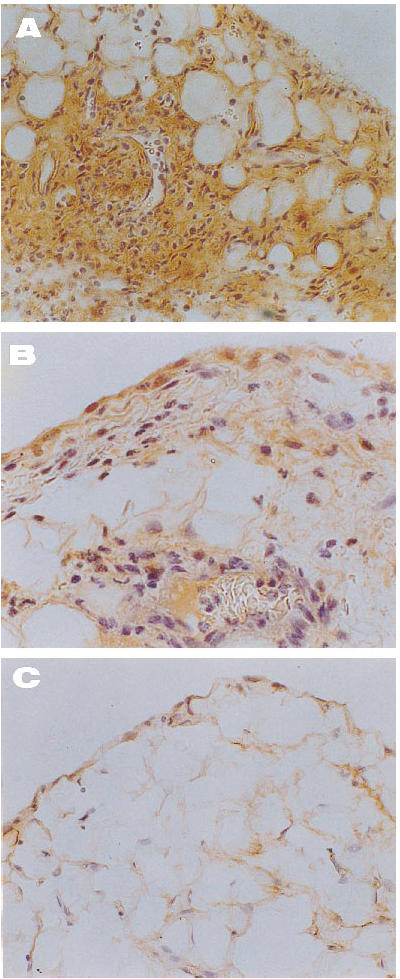
Immunolocalization of IL-8 in joint tissue from arthritic rabbits. (A) Intense and diffuse positive signal in an untreated rabbit, including a mural thrombosis and perivascular infiltration (×200). (B) Note the mild staining of superficial synovial cells and infiltrates in a tenidap-treated animal (×400). (C) A demonstrably healthy synovium where IL-8 was almost absent (×200). There was no staining in the negative controls included in each experiment (not shown).
Fig. 6.
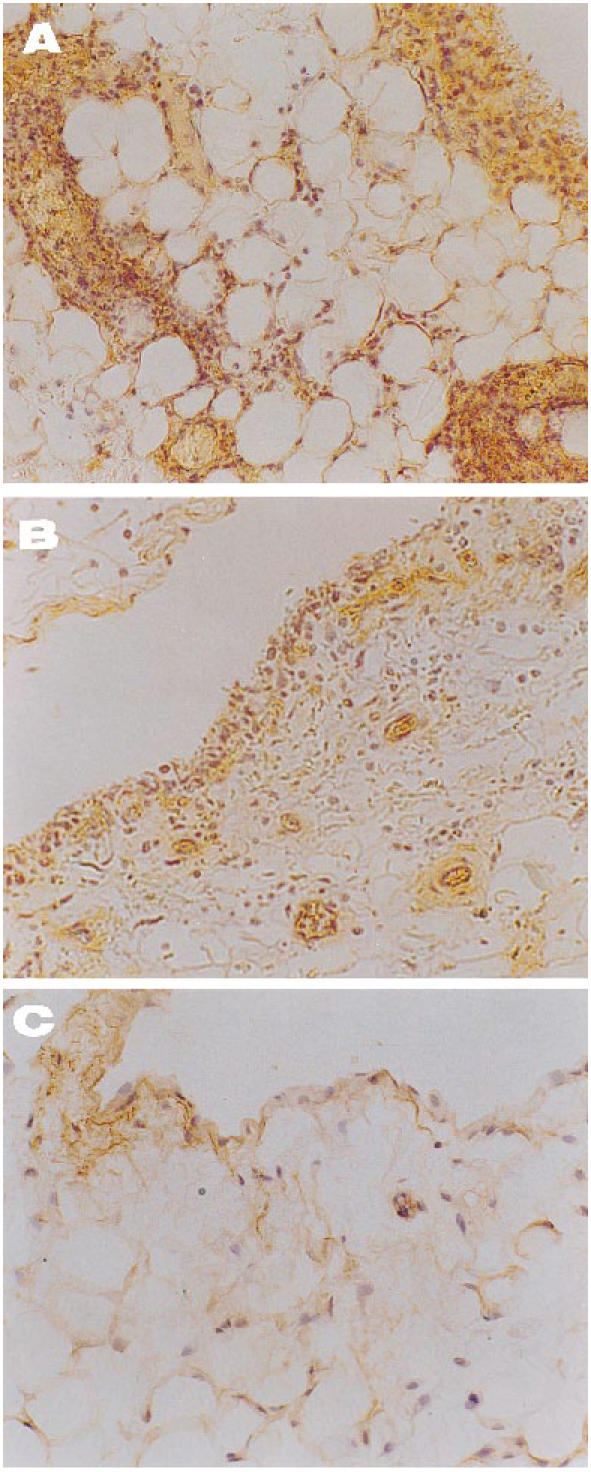
Immunolocalization of MCP-1 in joint tissue from arthritic rabbits. (A) An untreated specimen shows lining hyperplasia markedly positive for MCP-1 with several infiltrates which also bound the specific antibody (×200). (B) In a tenidap-treated synovium a diminution of the former signal is evident, with slight staining of endothelium and lining cells (×200). (C) Healthy control tissue, where minimal binding of anti-MCP-1 is seen at the lining layer (×200). There was no staining in the negative controls included in each experiment (not shown).
The increased chemokine protein expression was well correlated with the leucocyte infiltrates into the SF. These data show that during joint damage in acute antigen arthritis both IL-8 and MCP-1 are expressed, and probably play a crucial role in the recruitment of inflammatory cells.
Chemokine gene expression in cultured SC and monocytes
In the normal synovium, the membrane comprises principally two cell types, fibroblast-like and macrophage-like synoviocytes. In contrast, synovium from arthritis rabbits contains a large number of infiltrating cells. Both SC and monocytes may be a major source of chemokines. In order to ascertain if tenidap exerts a beneficial effect in RA by inhibiting the production of IL-8 and MCP-1, some experiments with cultured SC and U937 cells were done. The appropriate dose of TNF-α (100 U/ml), a potent inductor of chemokine gene expression, was used as a positive control. As seen in Fig. 7, the stimulation of quiescent rabbit SC with 100 U/ml TNF-α induced a four- and nine-fold increase in IL-8 and MCP-1 mRNA expression compared with control levels, respectively (n = 3, P < 0.01). The presence of tenidap in the culture medium significantly down-regulated IL-8 and MCP-1 mRNA expression induced by TNF-α (Fig. 7).
Fig. 7.
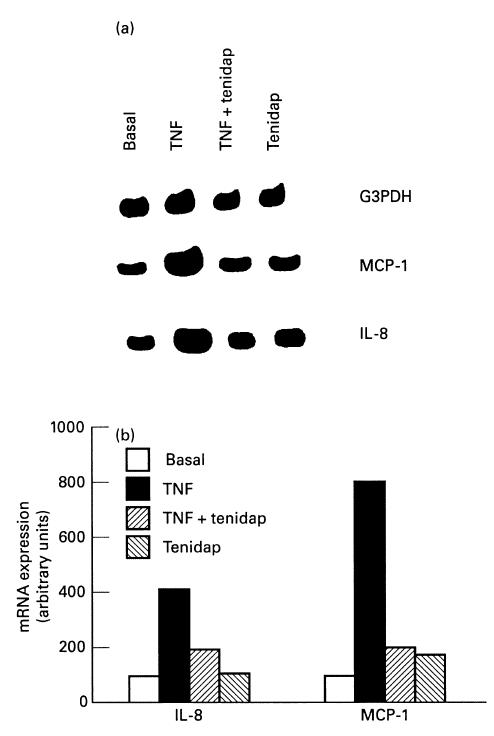
Effect of tenidap on tumour necrosis factor-alpha (TNF-α)-induced MCP-1 and IL-8 mRNA expression in rabbit fibroblast-like synoviocytes. (a) Fibroblast-like synoviocytes were stimulated for 4 h with TNF-α (100 U/ml) alone or in combination with 10 μm tenidap. Total RNA was extracted, and reverse transcriptase-polymerase chain reaction (RT-PCR) for MCP-1 and IL-8 mRNA expression was performed. This autoradiography is representative of a total of four experiments. (b) Densitometric analysis of the bands were conducted by computerized laser densitometry and normalized to the glyceraldehyde 3′-phosphate dehydrogenase (G3DPH) mRNA level. Values are expressed as the percentage over control.
In order to discover whether the IL-8 and MCP-1 mRNA expression elicited by TNF-α required the de novo synthesis of intermediate proteins, SC were treated with cycloheximide (CHX), a potent inhibitor of the peptidyltranferase. When SC were simultaneously incubated with TNF-α and CHX for 4 h, an over-expression in IL-8 and MCP-1 mRNA was seen (four- and eight-fold versus basal, respectively). These results suggest that transcription of chemokines in SC, elicited by TNF-α, may be partially repressed by some labile proteins.
Similar results were obtained in the human monocyte cell line (U937). IL-8 and MCP-1 mRNA expression induced in U937, when incubated for 4 h with TNF-α (100 U/ml), was significantly reversed by the presence of tenidap (10 μm) in the culture medium (Fig. 8).
Fig. 8.
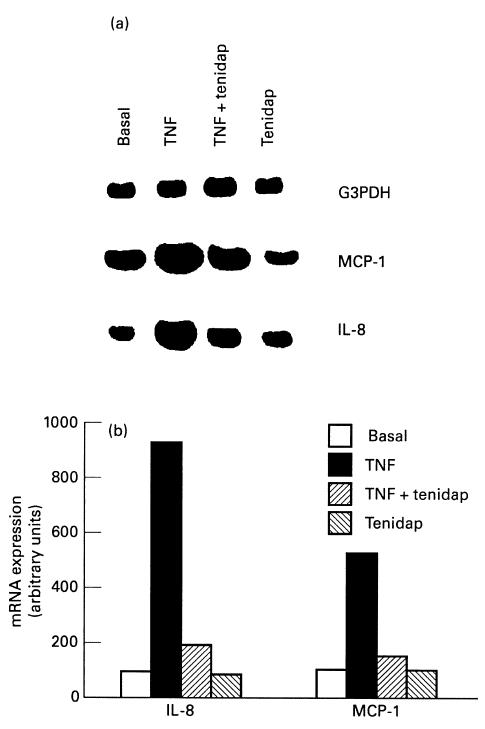
Effect of tenidap on tumour necrosis factor-alpha (TNF-α)-induced IL-8 and MCP-1 mRNA expression in monocytes. (a) U-937 cells were stimulated for 4 h with TNF-α (100 U/ml) alone or in combination with 10 μm tenidap. Total RNA was extracted, and reverse transcriptase-polymerase chain reaction (RT-PCR) for MCP-1 and IL-8 mRNA expression was performed. This autoradiography is representative of a total of four experiments. (b) Densitometric analysis of the bands was conducted by computerized laser densitometry and normalized to the glyceraldehyde 3′-phosphate dehydrogenase (G3DPH) mRNA level. Values are expressed as the percentage over control.
DISCUSSION
Tenidap is effective in the treatment of RA [18,27], reducing several biological markers of inflammation, such as erythrocyte sedimentation rate and serum levels of C-reactive protein (CRP) and serum amyloid A (SAA) protein, probably due to suppression of the synthesis of cytokines, such as IL-1 and IL-6 [18,28,29]. It is noteworthy that tenidap, but not non-steroidal anti-inflammatory drugs (NSAIDs), reduces leucocyte counts in knee effusions from RA patients [29]. This finding, together with the observation in this study that tenidap reduces the degree of inflammatory cell infiltration in the arthritic joint, may indicate that this drug exerts some action on chemotactic factors.
The central role of chemokines in the pathogenesis of chronic arthritis is supported by a number of studies showing that chemokines such as IL-8 and MCP-1 are present in the arthritic joint [1,7,10,11]. In this sense, injections of IL-8 into rabbit joints results in the accumulation of neutrophils and mononuclear cells [30]. In contrast, injection of anti-IL-8 antibodies into lipopolysaccharide (LPS)- or IL-1-induced arthritis resulted in the attenuation of the inflammation [9,24].
As occurs in other experimental models of RA [9], untreated rabbits presented in the synovial membrane, in parallel with the accumulation of leucocytes, a marked increase in IL-8 and MCP-1 mRNA expression. By immunoperoxidase staining, an increase in the deposition of the two chemokine proteins (IL-8 and MCP-1) was also observed. In arthritic rabbits receiving tenidap there was a diminution in IL-8 and MCP-1 protein levels, accompanied by a marked down-regulation of IL-8 and MCP-1 gene expression. These data suggest that the observed therapeutic effect on signs of inflammatory reaction in the synovium and SF may be due to reduced chemokine expression.
The mechanism of IL-8 and MCP-1 over-expression in the synovium of animals with arthritic joints is unclear. In several models of joint damage, including those used in this study, an increase in the synovial expression and synthesis of growth factors and cytokines, such as transforming growth factor-beta (TGF-β), TNF-α, IL-1 and IL-6, was observed, coinciding with maximal injury [31,32]. In this sense, the intra-articular injection of either TNF-α or of recombinant human IL-1 into animal joints produced an infiltration of leucocyte cells within the joint space [33]. Based on these and other studies, it was originally thought that TNF-α and IL-1 were responsible for mediating neutrophil chemotaxis. However, it has become clear that neither TNF-α nor rIL-1 are chemotactic in vitro for neutrophils [34]. This apparent paradox was solved by the discovery that cultured SC can synthesize chemokines in response to numerous proinflammatory stimuli, including TNF-α and IL-1 [10]. It is tempting to speculate that during joint injury, both infiltrating inflammatory and resident cells may release those mediators, stimulating SC to increase IL-8 and MCP-1 synthesis, that could contribute to the amplification of the inflammatory cascade by the production of adhesion molecules and other mediators of inflammation. In this sense, IL-8 induces not only neutrophil chemotaxis, but also activation of neutrophils, formation of superoxide anion [35], expression of LFA-1 [36] and adherence of monocytes to endothelium [37]. Furthermore, IL-8 stimulation of neutrophils results in induction of its own synthesis and secretion from these cells [38]. This autocrine loop could be important in the pathogenesis of arthritis, where neutrophils are present in the joint lumen together with a high amount of IL-8, thereby creating the possibility of cell stimulation, that could be down-regulated.
Studies in vitro suggest that tenidap exerts its anti-inflammatory activity by inhibiting the production of inflammatory cytokines induced by several stimuli [18,39]. Tenidap is a potent inhibitor of IL-6 in human peripheral blood mononuclear cells (PBMC) [20]. Interestingly, tenidap's activity was not mimicked by cyclooxygenase inhibitors, indicating that cytotoxic T lymphocyte (CTL)-induced IL-1β post-translational processing was not dependent on an effect on prostaglandin E2 (PGE2) production. Moreover, CHX did not inhibit CTL-induced cytokine processing, indicating that the ability of tenidap to reversibly suppress general translational activity was not responsible for this inhibitory activity [40].
The effects and mechanisms of action of tenidap on IL-8 and MCP-1 gene expression have not yet been elucidated. The data presented indicate that tenidap inhibits TNF-α-induced IL-8 and MCP-1 gene expression in rabbit fibroblast-like synoviocytes in a dose-dependent manner. Total protein synthesis was unaffected at concentrations and times at which effects on chemokine production were observed, suggesting that the inhibition of chemokine production is not a result of a generalized metabolic effect on the cells. Since tenidap is a potent anion-transport inhibitor and modulator of intracellular pH, it is possible that tenidap's effects on chemokine production, similar to what occurs with IL-6 and IL-1β production, could be attributed to changes in intracellular pH and anion transport. Tenidap also modifies the activation-induced calcium fluxes in several cells, such as mast cells and T cells [41] and the intracellular protein phosphorylation activity in activated macrophages [42].
The present studies do not exclude that tenidap may act on other potential cell recruitment mediators, such as leukotrienes, platelet-activating factor (PAF) and C5a. In fact, tenidap has been shown to inhibit lipoxygenase and consequently to decrease LTB4 levels in several cellular systems [43], including neutrophils from RA patient synovial fluids [44]. It has been shown that PAF, alone or with other mediators, could amplify the inflammatory joint reaction [45]. Tenidap also inhibits PAF-induced IL-8 and MCP-1 gene expression in rabbit monocytes in a dose-dependent manner (unpublished data).
On the whole, the present data show that in a rabbit model of acute antigen arthritis there was an increase in IL-8 and MCP-1 expression and leucocyte accumulation in the synovial membrane, that was prevented by tenidap treatment. Tenidap down-regulates TNF-α-induced IL-8 and MCP-1 gene expression in cultured mononuclear cells and SC. The current experiments afford an additional pathogenic mechanism supporting the known beneficial effect of tenidap in the treatment of RA.
Acknowledgments
This work has been supported by grant from FISS (94/0370), Ministerio de Educación y Ciencia (PM-95/93), Pfizer S. A. Spain and Fundación Iñigo Alvarez de Toledo. P.H. is a fellow from Fundación Conchita Rábago.
References
- 1.Brennan FM, Zachariae COC, Chantry D, Larsen CG, Turner M, Maini RN, Matsushima K, Feldmann M. Detection of interleukin 8 biological activity in synovial fluids from patients with rheumatoid arthritis and production of interleukin 8 mRNA by isolated synovial cells. Eur J Immunol. 1990;20:2141–4. doi: 10.1002/eji.1830200938. [DOI] [PubMed] [Google Scholar]
- 2.Khalil M, Al-Daccak R, Schall TJ, Mourad W. Induction of chemokine gene expression by major histocompatibility complex class II ligands in human fibroblast-like synoviocytes. Differential regulation by interleukin-4 and dexamethasone. J Biol Chem. 1994;269:32063–9. [PubMed] [Google Scholar]
- 3.Harris ED. Rheumatoid arthritis. Pathophysiology and implications for therapy. N Engl J Med. 1990;322:1277–89. doi: 10.1056/NEJM199005033221805. [DOI] [PubMed] [Google Scholar]
- 4.Bagiiolini M, Dewald D, Moser B. Interleukin-8 and related chemotactic cytokines CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 5.Matsushima K, Larsen CG, DuBois GC, Oppenheim JJ. Purification and characterization of a novel monocyte chemotactic and activating factor. [DOI] [PMC free article] [PubMed]
- 6.Leonard EJ, Yoshimura T. Human monocyte chemoattractant protein-1 (MCP-1) Immunol Today. 1990;11:97–101. doi: 10.1016/0167-5699(90)90035-8. [DOI] [PubMed] [Google Scholar]
- 7.Villiger PM, Terkeltaub R, Lotz M. Production of monocyte chemoattractant protein-1 by inflamed synovial tissue and cultured synoviocytes. J Immunol. 1992;149:722–7. [PubMed] [Google Scholar]
- 8.Harigai M, Hara M, Yoshimura T, Leonard EJ, Inoue K, Kashiwazaki S. Monocyte chemoattracttant protein 1 (MCP-1) in inflammatory joint diseases and its involvement in the cytokine network of rheumatoid synovium. Clin Immunol Immunopathol. 1993;69:83–91. doi: 10.1006/clin.1993.1153. [DOI] [PubMed] [Google Scholar]
- 9.Kasama T, Strieter RM, Lukacs NW, Lincoln PM, Burdick MD, Kunkel SL. Interleukin-10 expression and chemokine regulation during the evolution of murine type II collagen-induced arthritis. J Clin Invest. 1995;95:2868–76. doi: 10.1172/JCI117993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rathanaswami P, Hachicha M, Sadick M, Schall TJ, McColl SR. Expression of the cytokine RANTES in human rheumatoid synovial fibroblasts. Differential regulation of RANTES and interleukin-8 genes by inflammatory cytokines. J Biol Chem. 1993;268:5834–9. [PubMed] [Google Scholar]
- 11.Seitz M, Loetscher P, Dewald B, Towbin H, Rordorf C, Gallati H, Baggiolini M, Gerber NJ. Methotrexate action in rheumatoid arthritis: stimulation of cytokine inhibitor and inhibition of chemokine production by peripheral blood mononuclear cells. Br J Rheumatol. 1995;34:602–9. doi: 10.1093/rheumatology/34.7.602. [DOI] [PubMed] [Google Scholar]
- 12.Ortiz A, Bustos C, Alonso J, Alcazar R, López-Armada MJ, Gonzalez E, Egido J. Involvement of tumor necrosis factor alpha in the pathogenesis of experimental and human glomerulonephritis. Adv Nephrol. 1995;24:53–77. [PubMed] [Google Scholar]
- 13.Saxne T, Palladino MA, Heinegard D, Talal N, Wolheim FA. Detection of tumor necrosis factor α but not tumor necrosis factor β in rheumatoid arthritis synovial fluid and serum. Arthritis Rheum. 1988;31:1041–5. doi: 10.1002/art.1780310816. [DOI] [PubMed] [Google Scholar]
- 14.Keffer J, Probert L, Cazlaris H, Georgopoulos S, Kaslaris E, Kioussis D. Transgenic mice expressing human tumor necrosis factor: a predictive genetic model of arthritis. EMBO J. 1991;10:4025–231. doi: 10.1002/j.1460-2075.1991.tb04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elliot MJ, Maini RN, Feldmann M, et al. Treatment of rheumatoid arthritis with chimeric monoclonal antibodies to tumor necrosis factor α. Arthritis Rheum. 1993;36:1681–90. doi: 10.1002/art.1780361206. [DOI] [PubMed] [Google Scholar]
- 16.Hachicha M, Rathanaswami P, Schall TJ, McColl SR. Production of monocyte chemotactic protein-1 in human type B synoviocytes. Arthritis Rheum. 1993;36:26–34. doi: 10.1002/art.1780360106. [DOI] [PubMed] [Google Scholar]
- 17.Moilanen E, Alanko J, Asmawi MZ, Vapaatalo H. CP-66,248, a new anti-inflammatory agent, is a potent inhibitor of leukotriene B4 and prostanoid synthesis in human polymorphonuclear leukocytes in vitro. Eicosanoids. 1988;1:35–39. [PubMed] [Google Scholar]
- 18.Sipe JD, Bartle LM, Loose LD. Modification of proinflammatory cytokine production by the antirheumatic agents tenidap and naxopren. J Immunol. 1992;148:480–4. [PubMed] [Google Scholar]
- 19.Dingle JT, Leeming MRG, Martindale JJ. Effect of tenidap on cartilage integrity in vitro. Ann Rheum Dis. 1993;52:292–9. doi: 10.1136/ard.52.4.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blackburn WD, Loose LD, Heck LW, Chatham WW. Tenidap, in contrast to several available nonsteroidal antiinflammatory drugs, potently inhibits the release of activated neutrophil collagenase. Arthritis Rheum. 1991;34:211–6. doi: 10.1002/art.1780340213. [DOI] [PubMed] [Google Scholar]
- 21.Howson P, Shepard N, Mitchell N. The antigen induced arthritis model. The relevance of the method of induction to its use as a model of human disease. J Rheumatol. 1986;13:379–85. [PubMed] [Google Scholar]
- 22.Gutierrez S, Palacios I, Egido J, Zarco P, Miguelez R, Gonzalez E, Herrero-Beaumont G. IL-1β and IL-6 stimulate the production of platelet activating factor (PAF) by cultured rabbit synovial cells. Clin Exp Immunol. 1995;99:364–8. doi: 10.1111/j.1365-2249.1995.tb05559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocynate-phenol-chloroform extraction. Anal Biochem. 1987;162:155–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 24.Akahoshi T, Endo H, Kondo H, Kashiwazaki S, Kasahara T, Mukaida N. Essential involvement of interleukin-8 in neutrophil recruitment in rabbits with acute experimental arthritis induced by lipopolysaccharide and interleukin-1. Lymph Cyto Res. 1994;13:113–6. [PubMed] [Google Scholar]
- 25.Yoshimura T, Yuhki N. Neutrophil attractant/activation protein-1 and monocyte chemoattractant protein-1 in rabbit. cDNA cloning and their expression in spleen cells. J Immunol. 1991;146:3483–8. [PubMed] [Google Scholar]
- 26.Kaneto H, Morrisses J, Klark S. Increased expression of TGF-β1 in the obstructed kidney of rats with unilateral ureteral ligation. Kidney Int. 1993;44:313–21. doi: 10.1038/ki.1993.246. [DOI] [PubMed] [Google Scholar]
- 27.Wylie G, Appelboom T, Bolten W, et al. A comparative study of tenidap, a cytokine-modulating antirheumatic drug, and diclofenac in rheumatoid arthritis: 24-week analysis of a 1-year clinical trial. Br J Rheumatol. 1995;34:554–63. doi: 10.1093/rheumatology/34.6.554. [DOI] [PubMed] [Google Scholar]
- 28.Littman BH, Drury CE, Zimmerer RO, Stack CB, Law CG. Rheumatoid arthritis treated with tenidap and piroxicam: clinical associations with cytokine modulation by tenidap. Arthitis Rheum. 1995;38:29–37. doi: 10.1002/art.1780380105. [DOI] [PubMed] [Google Scholar]
- 29.Loose LD, Sipe JD, Kirby DS, et al. Reduction of acute-phase proteins with tenidap sodium, a cytokine-modulating antirheumatic drug. Br J Rheumatol. 1993;32(Suppl. 3):19–25. doi: 10.1093/rheumatology/32.suppl_3.19. [DOI] [PubMed] [Google Scholar]
- 30.Endo H, Akahoshi T, Takagishi K, Kashiwazaki S, Matsushima K. Elevation of interleukin 8 (IL-8) levels in joint fluids of patients with rheumatoid arthritis and the induction by IL-8 of leukocyte infiltration and synovitis in rabbit joints. Lymph Cyto Res. 1991;10:245–2. [PubMed] [Google Scholar]
- 31.Duff GW. Cytokines and acute phase proteins in rheumatoid arthritis. Scand J Rheumatol. 1994;23(Suppl. 100):9–19. doi: 10.3109/03009749409095197. [DOI] [PubMed] [Google Scholar]
- 32.Arend WP, Dayer JM. Inhibition of the production and effects of interleukin-1 and tumor necrosis factor in rheumatoid arthritis. Arthritis Rheum. 1995;2:151–60. doi: 10.1002/art.1780380202. [DOI] [PubMed] [Google Scholar]
- 33.Pettipher ER, Higgs GA, Henderson B. Interleukin-1 induces leukocyte infiltration and cartilage proteoglycan degradation in the synovial joint. Proc Natl Acad Sci USA. 1986;83:8749–53. doi: 10.1073/pnas.83.22.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshimura T, Matshushima K, Oppenheim JJ, Leonard EJ. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Natl Acad Sci USA. 1987;84:9233–7. doi: 10.1073/pnas.84.24.9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Djeu JY, Matsushima K, Oppenheim JJ, Shiotsuki K, Blanchard DK. Functional activation of human neutrophils by recombinant monocyte-derived neutrophil chemotactic factor/IL-8. J Immunol. 1990;144:2205–10. [PubMed] [Google Scholar]
- 36.Detmers PA, Lo SK, Olsen EE, Walz A, Baggiolini M, Cohn ZA. Neutrophil-activating protein 1/interleukin 8 stimulates the binding activity of the leukocyte adhesion receptor CD11b/CD18 on human neutrophils. J Exp Med. 1990;171:1155–62. doi: 10.1084/jem.171.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown KA, Le Roy F, Noble G, Bacon K, Camp R, Vora A. Interleukin 8 acts on monocytes to increase their attachment to cultured endothelium and enhance their expression of surface adhesion molecules. Adv Exp Cell Biol. 1991;305:161–2. [Google Scholar]
- 38.Strieter RM, Kasahara K, Allen RM, Standiford TJ, Rolfe MW, Becker FS. Cytokine-induced neutrophil-derived interleukin-8. Am J Pathol. 1992;141:397–407. [PMC free article] [PubMed] [Google Scholar]
- 39.Perregaux DG, Svensson L, Gabel CA. Tenidap and other anion transport inhibitors disrupt cytolytic T lymphocyte-mediated IL-1β post-translational processing. J Immunol. 1996;157:57–64. [PubMed] [Google Scholar]
- 40.McNiff P, Svensson L, Pazoles CJ, Gabel CA. Tenidap modulates cytoplasmic pH and inhibits anion transport in vitro. J Immunol. 1994;153:2180–93. [PubMed] [Google Scholar]
- 41.Cleveland PL, Millard PJ, Showell HJ, Fewtrell CMS. Tenidap: a novel inhibitor of calcium influx in a mast cell line. Cell Calcium. 1993;14:1–16. doi: 10.1016/0143-4160(93)90013-v. [DOI] [PubMed] [Google Scholar]
- 42.Bondeson J, Sundler R. Effects of tenidap on Ca++ and protein kinase C-mediated protein phosphorylation, activation of the arachidonate-mobilizing phospholipase A2 and subsequent eicosanoid formation in macrophages. Biochem Pharmacol. 1994;48:1171–9. doi: 10.1016/0006-2952(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 43.Panés J, Russel JM, Wolf RE, Wallace JL, Granger N. Effects of tenidap on leukocyte–endothelial cell adhesion in mesenteric venules. J Rheumatol. 1995;22:444–9. [PubMed] [Google Scholar]
- 44.Blackburn W, Heck LW, Loose LD, Eskra JD, Carty TJ. Inhibition of 5-lipoxygenase product formation and polymorphonuclear cell degranulation by tenidap sodium in patients with rheumatoid arthritis. Arthritis Rheum. 1991;34:204–10. doi: 10.1002/art.1780340212. [DOI] [PubMed] [Google Scholar]
- 45.Herrero-Beaumont G, Egido J. PAF, a potent proinflammatory mediator, looking for its role in the pathogenesis of joint damage. Ann Rheum Dis. 1997;56:211–3. doi: 10.1136/ard.56.4.211. [DOI] [PMC free article] [PubMed] [Google Scholar]


