Abstract
Imbalance in Th1 and Th2 subsets and their derived cytokines seems to be involved in the immune abnormalities underlying UC and CD. CD30 is a member of the tumour necrosis factor/nerve growth receptor superfamily expressed on T cells producing Th2 cytokines and released as a soluble form. In this study high levels of soluble CD30 were found in sera of UC patients independently of disease activity. Furthermore, increased titres of soluble CD30 molecule were shown, in the same patients, by mitogen-stimulated cultures of peripheral blood mononuclear cells. Our data seem to indicate that an activation of Th2 immune response is involved in the pathogenesis of UC, but not of CD. Furthermore, this finding indicates that serum soluble CD30 measurement may be helpful for differentiating these two forms of inflammatory bowel disease.
Keywords: inflammatory bowel disease, CD30, T cell
INTRODUCTION
The pathogenic mechanisms underlying UC and CD are unknown, although much evidence indicates that T cells play an important role in the immune events involved in the pathogenesis of these diseases. In fact, autoreactive T cells have been found in patients with inflammatory bowel disease (IBD) [1] and increased circulating and tissue-infiltrating T lymphocytes exhibiting an activated phenotype have been shown in the same patients [2,3].
CD4+ T cells constitute the predominant cell type infiltrating the bowel wall during IBD [4]. They can be divided into two functional subsets, based on their different cytokine production. Type 1 T helper (Th1) lymphocytes, secreting IL-2 and interferon-gamma (IFN-γ), are the prime movers of cell-mediated immunity, whereas Th2 cells, secreting IL-4, IL-5, IL-10 and IL-13, are mainly responsible for antibody responses [5]. Recently, it has been shown that Th2 lymphocytes co-express on their surface the CD30 antigen, which is a member of the tumour necrosis/nerve growth receptor superfamily originally described on Reed–Sternberg cells in Hodgkin's lymphoma [6,7]. In addition to its expression in a variety of malignancies of lymphoid origin, CD30 has been recognized on activated human T and B cells [8]. CD30 was found to be highly and consistently expressed on CD4+ T clones producing Th2 cytokines and virtually absent on Th1 lymphocytes [9]. The membrane-associated CD30 molecule is proteolytically cleaved to produce a soluble form (sCD30), detectable in culture supernatants of activated T and B cells and in the serum of patients with CD30+ neoplasia [10].
A different cytokine profile has been recently identified for CD and UC. IL-2 and IFN-γ are increased in the gut tissue of CD patients, while mucosal IL-4 and IL-10 mRNA are increased in UC but not in CD [11–14]. Thus, these data suggest a Th1/Th2 dichotomy in the immunopathogenesis of CD and UC. In this study, we evaluated sCD30 serum levels of IBD patients, both UC and CD, and correlated these findings with both disease activity and inflammatory markers such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). We also evaluated in the same patients the sCD30 titres in supernatants of phytohaemagglutinin (PHA)-stimulated and unstimulated peripheral blood mononuclear cells (PBMC). Furthermore, in order to assess the possible expansion of the Th2 subset and its relationship with the clinical stage of these diseases, we evaluated the percentage of circulating CD30+ T cells.
PATIENTS AND METHODS
Patients and controls
After informed consent, 35 consecutive patients meeting the inclusion criteria were entered into the study. Seventeen had UC and 18 CD (20 men and 15 women). IBD was diagnosed by the usual clinical, radiological, endoscopical and histological criteria. Disease activity was assessed in all patients at the time of immunological studies. In UC, disease activity was evaluated by Truelove's criteria [15], in CD using the Best's index (CDAI) [16], and in patients with colonic involvement by endoscopy. UC patients had a median age of 31.5 years (range 16–68 years), and the duration of the disease ranged from 1 to 26 years. Two patients had proctitis, 10 had proctosigmoiditis, and five had pancolitis. Eight patients had active, nine quiescent disease. CD patients had a median age of 35 years (range 18–63 years), and the disease had been diagnosed from 1 month to 24 years before. Ten patients had ileal involvement, six had ileocolitis and two colitis. Ten patients had active, eight quiescent disease. Two CD patients had been previously operated by ileocaecal resection. Steroid treatment was discontinued at least 3 months before the enrolment. Thirty-two patients were receiving oral salazopyrine or controlled-release 5-ASA tablets, and three patients were receiving no specific treatment. Twenty-eight healthy volunteers with age and sex distribution similar to IBD patients were enrolled as controls.
Serum samples
Serum samples were obtained from IBD patients and controls. All samples were aliquoted and stored at −80°C until used for sCD30 measurement.
Cell cultures
PBMC were separated from 27 IBD patients (14 UC and 13 CD) and 20 controls by standard Ficoll–Hypaque density gradient centrifugation. Their viability was routinely > 95%, as assessed by trypan blue dye exclusion. After two washes, PBMC were counted and resuspended at 2 × 106 cells/ml in RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum (FCS), 1% l-glutamine, 100 U/ml penicillin, 200 μg/ml streptomycin and 100 μg/ml gentamicin. The cells were cultured in the presence of medium alone or medium plus PHA (Biochrom KG, Berlin, Germany) at a final concentration of 10 μg/ml, at 37°C in a 5% CO2 atmosphere. Supernatants were harvested after 48 h of culture, aliquoted and stored at −80°C until use.
Assay for sCD30 in supernatants and blood samples
sCD30 was measured by ELISA test kits (Dako CD30, Ki-1 antigen ELISA; Glostrup, Denmark). The assay is based on the dual immunometric sandwich principle and was performed according to the manufacturer's instructions.
Cell phenotype
The surface co-expression of CD30 on PBMC was determined by two-colour immunofluorescence after incubation with a FITC-conjugated anti-CD30 MoAb (Ki-1 antigen, CD30/FITC; Dako) in combination with PE-conjugated MoAbs against CD3, CD4 and CD8 molecules (Leu-4, Leu-3 and Leu-2, respectively; Becton Dickinson, Mountain View, CA). The phenotypical analysis was performed by flow cytometry (FACScan; Becton Dickinson).
Statistical analysis
Because of the non-normal distribution of our results the following non-parametric tests were used for statistical analysis: Mann–Whitney U-test for non-paired samples, Wilcoxon's signed-rank test for paired data and Spearman's correlation coefficient.
RESULTS
sCD30 serum levels
Serum sCD30 values (expressed as median, range) were significantly higher in UC patients (23.3 U/ml, range 9.5–86 U/ml), both active (30 U/ml, range 9.5–86 U/ml) and quiescent (23.3 U/ml, range 10–65 U/ml), than in controls (6.93 U/ml, range 2–30 U/ml) (P = 0.0001, P = 0.0007, P = 0.0004, respectively). Furthermore, significant differences of sCD30 titres were observed between UC and CD patients (10.94 U/ml, range 3.6–22 U/ml), both active (10.44 U/ml, range 4.57–22 U/ml) and quiescent (12 U/ml, range 3.6–21 U/ml) (P = 0.002, P = 0.01, P = 0.04, respectively). sCD30 serum levels in CD patients were comparable to controls independently of disease activity (Fig. 1). No correlation was observed between sCD30 serum values and both ESR and CRP in IBD patients independently of the clinical phase of disease.
Fig. 1.
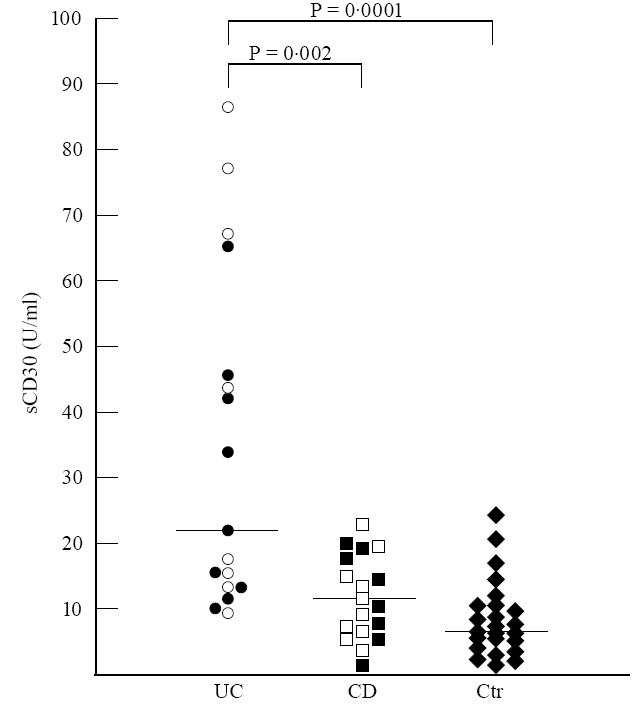
Serum levels of sCD30 in active (○) and quiescent (•) UC, active (□) and quiescent (▪) CD patients and controls (Ctr) (♦). Bars represent medians of total patients (active and quiescent). The titres are significantly higher in UC patients, independently of disease activity, compared with both CD patients and controls (P= 0.002 and P= 0.0001, respectively). No differences can be observed between CD patients and controls (Mann–Whitney U-test).
sCD30 levels in cell culture supernatants
Supernatants of unstimulated cultures of UC patients showed increased titres of sCD30 (8.25 U/ml, range 4.3–18 U/ml) compared with controls (4.9 U/ml, range 3.3–13.3 U/ml) (P = 0.0018), and this increase could be related to the higher levels of sCD30 observed in active UC patients (9 U/ml, range 6.5–18 U/ml) than in controls (4.9 U/ml, range 3.3–13.3 U/ml) (P = 0.001). In fact, there were no significant differences between quiescent patients (7.6 U/ml, range 4.3–12.5 U/ml) and controls. Unstimulated culture supernatants of UC patient sCD30 titres did not differ from CD patients (6 U/ml, range 2.51–9 U/ml) (P = 0.07). In contrast, active UC patients showed increased levels of sCD30 compared with active CD patients (6 U/ml, range 2.5–9 U/ml) (P = 0.037); no significant differences were observed between quiescent UC and CD (5.5 U/ml, range 2.98–14 U/ml). In unstimulated culture supernatants of CD patients, sCD30 levels were similar to controls independently of clinical status of the disease (Fig. 2).
Fig. 2.
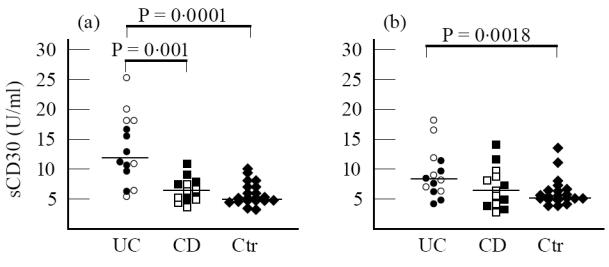
sCD30 levels in supernatants of both phytohaemagglutinin (PHA)-stimulated (a) and unstimulated (b) cultures of active (○) and quiescent (•) UC, active (□) and quiescent (▪) CD patients and controls (Ctr) (♦) after 48 h. Bars represent the medians of total patients (active and quiescent). (a) In PHA-stimulated cultures sCD30 levels were significantly increased in UC patients compared with both CD patients and controls (P= 0.001 and P= 0.0001, respectively). No differences could be observed between CD patients and controls. (b) In unstimulated cultures sCD30 levels were significantly increased only in active UC patients compared with controls (P= 0.0018). No significant differences could be observed between UC and CD patients and between CD patients and controls (Mann–Whitney U-test).
Raised sCD30 levels were detected in 48 h PHA-stimulated cultures of PBMC of UC patients (12 U/ml, range 5.18–25.3 U/ml), both active (18.3 U/ml, range 5.18–25.3 U/ml) and quiescent (11 U/ml, range 6.5–17.8 U/ml), compared with controls (5.03 U/ml, range 3–10 U/ml) (P = 0.0001, P = 0.002, P = 0.0005, respectively). These levels were significantly higher than those of CD patients (6.5 U/ml, range 3.8–11 U/ml), both active (4.7 U/ml, range 3.8–7.5 U/ml) and quiescent (7.5 U/ml, range 4.5–11 U/ml) (P = 0.001, P = 0.01, P = 0.035, respectively). Stimulated culture supernatants of CD patients, both active and quiescent, showed sCD30 values comparable to controls (Fig. 2).
UC stimulated supernatants showed increased levels of sCD30 compared with unstimulated culture supernatants (P = 0.04). Furthermore, we observed that sCD30 levels in supernatants of PHA-stimulated cultures of quiescent UC patients (11 U/ml, range 6.5–17.8 U/ml) were significantly increased compared with their unstimulated cultures (7.6 U/ml, range 4.3–12.5 U/ml) (P = 0.03). In contrast, no differences were observed in sCD30 titres between PHA-stimulated and unstimulated cultures of active UC patients. sCD30 values in PHA-stimulated and unstimulated culture supernatants of CD patients, both active and quiescent, were similar (Fig. 2).
Surface phenotype of PBMC
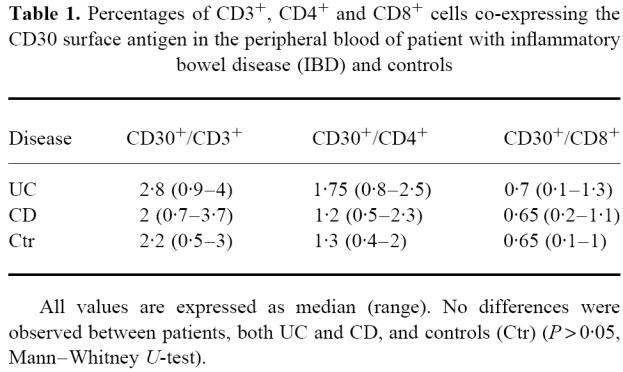
The percentage of peripheral blood CD3+/CD30+, CD4+/CD30+ and CD8+/CD30+ T lymphocytes in IBD patients did not significantly differ from controls (Table 1).
Table 1.
Percentages of CD3+, CD4+ and CD8+ cells co-expressing the CD30 surface antigen in the peripheral blood of patient with inflammatory bowel disease (IBD) and controls
DISCUSSION
Our study seems to indicate that serum levels of sCD30 are increased in UC patients independently of disease activity, while they are within normal range in CD patients. Increased sCD30 levels were found in several autoimmune diseases associated with Th2-like responses such as systemic lupus erythematosus (SLE) and systemic sclerosis (SSc) [17,18], thus suggesting the involvement of this subset in vivo. Similarly, our data suggest that the in vivo expansion and/or activation of the Th2 cell compartment may drive a Th2-dominated immune response in the pathogenesis of UC. Furthermore, the difference in sCD30 levels between UC and CD strongly suggests different pathogenic mechanisms in these diseases. Recently, it has been proposed that in CD, cell-mediated immune reactions play a key role; in contrast, during UC the humoral responses of the immune system could be involved in the pathogenesis of tissue damage [19–22]. Differences in the cytokine pattern between UC and CD, showing increased mucosal levels of IL-2 and IFN-γ in CD and of IL-4 and IL-10 in UC, are in keeping with the hypothesis that the immune response is mainly mediated by Th1 cells in CD, and by Th2 lymphocytes in UC [11–14]. Furthermore, accumulation of Th1 cells has been shown in gut biopsies of CD patients, and their mucosal CD4+ cells did not express CD30 [4]. Evidence that CD30+ T cells are more effective than Th1 in activating B cells [23] for immunoglobulin production suggests that this subset may be responsible for polyclonal activation of B cells and (auto)antibody production identified in UC patients [21,22,24]. In this light, our results suggest that CD30 molecule, in UC, may be associated with a Th2 cytokine production pattern, as previously reported, although recently some authors have proposed that CD30 molecule could be expressed by T cells producing both IL-4 and IFN-γ [25,26]. We found that higher serum sCD30 levels in UC patients did not correlate with the clinical status of the disease, suggesting a chronic activation of CD30-producing cells. A tentative explanation of these findings is that stimulating factors persist in vivo and activate these cells, inducing the secretion of sCD30 molecule. In the culture media assays we observed that the PBMC of active UC patients spontaneously produce higher levels of sCD30 in vitro, confirming that these cells have already been primed in vivo. In fact, our findings indicate that, in this phase of disease, PBMC are maximally activated in vivo and unresponsive to further in vitro mitogen stimulation. In contrast, PBMC of quiescent patients do not produce increased amounts of sCD30 spontaneously, but they appear more sensitive to mitogen stimulation than PBMC of both CD and controls. In fact, increased levels of sCD30 were found in culture media of mitogen-stimulated PBMC from UC patients, both in the active and quiescent phases of disease.
The discrepancy found in our study between the increased serum sCD30 levels and the normal percentage of circulating CD30+/CD3+ T cells in UC patients may have several explanations. First of all we have to consider that T cells releasing sCD30 could be confined to the specific target organs as found in inflamed joint effusions of rheumatoid arthritis patients [27]. Recently, it has been shown that CD30 expression is IL-4-dependent [28]. This cytokine has been found increased in UC gut tissue, and mucosal CD4+ T cells produced IL-4 [4,13]. These data suggest that IL-4 could induce in vivo local production and release of sCD30 by T cells into the circulation. On the other hand, a possible contribution of B cells to the sCD30 increase in UC sera cannot be excluded. Lastly, a defective detection of CD30 T cells has been found in healthy individuals with the use of FITC-based methods [29].
In IBD, differently from other autoimmune diseases such as SLE and SSc, no correlation between sCD30 and both ESR and CRP was observed. The relationship between sCD30 and inflammation is still unclear, and several questions concerning the pathophysiological role of CD30 remain to be answered.
Several lines of evidence indicate that different immunopathological mechanisms act in UC and CD. Our data provide further support to this view, and suggest that sCD30 serum values may be a helpful marker for differentiating these two forms of IBD.
Acknowledgments
The authors wish to thank Professor G. Corrao and B. Annibale MD for their helpful assistance in the statistical evaluation, and C. Petrucci and M. Termine for their technical assistance. This work was supported by MURST 40% and MURST 60% 1996 grants.
References
- 1.Okazaki K, Morita M, Nishimori I, et al. Major histocompatibility antigen-restricted cytotoxicity in inflammatory bowel disease. Gastroenterol. 1993;104:384–91. doi: 10.1016/0016-5085(93)90405-2. [DOI] [PubMed] [Google Scholar]
- 2.Pallone F, Fais S, Squarcia O, et al. Activation of peripheral blood and intestinal lamina propria lymphocytes in Crohn's disease. In vivo state of activation and in vitro response to stimulation as defined by the expression of early activation antigens. Gut. 1987;28:745–53. doi: 10.1136/gut.28.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schreiber S, MacDermott RP, Raedler A, et al. Increased activation of isolated intestinal lamina propria mononuclear cells in inflammatory bowel disease. Gastroenterol. 1991;101:1020–30. doi: 10.1016/0016-5085(91)90729-5. [DOI] [PubMed] [Google Scholar]
- 4.Parronchi P, Romagnani P, Annunziato F, et al. Type 1 T-helper cell predominance and interleukin-12 expression in the gut of patients with Crohn's disease. Am J Pathol. 1997;150:823–32. [PMC free article] [PubMed] [Google Scholar]
- 5.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 6.Falini B, Pileri S, Pizzolo G, et al. CD30 (Ki-1) molecule: a new cytokine receptor of the tumor necrosis receptor superfamily as a tool for diagnosis and immunotherapy. Blood. 1995;85:1–14. [PubMed] [Google Scholar]
- 7.Schwab U, Stein H, Gerdes G, et al. Production of a monoclonal antibody specific for Hodgkin and Sternberg–Reed cells of Hodgkin's disease and a subset of normal lymphoid cells. Nature. 1982;200:65–70. doi: 10.1038/299065a0. [DOI] [PubMed] [Google Scholar]
- 8.Schwarting R, Gerdes J, Durkop H, et al. BER-H2: a new anti-Ki-1 (CD30) monoclonal antibody directed at a formol-resistant epitope. Blood. 1989;74:1678–82. [PubMed] [Google Scholar]
- 9.Del Prete G, De Carli M, Almerigogna F, et al. Preferential expression of CD30 by human CD4+ T cells producing Th2 cytokines. FASEB. 1995;9:81–86. [PubMed] [Google Scholar]
- 10.Josimovic-Alasevic O, Durkop H, Schwarting R, et al. Ki-1 (CD30) antigen is released by Ki-1 positive tumor cells in vitro and in vivo. Partial characterisation of soluble Ki-1 antigen and detection of the antigen in cell culture supernatants and in serum by an enzyme-linked immunosorbent assay. Eur J Immunol. 1989;19:157–62. doi: 10.1002/eji.1830190125. [DOI] [PubMed] [Google Scholar]
- 11.Mullin GE, Lazenby AJ, Harris ML, et al. Increased interleukin-2 messenger RNA in the intestinal mucosal lesions of Crohn's disease but not in ulcerative colitis. Gastroenterol. 1992;102:1620–7. doi: 10.1016/0016-5085(92)91722-g. [DOI] [PubMed] [Google Scholar]
- 12.Breese E, Braegger CP, Corrigan CJ, et al. Interleukin-2 and interferon-γ secreting T cells in normal and diseased human intestinal mucosa. Immunol. 1993;78:127–31. [PMC free article] [PubMed] [Google Scholar]
- 13.Sartor RB. Cytokines in intestinal inflammation: pathophysiologic and clinical considerations. Gastroenterol. 1994;106:533–9. doi: 10.1016/0016-5085(94)90614-9. [DOI] [PubMed] [Google Scholar]
- 14.Niessner M, Volk BA. Altered Th1/Th2 cytokine profiles in the intestinal mucosa of patients with inflammatory bowel disease as assessed by quantitative reversed transcribed polymerase chain reaction (RT-PCR) Clin Exp Immunol. 1995;101:428–35. doi: 10.1111/j.1365-2249.1995.tb03130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Truelove SC, Witts LJ. Cortisone in ulcerative colitis. Final report on a therapeutic trial. Br Med J. 1955;2:1041–8. doi: 10.1136/bmj.2.4947.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Best W, Becktel J, Kern F. Development of Crohn's disease activity index. Gastroenterol. 1976;70:439–44. [PubMed] [Google Scholar]
- 17.Calligaris-Cappio F, Bertero MT, Converso M, et al. Circulating levels of soluble CD30, a marker of cells producing Th2-type cytokines, are increased in patients with systemic lupus erythematosus and correlate with disease activity. Clin Exp Rheumatol. 1995;13:339–43. [PubMed] [Google Scholar]
- 18.Giacomelli R, Cipriani P, Lattanzio R, et al. Circulating levels of soluble CD30 are increased in patients with systemic sclerosis (SSc) and correlate with serological and clinical features of the disease. Clin Exp Immunol. 1997;108:42–46. doi: 10.1046/j.1365-2249.1997.d01-991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raedler A, Fraenkel S, Klose G, et al. Involvement of the immune system in the pathogenesis of Crohn's disease. Gastroenterol. 1985;88:978–83. doi: 10.1016/s0016-5085(85)80017-2. [DOI] [PubMed] [Google Scholar]
- 20.Schreiber S, Raedler A, Stenson WF, et al. The role of the mucosal immune system in inflammatory bowel disease. Gastroenterol Clin North Am. 1992;21:451–502. [PubMed] [Google Scholar]
- 21.Das KM, Dasgupta A, Mandal A, et al. Autoimmunity to cytoskeletal protein tropomyosin. A clue to the pathogenic mechanism for ulcerative colitis. J Immunol. 1993;150:2487–93. [PubMed] [Google Scholar]
- 22.Halstensen TS, Das KM, Brandtzaeg P. Epithelial deposits of immunoglobulin G1 and activated complement colocalise with the Mr 40 kD putative autoantigen in ulcerative colitis. Gut. 1993;34:650–7. doi: 10.1136/gut.34.5.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romagnani S. Lymphokine production by human T cells in disease states. Annu Rev Immunol. 1994;12:227–57. doi: 10.1146/annurev.iy.12.040194.001303. [DOI] [PubMed] [Google Scholar]
- 24.Brandtzaeg P, Halstensen TS, Kett K. Immunopathology of inflammatory bowel disease. In: MacDermott RP, Stenson WF, editors. Inflammatory bowel disease. New York: Elsevier Science Publishers; 1992. pp. 95–136. [Google Scholar]
- 25.Hamann D, Hilkens CMU, Grogan JL, et al. CD30 expression does not discriminate between human Th1- and Th2-type T cells. J Immunol. 1996;156:1387–99. [PubMed] [Google Scholar]
- 26.Alzona M, Jack HM, Fisher RI, et al. CD30 defines a subset of activated human T cells that produce IFN-γ and IL-5 and exhibit enhanced B cell helper activity. J Immunol. 1994;153:2861–7. [PubMed] [Google Scholar]
- 27.Gerli R, Muscat C, Bistoni O, et al. High levels of the soluble form of CD30 molecule, in rheumatoid arthritis (RA) are expression of CD30+ T cell involvement in the inflamed joints. Clin Exp Immunol. 1995;102:547–50. doi: 10.1111/j.1365-2249.1995.tb03851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura T, Lee RK, Nam SY, et al. Reciprocal regulation of CD30 expression on CD4+ T cells by IL-4 and IFN-γ. J Immunol. 1997;158:2090–8. [PubMed] [Google Scholar]
- 29.Agrawal B, Reddish M, Longenecker BM. CD30 expression on human CD8+ T cells isolated from peripheral blood lymphocytes of normal donors. J Immunol. 1996;157:3229–34. [PubMed] [Google Scholar]


