Abstract
Anti-mitochondrial antibodies (anti-M7) in sera from patients with dilated cardiomyopathy and myocarditis recognize, besides mitochondrial antigens, bacterial sarcosine dehydrogenase. The common target antigen was identified as the covalently bound FAD of mitochondrial and bacterial flavoenzymes. Thus, anti-M7-positive serum reacted on Western blots exclusively with covalently flavinylated enzymes. The antigenic specificity of anti-M7 sera was reproduced by an antiserum raised in rabbits with 6-hydroxy-d-nicotine oxidase. The heart mitochondrial membrane antigen recognized by anti-M7 serum was identified as the flavoprotein subunit of succinate dehydrogenase, the antigens in rat liver mitochondrial matrix as the flavoenzymes dimethylglycine dehydrogenase and sarcosine dehydrogenase. Anti-M7 serum contained a specific anti-flavoenzyme antibody fraction. Nanomolar concentrations of FAD and riboflavin inhibited the immune reaction on Western blots and in ELISA, and incubation with FAD-agarose depleted the anti-M7 activity of the serum. N-terminally deleted dimethylglycine dehydrogenase proteins were only immunoprecipitated by anti-M7 sera when the FAD was covalently incorporated. An affinity constant (KD) of 10−8 m was established for the anti-flavoenzyme antibodies by competitive ELISA. Of patients with cardiomyopathy and myocarditis, 36% and 25%, respectively, were anti-flavoenzyme-positive by Western blot and ELISA, but only two of 15 patients with other heart diseases and none of 50 healthy controls.
Keywords: anti-flavin autoantibodies, dilated cardiomyopathy, flavoenzymes, mitochondria
INTRODUCTION
Recently, an anti-mitochondrial antibody was described in sera from patients with heart diseases of unknown aetiology which reacted with submitochondrial particles from bovine heart (HSMP) as shown by ELISA and Western blotting, and this antibody was named anti-M7 [1,2]. On Western blots of bovine heart and rat liver mitochondrial proteins, anti-M7-positive sera recognized an epitope of ≈ 64 kD, and in rat liver mitochondria a second band of ≈ 90 kD. Anti-M7 antibodies were detected in 60% of patients with acute myocarditis and 33% of patients with dilated cardiomyopathy [1]. The findings were suggestive of an organ-specific autoimmune reaction at least in some aetiologically undefined heart diseases [3]. At this time, the M7 antigen was identified as an epitope of bacterial sarcosine dehydrogenase (SaDHb) [1], which belongs to a class of flavoenzymes containing covalently bound FAD. In mammals, enzymes with covalently bound FAD are predominantly located to the mitochondria and are represented by monoamine oxidase of the outer mitochondrial membrane, by the flavoprotein subunit of succinate dehydrogenase (SDH) located in the inner mitochondrial membrane, as well as by the closely related mitochondrial matrix enzymes dimethylglycine dehydrogenase (DMGDH) and sarcosine dehydrogenase (SaDHm). Bacterial SaDH and mammalian DMGDH share sequence similarities, including the covalent type of FAD attachment to a histidine residue of the enzyme [4].
The present study was aimed at the identification of the mitochondrial anti-M7 antigens. It will be shown that the common epitope of mitochondrial and bacterial proteins recognized by anti-M7 sera represents the covalently bound FAD moiety of flavoenzymes.
PATIENTS AND METHODS
Patients
A marker serum with high anti-M7 titres from a patient with dilated cardiomyopathy as documented by heart catheterization, echocardiography, myocardial scintigraphy, and coronarography was used. Furthermore, sera from 25 patients with dilated cardiomyopathy as well as from 12 patients with myocarditis and 15 sera from patients with other heart diseases (coronary heart diseases, n = 5; hypertrophic obstructive cardiomyopathy, n = 3; postpartale cardiomyopathy, n = 2; not defined, n = 5) were tested. The criteria used for selection of the patients were left ventricular insufficiency and left ventricular end diastolic diameter (LVEDD) ≥ 60. As anti-mitochondrial-positive controls two sera were analysed from patients with syphilis stage II reacting with cardiolipin (M1) [5], two sera from patients with primary biliary cirrhosis (PBC) reacting with the pyruvate dehydrogenase complex (M2) [6] and two sera from patients with Venucuran-induced pseudolupus syndrome reacting with an antigen of the outer mitochondrial membrane (M3) [7].
In order to evaluate the specificity of anti-M7 for heart diseases, sera from four patients with systemic lupus erythematosus, four patients with cryptogenic liver cirrhosis, four patients with PBC, four patients with fibromyalgia syndrome, four patients with systemic sclerosis and 50 healthy controls were included in the study.
Chemicals
35S-methionine was from Amersham Corp. (Braunschweig, Germany). Immunodiffusion plates NOR-Partigen-IgG-HC were obtained from Behringwerke AG Diagnostika (Stuttgart, Germany). 5-Bromo-4-chloro-3-indolyl-phosphate, nitrotetrazolium blue and the chemiluminescence Western Blotting kit (rabbit) were purchased from Boehringer (Mannheim, Germany). Adenosine, AMP, ADP, ATP, riboflavin, FMN, FAD, FAD insolubilized on 4% beaded agarose, goat anti-rabbit, anti-human and anti-mouse IgGs conjugated to alkaline phosphatase, peroxidase-conjugated goat anti-human IgGs, bacterial sarcosine dehydrogenase, sarcosine oxidase (SaO), monoamine oxidase from bovine plasma, o-phenylenediamine and protein high molecular weight markers were from Sigma (Deisenhofen, Germany). Monoclonal mouse anti-human IgG1, IgG2, IgG3 and IgG4 were from Pharmingen (San Diego, CA).
Antisera
Antisera against DMGDH, 6-hydroxy-d-nicotine oxidase (6HDNO) and SDH (a kind gift of Dr Gary Cecchini, San Francisco, CA) were raised in rabbits according to standard protocols [8].
Isolation of mitochondria and mitochondrial fractions
Rat liver mitochondria were prepared as described [9]. Separation of rat liver mitochondria into a soluble and a membrane fraction was performed by four times freeze-thawing the washed mitochondria in liquid nitrogen, followed by centrifugation at 100 000 g for 30 min at 2°C. HSMP were prepared according to [10].
Purification of 6HDNO and 6-hydroxy-l-nicotine oxidase
6HDNO wild type and the 6HDNO.Cys mutant, which is unable to bind FAD covalently, were prepared as described [11]. 6-Hydroxy-l-nicotine oxidase (6HLNO) was purified from Arthrobacter nicotinovorans cells according to [12].
Western blotting
Mitochondrial proteins (50 μg) and 1 μg of the purified proteins were resolved by SDS–PAGE and transferred onto nitrocellulose (Optipran BA-S 85; Schleicher & Schuell, Dassel, Germany) using a semidry horizontal apparatus (BioRad, München, Germany). Individual lanes of the membrane were incubated with human sera at a dilution of 1:300 and with rabbit sera at a dilution of 1:1000. Second antibodies were used in a dilution of 1:3500. The membranes were stripped for redecoration by shaking at 50°C for 30 min in 50 ml stripping buffer (2% SDS, 60 mm Tris–HCl pH 6.7, 100 mm mercaptoethanol).
Isotypes of anti-M7 antibodies were determined using monoclonal mouse anti-human IgG1, IgG2, IgG3 and IgG4 antibodies (dilution 1:500) and alkaline phosphatase-conjugated anti-mouse antibodies (dilution 1:1000).
Immunoinhibition
The reaction of anti-M7 sera with purified 6HDNO as antigen was competed by incubating the serum overnight with various concentrations of adenosine, AMP, ADP, ATP, riboflavin and FAD. Depletion of anti-M7 sera of the anti-flavoprotein antibody fraction was performed by incubating the sera with FAD–agarose at room temperature for 15 min. The FAD–agarose beads were removed by centrifugation and the serum was used to decorate Western blots of mitochondrial proteins and purified bacterial flavoproteins.
Affinity purification of antibodies
Affinity purification of the anti-flavoenzyme antibody fraction of anti-M7 sera was performed with the aid of 6HDNO immobilized to nitrocellulose membrane, as described [13].
Single radial immunodiffusion
Immunodiffusion was performed on NOR-Partigen-IgG-HC plates to determine the IgG concentration in the sera according to the supplier's instructions (Behringwerke AG Diagnostika, Stuttgart, Germany).
ELISA
An ELISA was performed according to standard methods [14]. Maxisorb microtitre plates (Nunc, Roskilde, Denmark) were coated with 500 ng purified 6HDNO per well. Sera were employed in dilutions of 1:1000. Bound antibodies were visualized using peroxidase-conjugated goat anti-human IgG antibodies at a dilution of 1:3500 and o-phenylenediamine as substrate. A competitive ELISA with 6HDNO as antigen and FAD was performed according to [15].
N-terminal deletions of DMGDH, in vitro transcription-translation and immunoprecipitation
N-terminal deletions of DMGDH2 (96 kD) [16,17] resulting in proteins of 93 kD (DMGDH1), 91 kD (DMGDH3) and 74 kD (DMGDH4) were in vitro translated in the rabbit reticulocyte lysate (RL) in the presence of 35S-Met according to the supplier's instructions (Promega, Madison, WI). Holoenzyme formation was tested by trypsin resistance. Translated proteins (4 μl) were immunoprecipitated under denaturing conditions with 20 μl anti-M7 or control serum according to [16], analysed by SDS–PAGE on 7.5% polyacrylamide gels [18], followed by autoradiography. The intensity of the bands on the exposed films was quantified by densitometry.
RESULTS
Characterization of the M7 antigen as the covalently bound flavin of flavoenzymes
Mitochondrial extracts were applied to Western blotting using the anti-M7-positive marker serum in order to identify the proteins which were recognized by anti-M7. As shown in Fig. 1, the serum reacted with two proteins of 90–110 kD in the matrix fraction of rat liver mitochondria (Fig. 1a, lane 1) and with one prominent protein of around 66 kD in the membrane fraction (Fig. 1a, lane 2). The 66-kD antigen was also present in submitochondrial particles prepared from bovine heart mitochondria (Fig. 1a, lane 3), in accordance with the results of the original work defining the anti-M7 serum [1]. In addition, the bacterial enzymes SaDH of Pseudomonas sp. (45 kD), SaO of Bacillus subtilis (42 kD) and 6HDNO of A. nicotinovorans (48 kD), all flavoenzymes with FAD covalently bound to a histidine residue [19], reacted on Western blots with the anti-M7 marker serum (Fig. 1a, lanes 4, 5 and 6, respectively). In contrast, 6HLNO, the enantiozyme of 6HDNO [20], which loses the non-covalently bound FAD by denaturation of the protein during SDS–PAGE, gave no reaction (Fig. 1a, lane 7). Similarly, a 6HDNO mutant, incapable of binding FAD covalently because of the replacement of the FAD-binding His by Cys [21], gave no immune reaction on Western blot with anti-M7 serum (results not shown). These data may indicate that the covalently bound flavin is the common epitope of bacterial and mitochondrial proteins recognized by the marker serum. In order to substantiate this assumption, antibodies to 6HDNO were raised by immunizing rabbits with the highly purified bacterial protein. Surprisingly, the rabbit serum exhibited the same antigenic recognition pattern as the human anti-M7 sera with the exception of 6HLNO, the enantiozyme of 6HDNO, which was known to be recognized by the anti-6HDNO serum (Fig. 1b, lane 7).
Fig. 1.
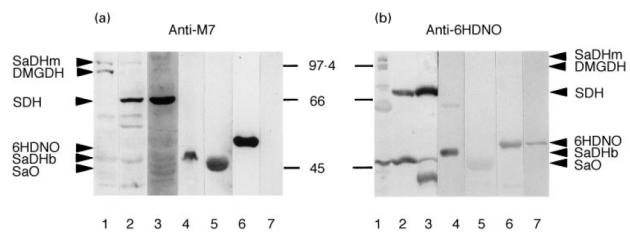
Mitochondrial antigens reactive with anti-M7 and anti-6-hydroxy-d-nicotine oxidase (6HDNO) sera. Rat liver mitochondrial matrix (lane 1), mitochondrial membrane (lane 2), bovine heart submitochondrial particles (HSMP) (lane 3), sarcosine dehydrogenase (SaDH) of Pseudomonas sp. (lane 4), sarcosine oxidase (SaO) of Bacillus subtilis (lane 5), 6HDNO (lane 6) and 6-hydroxy-l-nicotine oxidase (6HLNO) of Arthrobacter nicotinovorans (lane 7) were subjected to Western blot analysis with anti-M7 serum (a) and with anti-6HDNO serum (b). Arrowheads indicate the position of migration of the major antigens recognized by the antisera; the molecular weights of marker proteins are indicated between the two panels.
Identity of the mitochondrial antigens
To identify the predominant mitochondrial antigens recognized by anti-M7 and anti-6HDNO serum, two antisera were employed, one specifically established to the flavoprotein subunit of bovine SDH (mitochondrial membrane protein) and the other to DMGDH (mitochondrial matrix protein). Figure 2a shows that the antigen reacting with the anti-M7 and anti-6HDNO serum in the mitochondrial membrane was identical to the antigen recognized by anti-SDH serum. One of the high molecular weight bands of rat liver mitochondrial matrix corresponded to DMGDH (Fig. 2b). The second high molecular weight antigen most probably represents SaDHm, which migrates on SDS–PAGE as a 105-kD protein band [22]. This protein is not recognized as antigen by the DMGDH antiserum (Otto and Brandsch, unpublished observation). In agreement with previous reports [2], the anti-M7 and anti-6HDNO sera failed to recognize the two high molecular weight proteins in the matrix fraction of bovine heart mitochondria (data not shown), which is in line with the tissue-specific expression of DMGDH and SaDHm in the liver but not in the heart.
Fig. 2.
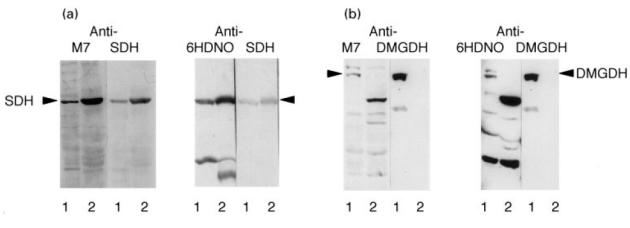
Identity of the mitochondrial antigens. (a) Rat liver mitochondrial membrane proteins (lane 1) and heart submitochondrial particles (HSMP) (lane 2) were analysed by Western blots decorated with anti-M7 serum. The blot was stripped and redecorated with an antiserum against succinate dehydrogenase (SDH). In parallel a Western blot with the same antigens was first decorated with anti-6-hydroxy-d-nicotine oxidase (6HDNO) serum, stripped and redecorated with anti-SDH serum. (b) Rat liver mitochondrial matrix (lane 1) and mitochondrial membrane (lane 2) proteins were analysed by Western blots and decorated first with anti-M7 serum, stripped and redecorated with anti- dimethylglycine dehydrogenase (DMGDH) serum. The same experiment is shown in the second half of the figure, except that the membrane was first decorated with anti-6HDNO serum, stripped and developed with anti-DMGDH serum.
Isolation of the anti-M7 antibody fraction
In order to evaluate the specificity of anti-M7 sera to flavoenzymes with covalently bound FAD, the marker serum was incubated with 6HDNO blotted onto a nitrocellulose membrane. An antibody fraction that specifically reacted with the mitochondrial and bacterial flavoenzymes could be isolated (Fig. 3a). No such antibody fraction was present in control sera from healthy people (Fig. 3b).
Fig. 3.
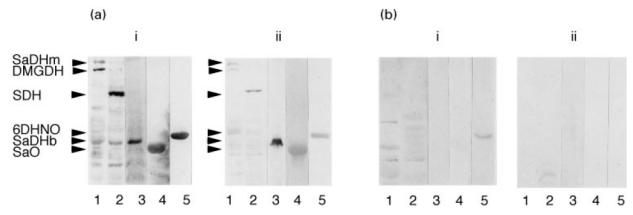
Isolation of the anti-M7 antibody fraction. The antigens tested in this experiment were rat liver mitochondrial matrix (lane 1), mitochondrial membrane (lane 2), sarcosine dehydrogenase from bacteria (SaDHb) (lane 3), sarcosine oxidase (SaO) (lane 4) and 6-hydroxy-d-nicotine oxidase (6HDNO) (lane 5). (a)i Western blot analysis with anti-M7 serum. (a)ii Incubation of the identical Western blot with the affinity-purified fraction of the anti-M7 serum. (b) Western blot developed with control serum (bi) and with the affinity-purified fraction of control serum (bii). The weak reaction shown in lane 5 (bi) was unspecific, since it disappeared after affinity purification.
FAD as antigenic determinant of anti-mitochondrial autoantibodies
To prove that the mitochondrial antigens were identified by the anti-M7 and anti-6HDNO serum because they carry covalently bound FAD, the reaction of the anti-M7 antibodies on Western blots with mitochondrial antigens was competed with FAD. Besides FAD, adenosine and adenosine nucleotides, which represent the moiety of the second nucleotide of FAD, as well as riboflavin were employed in the competition. As shown in Fig. 4, nanomolar concentrations of riboflavin and FAD, but not adenosine derivatives, were able to neutralize the anti-flavoenzyme antibodies of the anti-M7 sera. Incubation of antisera specific for different antigens with FAD did not affect the immune reaction (results not shown). FMN had the same effect as FAD and riboflavin. An affinity constant KD = 10−8 mwas determined for the anti-flavoenzyme antibodies by competitive ELISA. Apparently the riboflavin moiety of FAD represented the main mitochondrial antigenic determinant of the covalently flavinylated enzymes. In addition, incubation of the anti-M7 and anti-6HDNO serum with FAD–agarose abolished their anti-flavoenzyme activity (Fig. 5a,b), while agarose itself had no effect. Incubation of an anti-M2-positive PBC serum with FAD–agarose did not affect the anti-M2 activity.
Fig. 4.
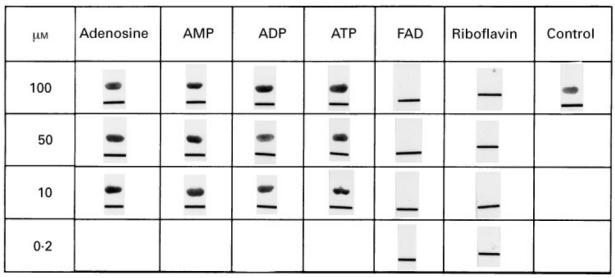
Inhibition of the immune reaction by incubation with FAD. Purified 6-hydroxy-d-nicotine oxidase (6HDNO) was blotted onto a nitrocellulose membrane and developed with anti-M7 serum preincubated overnight with different concentrations of adenosine, AMP, ADP, ATP, FAD and riboflavin. As control the anti-M7 serum was incubated without nucleotides overnight. To identify the protein-bound site the membrane was marked by a lane.
Fig. 5.
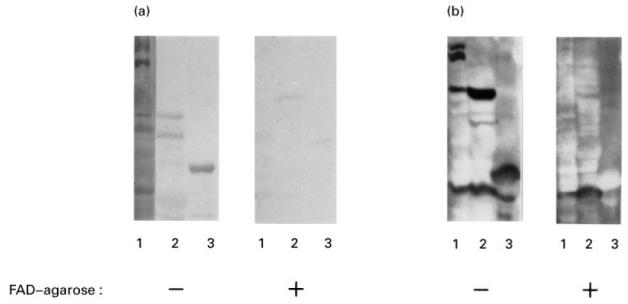
Depletion of the anti-flavoenzyme reactivity by incubation with FAD–agarose. (a) Rat liver mitochondrial matrix (lane 1), mitochondrial membrane proteins (lane 2) and 6-hydroxy-d-nicotine oxidase (6HDNO) (lane 3) were Western blotted and decorated with anti-M7 serum (−). The second part of the Western blot which carries the same pattern of proteins was decorated with an anti-M7 serum depleted of the anti-flavoenzyme fraction by incubation with FAD–agarose (+). (b) The same experiment as in (a) was performed with the anti-6HDNO serum. Lanes 1 and 2 show the mitochondrial matrix and membrane proteins and lane 3 sarcosine oxidase (SaO).
Immunoprecipitation of N-terminally truncated DMGDH proteins
The amino acid fingerprint sequence of the dinucleotide binding site of flavoenzymes is found in DMGDH2 between Gly49 and Gly54. The residue to which FAD is covalently attached is His85[17]. The dinucleotide binding site and His85 are present in DMGDH2, DMGDH1 and DMGDH3, but absent from DMGDH4. The N-terminally truncated DMGDH proteins were transcribed and translated in the rabbit RL in vitro system in the presence of 35S-methionine. The trypsin resistance of the translation products is an indication of the extent of holoenzyme formation, since incorporation of FAD present in the RL into DMGDH renders the protein protease-resistant [16]. Of the translation product of DMGDH1, DMGDH2 and DMGDH3, 20–40% adopted the trypsin-resistant holoenzyme conformation (Fig. 6a), but DMGDH4 formed no protease-resistant molecules. Immunoprecipitations under denaturing conditions revealed that only those proteins which were able to form holoenzyme were precipitated by the anti-M7 serum (Fig. 6b). Similarly, the 6HDNO His71 to Cys mutant protein which had lost the ability to bind FAD covalently was not precipitated by anti-M7 sera (results not shown). This is in accordance with the assumption that the protein-bound FAD is the major antigenic determinant of the anti-flavoenzyme antibodies of the anti-M7 sera. Attempts to demonstrate the formation of immunocomplexes by double diffusion techniques or to inhibit DMGDH and 6HDNO activity by anti-M7 sera were unsuccessful.
Fig. 6.
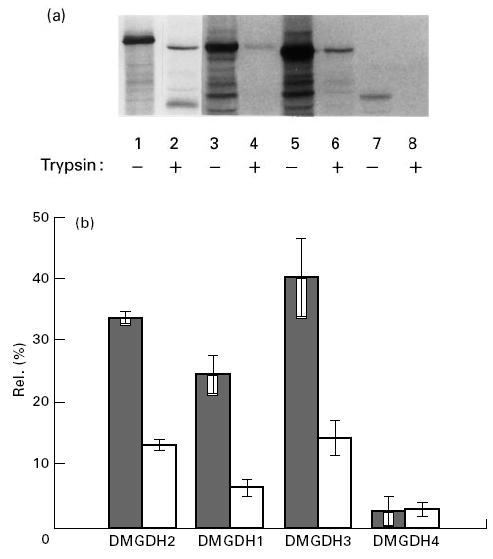
Trypsin digestion and immunoprecipitation of N-terminally truncated dimethylglycine dehydrogenase (DMGDH) proteins. (a) Coupled transcription-translation of DMGDH2 (lanes 1 and 2), DMGDH1 (lanes 3 and 4), DMGDH3 (lanes 5 and 6) and DMGDH4 (lanes 7 and 8) was carried out in rabbit reticulocyte lysate (RL) as described in Patients and Methods. After 3 h, 2 μl of the assays were subjected to trypsin digestion (lanes 2, 4, 6 and 8). The labelled proteins were separated by SDS–PAGE and autoradiographed. (b) In vitro labelled DMGDH proteins were immunoprecipitated under denaturing conditions with anti-M7 or control serum and analysed as in (a). The amount of precipitated proteins was expressed as percentage of DMGDH proteins immunoprecipitated with anti-DMGDH serum. Three independent experiments were performed. ▪, Anti-M7; □, control.
Anti-flavoenzyme antibodies detected by ELISA in anti-M7 sera
An ELISA with recombinant purified 6HDNO holoenzyme as antigen was performed with various sera (Fig. 7). The results of both methods, ELISA and Western blot, used to detect anti-M7-positive sera were consistent. Thirty-six percent anti-M7-positive sera were found in patients with dilated cardiomyopathy (n = 25) (Fig. 7, DCM) and 25% in patients with myocarditis (n = 12) (Fig. 7, myocarditis). Among patients suffering from cardiac diseases of other origin (n = 15), two were found to be anti-M7-positive (13%) (Fig. 7, OHD). One suffered from hypertrophic obstructive cardiomyopathy, the other from postpartale myopathy. No anti-M7-positive serum was detected in control sera (n = 50). In addition, no significant difference was observed in the concentration of the IgG fraction between sera from healthy and affected people. The anti-flavoenzyme antibodies of the anti-M7 sera belonged to the IgG class. No anti-M7 antibodies of the IgM type were found. When they were tested for subtypes on Western blots with MoAbs it was found that in addition to IgG1, 31% of the sera contained IgG2 and 38% contained IgG3. The IgG4 subtype was not detected.
Fig. 7.
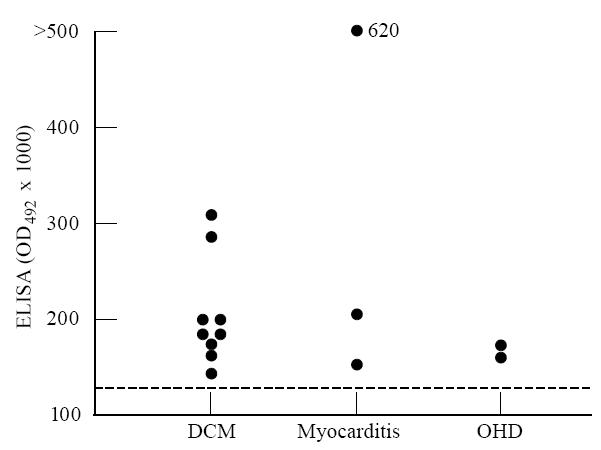
Activity of anti-flavoenzyme antibodies in patients with various heart diseases who were positive for anti-6-hydroxy-d-nicotine oxidase (6HDNO). ELISAs were performed with 6HDNO as antigen and sera from patients with dilated cardiomyopathy (DCM), myocarditis and other heart diseases (OHD) as described in Patients and Methods. The broken line represents the activity level in sera from 50 healthy controls. •, The result obtained with the serum from one patient.
Antibodies to the covalently flavinylated enzymes were confined to patients with myocarditis and dilated cardiomyopathy, and were not found in sera from patients suffering from various autoimmune and non-autoimmune disorders (data not shown).
DISCUSSION
In the present study it is shown that anti-M7 antibodies in sera from patients with acute and chronic heart diseases react with enzymes containing covalently bound FAD, and that the covalently bound FAD moiety is essential for antibody binding. This conclusion is supported by similar findings made with the antiflavin antibodies raised by FAD attached to albumin [23]. These antibodies failed to inhibit the enzyme activity of tested flavoenzymes. From the structure of many flavoenzymes it is known that the cofactor is buried in the protein in a hydrophobic pocket. It seems that the native conformation of the enzymes had to be unfolded and the FAD moiety to be exposed in order to be recognized by the anti-M7 antibodies. The quantitatively predominant antigen recognized by the anti-M7 sera in mitochondria was identified as the flavoprotein subunit of the succinate dehydrogenase complex. This is not surprising considering the large amount of the respiratory chain components in the inner mitochondrial membrane. No antigen corresponding to monoamine oxidase, which contains FAD bound to a cysteinyl residue, was recognized by the anti-M7 or anti-6HDNO sera. The reason could be the different type of FAD attachment. The report that the antiflavin antibodies generated against FAD–albumin showed a certain specificity for the type of FAD attachment to the protein supports this assumption. They reacted most strongly with the FAD–His complex [23]. When an antiflavin antiserum was induced with fumarate reductase, which contains FAD bound to a histidine residue, the antiserum reacted with enzymes containing a covalent FAD–histidyl complex, but not with a FAD–cysteinyl attached cofactor [24]. The specificity of the anti-flavoenzyme antibodies towards the nature of the FAD–amino acid complex may indicate that a conformational epitope of the flavinylated enzymes forms part of the antigenic determinant.
Riboflavin, the predominant flavin form in human serum, is transported in the blood at a concentration of ≈ 30 nm in a loosely associated form with albumin and in a more tightly bound form with immunoglobulins (see [25] for a review). However, the anti-flavoenzyme antibodies are not identical to the riboflavin-binding immunoglobulin fraction. A specific anti-flavoenzyme antibody fraction could be isolated from anti-M7 sera but not from sera of healthy individuals. In addition, the antiserum to 6HDNO raised in rabbits had the same immunological properties as human anti-M7 antibodies.
Our results revealed that at least one third of the sera from patients with defined dilated cardiomyopathy were anti-flavoenzyme-positive. One could argue that a peptide derived from a covalently flavinylated enzyme either of bacterial or endogenous origin acted as a true autoantigen or as neoantigen when presented to autoreactive T and B cells via antigen-presenting cells.
It is a well established phenomenon that epitopes being complexed with carrier proteins become immunogenic, as documented for instance in drug-induced human diseases, and good evidence for this concept are the clinical observations that patients with pseudolupus syndrome caused by the intake of Venocuran had anti-mitochondrial antibodies to an outer mitochondrial membrane antigen which disappeared when the offending drug was withdrawn [7,26]. Furthermore, several protein hapten components occur in bacterial and mammalian cells in the form of enzymes with covalently bound prosthetic groups. Thus, FAD in some flavoenzymes, lipoic acid in pyruvate dehydrogenase and 2-oxo-glutarate dehydrogenase, biotin in carboxylases and the heme group of cytochrome C are covalently attached to the apoenzyme. These enzyme-prosthetic group compounds may be of potential hazard to the organism and lead to a humoral response, as demonstrated by anti-mitochondrial antibodies to pyruvate dehydrogenase in PBC [6,27] and those against cytochrome C [28] and biotinylated proteins in human sera [29], and as shown in this work, by antibodies to flavinylated proteins.
The clinical relevance of anti-flavoenzyme antibodies remains obscure, and considering the fact of a strict organ-specific reaction a pathogenic role seems rather unlikely. The demonstration of anti-M7 of the IgG3 type may be interpreted in such a way that a certain T cell subpopulation is activated, inducing cytotoxic reactions, a characteristic phenomenon in organ-specific autoimmune disorders [30,31]. The identification of M7 at a molecular level provides new avenues in studying FAD-specific immune responses of autoreactive T cells in patients with dilated cardiomyopathy, hereby facilitating more refined immunological studies on the T cell level to obtain a better insight into the aetiopathogenesis of this chronic heart process of unknown aetiology.
Screening patients with acute myocarditis for this antibody type may be another diagnostic approach to recognize at early stages the transition to the chronic stages. The diagnostic specificity of anti-flavoenzyme antibodies of the IgG type is without question considering their lack in other autoimmune and non-autoimmune disorders as well as in healthy individuals. Furthermore, the screening of patients with acute and chronic heart diseases for these antibodies may help to identify patients with an underlying autoimmune condition.
Acknowledgments
The authors would like to thank Professor W. Kreisel and Dr A. van de Loo (University of Freiburg) for their support, and Professor B. Maisch (University of Marburg) for his interest in this work. This work was funded by grants of the Deutsche Forschungsgemeinschaft to R.B. and P.A.B.
References
- 1.Klein R, Maisch B, Kochisek K, Berg PA. Demonstration of organ specific antibodies against heart mitochondria (anti-M7) in sera from patients with some forms of heart diseases. Clin Exp Immunol. 1990;82:283–92. [PMC free article] [PubMed] [Google Scholar]
- 2.Klein R, Maisch B, Kochisek K, Berg PA. Demonstration of organ specific antibodies against heart mitochondria (anti-M7) in sera from patients with some forms of heart diseases. Clin Exp Immunol. 1984;48:283–92. [PMC free article] [PubMed] [Google Scholar]
- 3.Berg PA, Klein R. Antimitochondrial antibodies in primary biliary cirrhosis, other disorders: definition and clinical relevance. Dig Dis. 1992;10:85–101. doi: 10.1159/000171347. [DOI] [PubMed] [Google Scholar]
- 4.Chlumsky LJ, Zhang L, Schuman Jorns MJ. Sequence analysis of sarcosine oxidase and nearby genes reveals homologies with key enzymes of folate one-carbon metabolism. Biol Chem. 1995;270:18252–9. doi: 10.1074/jbc.270.31.18252. [DOI] [PubMed] [Google Scholar]
- 5.Berg PA, Klein R. Heterogeneity of antimitochondrial antibodies. Sem Liver Dis. 1989;9:103–16. doi: 10.1055/s-2008-1040501. [DOI] [PubMed] [Google Scholar]
- 6.Baum H. Mitochondrial antigens, molecular mimicry and autoimmune disease. Biochem Biophys Acta. 1995;1271:111–21. doi: 10.1016/0925-4439(95)00017-x. [DOI] [PubMed] [Google Scholar]
- 7.Schuff-Werner P, Berg PA. Immunreaktionen beim Pseudo-Lupus-Syndrom. Klin Wochenschr. 1980;58:935–41. doi: 10.1007/BF01477051. [DOI] [PubMed] [Google Scholar]
- 8.Harlow E, Lane D. A laboratory manual. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1988. Antibodies; pp. 92–135. [Google Scholar]
- 9.Conboy JG, Fenton WA, Rosenberg LE. Processing of pre-ornithine transcarbamylase requires a zinc-dependent protease localized to the mitochondrial matrix. Biochem Biophys Res Commun. 1982;105:1–7. doi: 10.1016/s0006-291x(82)80002-8. [DOI] [PubMed] [Google Scholar]
- 10.Smith AL. Preparation, properties, and conditions for assay of mitochondria. slaughterhouse material, small-scale. Methods Enzymol. 1976;10:81–86. [Google Scholar]
- 11.Berthold H, Scanarini M, Abney CC, Frorath B, Northemann W. Purification of recombinant antigenic epitopes of the human 68-kDa (U1) ribonucleoprotein antigen using the expression system pH6EX3 followed by metal chelating affinity chromatography. Prot Exp Purif. 1992;3:50–56. doi: 10.1016/1046-5928(92)90055-2. [DOI] [PubMed] [Google Scholar]
- 12.Hinkkanen A, Lilius E-M, Nowack J, Maas R, Decker K. Purification of the flavoproteins 6-hydroxy-D- and 6-hydroxy-L-nicotine oxidase using hydrophobic affinity chromatography. Hoppe-Seyler's Z Physiol Chem. 1983;364:801–6. doi: 10.1515/bchm2.1983.364.2.801. [DOI] [PubMed] [Google Scholar]
- 13.Sambrook J, Fritsch EF, Maniatis T. A laboratory manual. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1989. Molecular cloning. [Google Scholar]
- 14.Engvall E. Enzyme immunoassay ELISA and EMIT. Methods Enzymol. 1980;70:419–39. doi: 10.1016/s0076-6879(80)70067-8. [DOI] [PubMed] [Google Scholar]
- 15.Friguet B, Chaffotte AF, Djavadi-Ohaniance L, Goldberg ME. Measurements of the true affinity constant in solution of antigen–antibody complexes by enzyme-linked immunosorbent assay. J Immunol Methods. 1985;77:305–19. doi: 10.1016/0022-1759(85)90044-4. [DOI] [PubMed] [Google Scholar]
- 16.Otto A, Stoltz M, Sailer H-P, Brandsch R. Biogenesis of the covalently flavinylated mitochondrial enzyme dimethylglycine dehydrogenase. J Biol Chem. 1996;271:9823–9. doi: 10.1074/jbc.271.16.9823. [DOI] [PubMed] [Google Scholar]
- 17.Lang H, Polster M, Brandsch R. Rat liver dimethylglycine dehydrogenase. Flavinylation of the enzyme in hepatocytes in primary culture and characterization of a cDNA clone. Eur J Biochem. 1991;198:793–9. doi: 10.1111/j.1432-1033.1991.tb16083.x. [DOI] [PubMed] [Google Scholar]
- 18.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Decker K, Brandsch R. Flavoproteins with a covalent histidyl(N3)-8α-riboflavin linkage. BioFactors. 1991;3:69–81. [PubMed] [Google Scholar]
- 20.Dai D, Decker K, Sund H. Purification and properties of L-6-hydroxynicotine oxidase. Eur J Biochem. 1968;4:95–102. doi: 10.1111/j.1432-1033.1968.tb00177.x. [DOI] [PubMed] [Google Scholar]
- 21.Stoltz M, Henninger H-P, Brandsch R. The design of an alternative, covalently flavinylated 6-hydroxy-D-nicotine oxidase by replacing the FAD-binding histidine by cysteine and reconstruction of the holoenzyme with 8-(methylsulfonyl)FAD. FEBS Letters. 1996;386:194–6. doi: 10.1016/0014-5793(96)00438-3. [DOI] [PubMed] [Google Scholar]
- 22.Wittwer AJ, Wagner C. Identification of the folate-binding proteins of rat liver mitochondria as dimethylglycine dehydrogenase and sarcosine dehydrogenase. J Biol Chem. 1981;256:4102–8. [PubMed] [Google Scholar]
- 23.Barber MJ, Eichler DC, Solomonson LP, Ackrell BA. Anti-flavin antibodies. Biochem J. 1987;242:89–95. doi: 10.1042/bj2420089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robinson KM, Lemire BD. Flavinylation of succinate: ubiquinone oxidoreductase from Saccharomyces cerevisiae. Methods Enzymol. 1995;260:34–51. doi: 10.1016/0076-6879(95)60128-7. [DOI] [PubMed] [Google Scholar]
- 25.McCormick DB. Two interconnected B vitamins: riboflavin and pyridoxine. Physiol Rev. 1989;69:1170–98. doi: 10.1152/physrev.1989.69.4.1170. [DOI] [PubMed] [Google Scholar]
- 26.Pons C, Dansette PM, Gregeois J, Homberg JC, Billett EE, Mansuy D. Human anti-mitochondria autoantibodies appearing in iproniazid-induced immunoallergic hepatitis recognize human liver monoamine oxidase B. Biochem Biophys Res Commun. 1996;218:118–24. doi: 10.1006/bbrc.1996.0021. [DOI] [PubMed] [Google Scholar]
- 27.Berg PA, Klein R. Mitochondrial antigen/antibody systems in primary biliary cirrhosis: revisited. Liver. 1995;15:281–92. doi: 10.1111/j.1600-0676.1995.tb00687.x. [DOI] [PubMed] [Google Scholar]
- 28.Mamula MJ, Janeway CA. Do B-cells drive the diversification of immune responses? Immunol Today. 1993;14:104–6. doi: 10.1016/0167-5699(93)90274-O. [DOI] [PubMed] [Google Scholar]
- 29.Dale GL, Gaddy P, Pikul FJ. Antibodies against biotinylated proteins are present in normal humans. J Lab Clin Med. 1994;123:365–71. [PubMed] [Google Scholar]
- 30.Mosmann TR, Sad S. The expanding universe of T-cell subsets: TH1, TH2 and more. Immunol Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 31.Berg PA, Klein R, Röcken M. Cytokines in primary biliary cirrhosis. Sem Liver Dis. 1997;17:115–23. doi: 10.1055/s-2007-1007189. [DOI] [PubMed] [Google Scholar]


