Abstract
Impaired T cell function has been reported to predispose women to recurrent vulvovaginal candidiasis, but conflicting results have been noted in the literature. Most clinical episodes occur in the late luteal phase, suggesting hormonal influence on host resistance. The present study assesses the cellular immune responses of 28 women with recurrent vaginal candidiasis (patients) and 25 control women (controls), noting results in relation to whether the women were in the follicular or luteal phase of the menstrual cycle at the time of sampling. Candida-stimulated peripheral blood lymphocyte proliferation was significantly reduced in patients compared with controls. Interferon-gamma (IFN-γ) production in response to both Candida and purified protein derivative (PPD) stimulation was significantly lower in patients compared with controls. Skin test responses were comparable in both groups. A significant reduction in Candida-stimulated IFN-γ production was seen in patients but not controls in the follicular phase compared with those in the luteal phase. There was also a trend towards lower proliferation in response to Candida in patients but not controls in the follicular phase compared with patients in the luteal phase. These results suggest that there is a partial T cell dysregulation in recurrent vaginal candidiasis which may be exacerbated by the hormonal balance present during the follicular phase, correlating with the risk of clinical infection.
Keywords: cell-mediated immunity, Candida albicans, interferon-gamma, DTH, menstrual cycle
INTRODUCTION
Candidal vaginitis is a common mucosal infection principally caused by the opportunistic yeast-like fungus Candida albicans. While ∼ 75% of women will experience a single episode of candidal vaginitis in their lifetime, a significant percentage of these women (5%) will subsequently experience recurrent Candida infections [1]. The high incidence of mucosal candidiasis in immunocompromised individuals, such as those with AIDS and patients being treated with immunosuppressive agents [2,3] emphasizes the potential importance of cell-mediated immunity (CMI) in protection against mucosal C. albicans infections.
Reports from most studies of in vivo skin test reactivity suggest that women with recurrent vulvovaginal candidiasis (RVVC) are anergic to Candida antigens at least during a symptomatic infection, but that they do react to other antigens [4–6]. These results would support the hypothesis that there is specific impaired T lymphocyte function in RVVC, but the results of studies of in vitro CMI reactivity have yielded conflicting results. In some studies most women with RVVC had a significantly lower lymphoblastic response to C. albicans antigen than control women [7–9], while in other investigations they were found to have normal levels of in vitro responsiveness to Candida [4–6].
It has been suggested that factors associated with the various stages of the menstrual cycle may modulate local immune responses [10,11]. Studies in rats have found that endocrine changes during the reproductive cycle regulate the levels of IgA, secretory component and IgG in uterine and cervicovaginal secretions [12,13]. Oestradiol and progesterone were also shown to interact in the regulation of antigen presentation in the lower female reproductive tract [14]. It is possible that T cell function may be similarly influenced by reproductive hormones, as cytotoxic T lymphocyte (CTL) activity in human uterine T cells, measured in a redirected lysis assay, varies markedly depending on the stage of cycle [15]. In this study, we demonstrate significant differences in T cell function in the circulating T cell pool in women with RVVC.
PATIENTS AND METHODS
Subjects
The patient population consisted of 28 pre-menopausal women with at least a 2-year history of RVVC and a frequency of six or more episodes per year. Twenty-five healthy pre-menopausal women with no known history of allergy or candidal vaginitis made up the control group. Women with diabetes mellitus or on immunosuppressive medication were excluded from the study, as were those with other infections. Thirty-six percent of control women and 18% of patients were taking oral contraceptives. The patient group ranged in age from 19 to 47 years, with a mean age of 34 years. The age range of the control group was from 20 to 42 years with a mean age of 28 years. The mean age of the patients was statistically higher, but was not considered to be of physiological importance.
Skin testing
Delayed cutaneous skin test reactivity was tested by intradermal injection of 0.1 ml of fresh non-glycerinated C. albicans extract (Bayer Corp., Elkhart, IN) into the forearm. Neat C. albicans extract (1000 protein nitrogen units (PNU)/ml) and a 1:10 dilution of C. albicans extract in sterile saline (100 PNU/ml) were tested. Saline was injected as a control. Forty-eight hours later, the long and the short diameters of induration and erythema were measured and the average of these diameters was calculated.
Lymphocyte proliferative response to C. albicans, purified protein derivative and phytohaemagglutinin
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood by density gradient centrifugation using Ficoll–Paque (Pharmacia Biotech AB, Uppsala, Sweden). The proliferative response of PBMC to Candida, purified protein derivative (PPD) and phytohaemagglutinin (PHA) was detected by 3H-thymidine incorporation. Cells were resuspended at 1 × 106/ml in AIM V serum-free lymphocyte medium (Life Technologies, Mt Waverley, Australia) containing 5 × 10−5 m 2-mercaptoethanol (2-ME; Sigma-Aldrich, Castle Hill, Australia), 100 U/ml penicillin and 100 μg/ml streptomycin (Trace Biosciences, Castle Hill, Australia). Cells were added in quadruplicate to round-bottomed microtitre wells (100 μl/well). To each set of wells 100 μl of varying concentrations of Candida (Bayer), PPD (Commonwealth Serum Laboratories (CSL), Parkville, Australia) or PHA (Sigma-Aldrich) were added. The antigens were dialysed before use in culture. Control wells (unstimulated) received the same volume of tissue culture medium. Cultures were incubated at 37°C in 5% CO2 for 3 days with PHA and for 6 days with antigens. Six hours before the termination of each experiment each well was pulsed with 1 μCi 3H-thymidine (Amersham, Baulkham Hills, Australia). Cells were harvested onto glassfibre filters, and radioactivity incorporated into cellular DNA was measured in a liquid scintillation counter (Canberra-Packard Topcount microplate scintillation counter). Results are expressed as stimulation indices (SIs) (mean ct/min of stimulated wells/mean ct/min of unstimulated wells) to account for the variability in background proliferation among individuals.
Cytokine production
PBMC at 1 × 106/ml in AIM V were added to 24-well plates in a volume of 1 ml/well. To each well, 1 ml of PPD or Candida antigen was added to give final concentrations of 14.5 μg/ml PPD and 1.6 μg/ml Candida. Control wells received the same volume of tissue culture medium. After 6 days of culture, supernatants were collected by centrifugation and stored at −70°C. Interferon-gamma (IFN-γ) was detected by ELISA (CSL human γ interferon test) which can detect < 1 U/ml IFN-γ. IL-4 was measured using an ELISA (Biokine IL-4 Test Kit; T Cell Diagnostics, Inc., Woburn, MA), which has a sensitivity of 50 pg/ml of IL-4.
Statistical analysis
Results were compared using the non-parametric Mann–Whitney rank test. Associations between lymphoproliferative responses to Candida antigen and skin test results were examined using the Spearman rank correlation coefficient.
RESULTS
DTH to Candida antigen
There were no significant differences between patients and controls in skin test reactivity to Candida antigen (results not shown). Intermediate (5–10 mm) and positive (≥ 10 mm) responses to C. albicans antigen (1000 PNU/ml) totalled 56% in control subjects and 54% in RVVC patients. Intermediate and positive responses to C. albicans antigen at a concentration of 100 PNU/ml were also very similar in the two groups, totalling 20% in controls and 18% in RVVC patients. Only one subject, a member of the control group, showed signs of a type I skin response, defined as significant erythema developing within 15 min of C. albicans injection (data not shown).
Proliferative response to Candida, PPD and PHA
The level of proliferation in response to Candida (1.6 μg/ml and 0.4 μg/ml) was significantly lower in RVVC patients compared with controls (P < 0.003 and P < 0.03, respectively). There was no significant difference between patients and controls in their response to PPD at concentrations of 14.5 μg/ml, 3.6 μg/ml or 0.9 μg/ml. Figure 1 shows the proliferation of PBMC from patients and controls in response to stimulation with Candida (1.6 μg/ml) and with PPD (14.5 μg/ml). No significant difference was seen between patients and controls in their proliferation in response to PHA at any of the concentrations tested (10, 2.5 and 0.6 μg/ml) (results not shown).
Fig. 1.

Proliferation of peripheral blood mononuclear cells (PBMC) from recurrent vulvovaginal candidiasis (RVVC) patients (▪) and normal controls (□) in response to stimulation with Candida and purified protein derivative (PPD). Cells were incubated with Candida antigen (1.6 μg/ml) or PPD (14.5 μg/ml) for 6 days and proliferation was detected by 3H-thymidine incorporation. This graph shows the mean stimulation index (SI) + s.e.m. for each group. *P < 0.003.
There was low correlation between lymphocyte proliferation in response to Candida antigen and skin test responses to Candida antigen as assessed using the Spearman rank correlation coefficient, ρ. Correlation was somewhat higher in the control group than in the RVVC group (ρ = 0.403 for controls and 0.125 for RVVC subjects).
Cytokine production
The production of IFN-γ in response to Candida stimulation was lower in patients with RVVC compared with controls (P < 0.05). The level of IFN-γ produced in response to PPD stimulation was also significantly lower in patients compared with controls (P < 0.03) (Fig. 2). No significant difference was seen between patients and controls in the production of IFN-γ in response to stimulation with PHA (results not shown). IL-4 production in response to stimulation with Candida and with PPD was also measured by ELISA. The level of IL-4 detected was < 50 pg/ml in supernatants from both RVVC subjects and control subjects (results not shown).
Fig. 2.

IFN-γ produced in vitro by peripheral blood mononuclear cells (PBMC) from controls and recurrent vulvovaginal candidiasis (RVVC) patients in response to stimulation with (a) Candida antigen and (b) purified protein derivative (PPD). Cells were stimulated for 6 days with Candida (1.6 μg/ml) or PPD (14.5 μg/ml), after which supernatants were harvested for measurement of IFN-γ by ELISA. Results shown are mean + s.e.m. *P < 0.05; **P < 0.03.
Comparison of follicular and luteal phases of the menstrual cycle
Table 1 compares those patients in the follicular phase at the time of sampling with those in the luteal phase and compares controls in the follicular phase at the time of sampling with controls in the luteal phase. Skin test reactivity to Candida antigen was not significantly different between the two groups of patients. While the levels of proliferation detected in patients in the follicular and luteal phase in response to Candida at a concentration of 1.6 μg/ml were not significantly different, there was a trend towards a lower proliferative response to Candida in patients studied during the follicular phase (P = 0.052). The proliferative response of patients in the follicular phase to Candida antigen at concentrations of 0.4 μg/ml and 0.1 μg/ml was significantly lower than that of patients in the luteal phase (P < 0.008 and P < 0.02, respectively).
Table 1.
Recurrent vulvovaginal candidiasis (RVVC) patients and healthy controls studied during the follicular phase compared with those patients and controls studied in the luteal phase
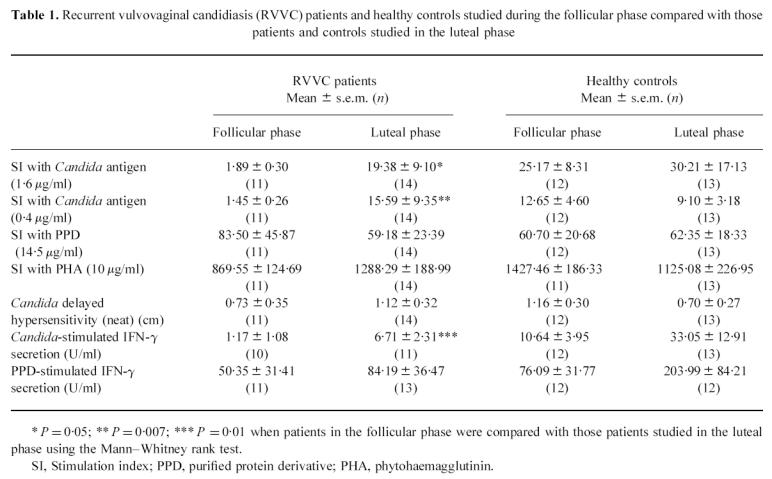
The level of IFN-γ produced in response to Candida stimulation was significantly lower in patients studied while they were in the follicular phase compared with those studied during the luteal phase (P = 0.01). No difference was seen between patients in the two phases in the levels of IFN-γ secreted in response to PPD stimulation or in levels of proliferation in response to PPD. Levels of IFN-γ produced by PBMC from patients in the follicular and luteal phases in response to stimulation with Candida and PPD are shown in Fig. 3.
Fig. 3.

IFN-γ produced in vitro by peripheral blood mononuclear cells (PBMC) from recurrent vulvovaginal candidiasis (RVVC) patients in the follicular phase (days 1–15) and in the luteal phase (days 16–30) in response to stimulation with (a) Candida antigen and (b) purified protein derivative (PPD). Cells were stimulated for 6 days with Candida (1.6 μg/ml) or PPD (14.5 μg/ml), after which supernatants were harvested for measurement of IFN-γ by ELISA. Results shown are mean + s.e.m. *P = 0.010; **P = 0.077.
There were no significant differences between the controls in the follicular phase compared with controls studied in the luteal phase for any of the parameters examined.
DISCUSSION
In this study of subjects with RVVC, we assessed T lymphocyte function in the circulating T cell pool and demonstrated no significant differences in skin test reactivity to Candida antigen, but a reduction in T cell proliferation and IFN-γ production in response to stimulation with Candida. Subjects with RVVC tested during the follicular phase of the menstrual cycle selectively had less proliferation and reduced IFN-γ production in response to Candida antigen when compared with those studied during the luteal phase.
There is considerable conflict in the literature as it relates to T lymphocyte function in RVVC. In selecting patients for this type of study it is important to identify those patients who truly have a propensity to recurrent Candida infection. In addition, any effect of the contraceptive pill needs to be assessed, and subjects with complicating infection with additional pathogens need to be excluded. Patients in the present study had at least a 2-year history of Candida infections and a frequency of six or more episodes a year. They did not have concurrent infection with other pathogens and their menstrual cycle details were noted.
In this study RVVC patients were found to have reduced Candida-stimulated T cell proliferation, but responses to PHA and PPD were similar to normals, suggesting a Candida-specific defect. The reduced IFN-γ production by RVVC patients' cells in response to Candida is consistent with the proliferation defect, but significant reductions in IFN-γ secretion from PPD-stimulated T cells were also seen, suggesting that a broader T cell defect may exist. Alternatively, this could indicate that RVVC patients tend to mount more of a Th2 response to Candida and mycobacterial antigens. Such a polarization of T cell responses is well documented in the case of Mycobacterium leprae infection, where subjects with lepromatous leprosy mount strong Th2 responses (IL-4, IL-5 and IL-10), while those with tuberculoid leprosy mount strong Th1 responses (IL-2 and IFN-γ) [16]. However, in the present study, levels of IL-4 produced by PBMC in response to stimulation with Candida, PPD or PHA were below the level of detection of the assay in both RVVC subjects and controls.
The finding of a reduction in Candida-specific lymphocyte proliferation in RVVC patients is in agreement with the results of the studies of Witkin et al. [9]. However, other groups have reported that lymphocyte transformation [4–6], leucocyte migration inhibition [4], and Th1 type lymphokine production [6] responses to Candida antigen by RVVC patients were not significantly different from responses seen in normal controls.
There is a long-standing impression that subjects with RVVC have selective anergy to Candida, though recent studies suggest that anergy is restricted to a time of acute infection [6]. In contrast to previous reports, we found no significant difference between patients and controls in response to skin testing with Candida antigen. Ten of the patients were studied while infected with Candida, and of these only six (60%) had negative skin test responses to neat (1000 PNU/ml) C. albicans extract. There was low correlation between skin test results and in vitro lymphocyte proliferative responses to Candida antigen. Correlation was found to be somewhat higher in controls than in RVVC subjects.
An important clinical observation in subjects with RVVC is the regular recurrence of episodes of infection in the immediate pre-menstrual period [17], a time of low oestrogen levels. In the early studies that demonstrated participation of the female reproductive tract in the common mucosal immune system, migration of plasma cell precursors into the mucosa was found to be oestrogen-dependent [18]. If T cell migration into mucosal sites was controlled in a similar way, clinical episodes of vaginitis in women with a reduced pool of Candida-specific T lymphocytes would predictably occur when protective T lymphocytes are least able to enter the mucosa.
The influence of the stage of the menstrual cycle on PBMC proliferation and production of IFN-γ in response to Candida stimulation was examined and it was found that both responses were reduced in patients studied while they were in the follicular phase compared with patients studied during the luteal phase of the cycle. However, the differences in the cellular immune response to Candida seen in the two phases were not observed in the control group. These results suggest that there is a reduced T cell pool in subjects with RVVC which is influenced by the hormonal balance, so that recruitment into the reproductive tract (the site of infection) may be impaired during the luteal phase, the time infection is most likely to occur. The lack of differences in skin test results between subjects in the follicular and luteal phase indicates that recruitment of Candida-reactive T lymphocytes into the skin is not dependent on the levels of sex hormones.
These results contrast with the results of a study of six healthy women by Kalo-Klein & Witkin, which found that proliferation of PBMC to C. albicans was decreased during the luteal phase of the menstrual cycle, when progesterone levels were high and the level of oestradiol was decreasing [19]. Weekly fluctuations in proliferative responses to Candida were much less obvious in women taking oral contraceptives, suggesting that it was the changing levels of oestradiol and progesterone during the menstrual cycle that influenced the response to Candida [19].
The incidence of acute episodes of Candida vaginitis does seem to be influenced by changes in hormone levels, particularly levels of oestrogen. Menarchal females are most at risk of Candida vaginitis, while the incidence rate drops significantly in post-menopausal women, with the exception of those on oestrogen hormone replacement therapy [20]. An increased incidence of infection in pregnant women and women on oral contraceptives with high oestrogen content has been reported [21]. There is a requirement for pseudoestrus in animal models of vaginitis [22]. Oestrogen has also been shown to reduce cutaneous skin test reactivity, impair the activity of natural killer cells and suppress the action of neutrophils [23,24]. In addition, it has been reported that oestrogen can regulate the IFN-γ promoter [25].
Progesterone has also been shown to be immunosuppressive, possibly by inhibiting monocyte function. Candida-specific peripheral blood lymphocyte proliferation was found to be ∼ 50% lower in the presence of luteal-phase levels of progesterone when compared with proliferation measured in the presence of follicular phase levels of progesterone [26]. This effect was abrogated when monocytes were removed from the cultures, suggesting that progesterone inhibited lymphocyte proliferation through a monocyte-dependent mechanism [26]. The latter study involved healthy subjects with no history of Candida vaginitis, and follicular-phase or luteal-phase levels of oestrogen were not found to be inhibitory.
In addition to effects on the host, reproductive hormones may directly influence C. albicans. An oestrogen-binding protein with high affinity for oestradiol and oestrone has been found in C. albicans [27]. Oestradiol has been shown to directly stimulate the transition of C. albicans from the yeast form to the more invasive hyphal form [28]. Candida albicans has also been found to have a corticosteroid-binding protein that shows high affinity for corticosterone and progesterone [29,30].
In conclusion, we have shown that RVVC patients have reduced Candida-specific T cell function (both proliferation and IFN-γ secretion) compared with controls. Further, the magnitude of this defect is influenced in patients by hormonal changes occurring during the menstrual cycle. These changes could predispose individuals to RVVC at three levels: (i) reduced recruitment of T cells into the vaginal mucosa during infection; (ii) reduced effector function—possibly decreased CMI due to production of Th2 cytokines; and (iii) hormonal modulation of the pathogen in favour of the more pathogenic hyphal form. While not mutually exclusive, further studies of the local vaginal immune response are needed to define the immune dysfunction in RVVC. These studies should take into consideration the hormonal status of the women.
Acknowledgments
This work was supported by the Australian Institute of Mucosal Immunology. Trial co-ordination and sample collection were performed by Felicity Miners, Karin Black and Marianne Schreuder.
References
- 1.Hurley R. Recurrent candida infection. Clin Obstet Gynecol. 1981;8:209–13. [PubMed] [Google Scholar]
- 2.Klein RS, Harris CA, Small CB, et al. Oral candidiasis in high-risk patients as the initial manifestation of the acquired immunodeficiency syndrome. N Engl J Med. 1984;311:354–8. doi: 10.1056/NEJM198408093110602. [DOI] [PubMed] [Google Scholar]
- 3.Clift RA. Candidiasis in the transplant patient. Am J Med. 1984;77(Suppl. 4D):34–38. [PubMed] [Google Scholar]
- 4.Syverson RE, Buckley H, Gibian J, Ryan GM. Cellular and humoral immune status in women with chronic Candida vaginitis. Am J Obstet Gynecol. 1979;134:624–7. doi: 10.1016/0002-9378(79)90641-0. [DOI] [PubMed] [Google Scholar]
- 5.Fong IW, McCleary P, Read S. Cellular immunity of patients with recurrent or refractory vulvovaginal moniliasis. Am J Obstet Gynecol. 1992;166:887–90. doi: 10.1016/0002-9378(92)91356-f. [DOI] [PubMed] [Google Scholar]
- 6.Fidel PL, Jr, Lynch ME, Redondo-Lopez V, et al. Systemic cell-mediated immune reactivity in women with recurrent vulvovaginal candidiasis. J Infect Dis. 1993;168:1458–65. doi: 10.1093/infdis/168.6.1458. [DOI] [PubMed] [Google Scholar]
- 7.Hobbs JR, Briden D, Davidson F, et al. Immunological aspects of candidal vaginitis. Proc R Soc Med. 1977;70:11–14. doi: 10.1177/00359157770700S404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Witkin SS, Yu IR, Ledger WJ. Inhibition of Candida albicans-induced lymphocyte proliferation by lymphocytes and sera from women with recurrent vaginitis. Am J Obstet Gynecol. 1983;147:809–11. doi: 10.1016/0002-9378(83)90044-3. [DOI] [PubMed] [Google Scholar]
- 9.Witkin SS, Hirsch J, Ledger WJ. A macrophage defect in women with recurrent Candida vaginitis and its reversal in vitro by prostaglandin inhibitors. Am J Obstet Gynecol. 1986;155:790–5. doi: 10.1016/s0002-9378(86)80022-9. [DOI] [PubMed] [Google Scholar]
- 10.Mathur S, Mathur RS, Dowda H, et al. Sex steroid hormones and antibodies to Candida albicans. Clin Exp Immunol. 1978;33:79–87. [PMC free article] [PubMed] [Google Scholar]
- 11.Wira CR, Stern JE. Endocrine regulation of the mucosal immune system in the female reproductive tract. Control of IgA, IgG and secretory component during the reproductive cycle, at implantation and throughout pregnancy. In: Pasqualini JR, Scholler R, editors. Hormones and fetal pathophysiology. New York: M. Dekker; 1992. pp. 343–67. [Google Scholar]
- 12.Wira CR, Sandoe CP. Sex steroid hormone regulation of IgA and IgG in rat uterine secretions. Nature. 1977;268:534–6. doi: 10.1038/268534a0. [DOI] [PubMed] [Google Scholar]
- 13.Wira CR, Sullivan DA. Estradiol and progesterone regulation of immunoglobulin A and G and secretory component in cervicovaginal secretions of the rat. Biol Reprod. 1985;32:90–95. doi: 10.1095/biolreprod32.1.90. [DOI] [PubMed] [Google Scholar]
- 14.Wira CR, Rossoll RM. Antigen-presenting cells in the female reproductive tract: influence of sex hormones on antigen presentation in the vagina. Immunology. 1995;84:505–8. [PMC free article] [PubMed] [Google Scholar]
- 15.Wira CR, White H, Yeaman GR. Endocrine regulation of mucosal immunity in the human female reproductive tract. Immunol Cell Biol. 1997;75(Suppl. 1):A10. doi: 10.1111/j.1600-0897.1997.tb00190.x. (Abstr.) [DOI] [PubMed] [Google Scholar]
- 16.Modlin RL. Th1-Th2 paradigm: insights from leprosy. J Invest Dermatol. 1994;102:828–32. doi: 10.1111/1523-1747.ep12381958. [DOI] [PubMed] [Google Scholar]
- 17.Kent HL. Epidemiology of vaginitis. Am J Obstet Gynecol. 1991;165:1168–76. doi: 10.1016/s0002-9378(12)90722-x. [DOI] [PubMed] [Google Scholar]
- 18.McDermott MR, Clark DA, Bienenstock J. Evidence for a common mucosal immunologic system II. Influence of the estrous cycle on B lymphoblast migration into genital and intestinal tissues. J Immunol. 1980;124:2536–9. [PubMed] [Google Scholar]
- 19.Kalo-Klein A, Witkin SS. Candida albicans: cellular immune system interactions during different stages of the menstrual cycle. Am J Obstet Gynecol. 1989;161:1132–6. doi: 10.1016/0002-9378(89)90649-2. [DOI] [PubMed] [Google Scholar]
- 20.Sobel JD. Candidal vulvovaginitis. Clin Obstet Gynecol. 1993;36:153–65. doi: 10.1097/00003081-199303000-00021. [DOI] [PubMed] [Google Scholar]
- 21.Odds FC. Factors that predispose the host to candidosis. In: Odds FC, editor. Candida and candidosis. Baltimore: University Park Press; 1979. pp. 74–91. [Google Scholar]
- 22.Sobel JD, Muller G, McCormick JF. Experimental chronic vaginal candidosis in rats. Sabouraudia. 1985;23:199–206. doi: 10.1080/00362178585380301. [DOI] [PubMed] [Google Scholar]
- 23.Carlsten H, Holmdahl R, Tarkowski A. Analysis of the genetic encoding of oestradiol suppression of delayed-type hypersensitivity in (NZB x NZW) F1 mice. Immunology. 1991;73:186–90. [PMC free article] [PubMed] [Google Scholar]
- 24.Styrt B, Sugarman B. Estrogens and infection. Rev Infect Dis. 1991;13:1139–50. doi: 10.1093/clinids/13.6.1139. [DOI] [PubMed] [Google Scholar]
- 25.Fox HS, Bond BL, Parslow TG. Estrogen regulates the IFN-γ promoter. J Immunol. 1991;146:4362–7. [PubMed] [Google Scholar]
- 26.Kalo-Klein A, Witkin SS. Regulation of the immune response to Candida albicans by monocytes and progesterone. Am J Obstet Gynecol. 1991;164:1351–4. doi: 10.1016/0002-9378(91)90712-z. [DOI] [PubMed] [Google Scholar]
- 27.Skowronski R, Feldman D. Characterization of an estrogen-binding protein in the yeast Candida albicans. Endocrinology. 1989;124:1965–72. doi: 10.1210/endo-124-4-1965. [DOI] [PubMed] [Google Scholar]
- 28.Kinsman OS, Pitblado K, Coulson CJ. Effect of mammalian steroid hormones and luteinizing hormone on the germination of Candida albicans and implications for vaginal candidosis. Mycoses. 1988;31:617–26. doi: 10.1111/j.1439-0507.1988.tb04416.x. [DOI] [PubMed] [Google Scholar]
- 29.Loose DS, Schurman DJ, Feldman D. A corticosteroid binding protein and endogenous ligand in C. albicans indicating a possible steroid-receptor system. Nature. 1981;293:477–9. doi: 10.1038/293477a0. [DOI] [PubMed] [Google Scholar]
- 30.Loose DS, Feldman D. Characterization of a unique corticosterone binding protein in Candida albicans. J Biol Chem. 1982;257:4925–30. [PubMed] [Google Scholar]


