Abstract
The effectiveness of polyvalent plasma-derived human immunoglobulins (IVIG) in passive immunotherapy of influenza virus pneumonia was assessed, using the Strain Scotland (A/Scotland/74 (H3N2)) adapted to BALB/c mice by repeated lung passages. Haemagglutinin antibodies in two batches of IVIG at 10 mg/ml had a titre of 1/16. Intravenous injection of 1000–5000 μg of IVIG, 3 h after infection, gave 60–70% protection, whereas intranasal injection of 25–50 μg protected 90% of mice infected with a lethal dose of influenza virus. F (ab′)2 fragments were at least as protective as intact IVIG, suggesting that complement or Fcγ receptor-bearing cells were not required. Topical passive immunotherapy with IVIG or F(ab′)2 gave protection up to 8 h after infection, but not at 24 h, suggesting that anti-influenza A antibodies in IVIG, delivered locally, are only effective at early stages of the infectious process. The potential value of topical administration of IVIG or F(ab′)2 fragments for influenza A pneumonia prophylaxis was further demonstrated by the protective effects of their intranasal administration 24 h before challenge.
Keywords: influenza A virus pneumonia, immunoprophylaxis, mouse model
INTRODUCTION
Protective immune responses to influenza virus pneumonia include both cell-mediated immunity involving cytolytic T cells [1,2], and neutralizing antibodies [3–6]. Specific antibodies directed against the viral haemagglutinin passively protect experimentally infected mice [3–7]. Human IgG for intravenous use (IVIG) prepared from pools of plasma from healthy adult donors are used to prevent and treat a variety of communicable infections, because of their wide-spectrum neutralizing activity against common infective agents [8,9]. They are usually administered intravenously, but they have been shown to be effective when administered topically [10–12].
In this study a mouse-adapted influenza A virus strain was used to induce influenza pneumonia in BALB/c mice. We evaluated the effectiveness of IVIG or their F(ab′)2 fragments in preventing and curing viral infection at various times during the early infectious process.
MATERIALS AND METHODS
Viruses
The influenza A virus strains used were obtained from the Collection of the French National Reference Centre for Influenza (Institut Pasteur, Paris). The strain, A/Scotland/20/74 (H3N2), originating from a lyophilized stock of a culture in the allantoic cavity of embryonated hen's egg, was adapted to BALB/c mice by serial lung passages and recovery from lung homogenates until it caused 100% influenza virus pneumonia. This was assessed by detecting lobar haemorrhagic foci from day 4, under experimental conditions similar to those used for the mouse-adapted strain, PR8 (A/PR/8/34 (H1N1)) [4–7], which we used as a reference. A stock of virus was made from lung homogenates in 30% glycerol and stored at −80°C. Inocula for infectious challenges were prepared from the virus stock after one passage in a mouse and recovery from the supernatants of lung homogenates, at day 4 before each experiment. Virus titres were determined from 50 μl of the supernatants of lung homogenates centrifuged at 700 g for 15 min, serially diluted 1:10 in minimal essential medium (MEM; Eurobio, Les Ulis, France) on Madin Darby canine kidney (MDCK) cells grown in MEM containing 5% fetal calf serum (FCS; Bio-Media, Boussens, France) in flat-bottomed 96-well microtitre plates, in duplicate. The cells were incubated overnight at 37°C in humidified air/5% CO2 and the culture medium was then replaced with MEM containing 2 μg/ml TPCK-treated trypsin (Worthington Biochemical Corp. Freehold, NJ). The influenza virus in each culture was titrated by checking the haemagglutinin activity (HA) of the supernatants after 3 days of incubation, using a 1% suspension of chicken erythrocytes (Technique Biologique, Paris, France). Virus titres are expressed as the log10 reciprocal end-point dilution of the preparation causing 50% HA (TCID50).
Mice
Five-week-old female BALB/c mice (Centre d'Élevages René Janvier, Le Genest St Isles, France) were kept in a biosafety containment facility in groups of five, in filter-topped cages with sterile litter, water and food. Influenza pneumonia was induced by intranasal administration of 50 μl of viral inoculum standardized to about 1000 × TCID50 (about ten 50% lethal doses by day 20) in mice lightly anaesthetized with sodium pentobarbital (Sanofi, Santé Animale, Libourne, France). The survival of the challenged mice (groups of 10 mice per experiment per assay) was scored each day for 20 days.
Immunoglobulins and F(ab′)2 fragments
IVIG lots 500 750 20 and 500 353 11 were obtained from Biotransfusion (Les Ulis, France). According to the manufacturer, IVIG, obtained by cold ethanol precipitation and pepsin treatment at pH 4 following the criteria and recommendations of the International Union of Immunological Societies and the World Health Organization [13], consists of 98% IgG, including 70% IgG, 20% IgG2, 8% IgG3 and < 2% IgG4. The certificate of analysis of the lots tested stated that: they were 95.4% pure, as assessed by electrophoresis; they were stabilized with 34 g/l sucrose; the final preparation contained 7.75 g/l glucose, 2.5 mg/l pepsin and 9.65 mg/ml glycine. F(ab′)2 fragments from IVIG lot 500 353 11 were obtained from the Laboratoire Français du Fractionnement et des Biotechnologies (Les Ulis, France) by pepsin digestion and purification using Staphylococcus aureus protein A-Sepharose chromatography (Affi-gel Hz immunoaffinity kit; Biorad, Ivry-sur-Seine, France). Their purity and homogeneity were tested by SDS–PAGE. Stock preparations at concentrations of 50 mg/ml for IVIG and 30 mg/ml for F(ab′)2 in PBS (Sigma, Saint Quentin-Fallavier, France) were stored at −80°C until required.
Haemagglutination inhibition titration
IVIG and their F(ab′)2 fragments were tested for antibodies to the influenza virus strains Scotland and PR8 by haemagglutination inhibition (HI) using a viral suspension recovered by centrifugation at 1000 g of a culture from the allantoic cavity of an embryonated egg. HI was also assessed using commercial HA antigens (0.1% formaldehyde-inactivated virus) from influenza virus types A (Eurobio, reference 906013 strain A/Kumamoto/22/7 (H3N2)) or B (Eurobio, reference 906014 strain B/Kanagawa/3/76), according to the manufacturer's instructions. IVIG was treated to eliminate non-specific HI inhibitors by adding 0.6 ml of receptor-destroying enzyme (neuraminidase from Vibrio cholerae 4Z) (Eurobio) to 0.2 ml of IVIG, incubating the mixture overnight at 37°C, and heating the mixture at 56°C for 30 min. Anti-chicken erythrocyte agglutinins were adsorbed to 5% erythrocytes by incubation at room temperature for 1 h. HI was assessed using 25 μl each of a series of IVIG dilutions 1:2, into round-bottomed polystyrene microtitre plates, and 25 μl of HA antigen, standardized at 4 haemagglutination units (HAU) by haemagglutination titration, were added [14]. The mixture was incubated for 1 h at room temperature, 50 μl of 1% chicken erythrocytes were added and the plate was gently shaken. The HI titre was recorded after incubation for 1 h at room temperature and is expressed as the reciprocal of the IVIG dilution that inhibited haemagglutination.
Passive immunotherapy with IVIG and its F(ab′)2 fragments
IVIG and its F(ab′)2 fragments were diluted in PBS and administered to mice at the desired concentrations either intravenously (250 μl in a tail vein) or intranasally (50 μl in mice anaesthetized with sodium pentobarbital (Sanofi)) at the stated times before or after intranasal challenge with the virus. The effectiveness of the passive immunotherapies was assessed by the survival of mice following a lethal challenge, and by virus titrations in lung homogenates (the trachea and the main bronchia were omitted), of mice challenged with an inoculum of about 100 TCID50, equivalent to LD50 by day 20.
A monoclonal human IgG1 anti-human erythrocyte rhesus D antigen (RhD) (provided by J. Bartholeyns, Laboratoire Français du Fractionnement et des Biotechnologies), known to bind efficiently to Fcγ receptor I [15], was used as an unrelated human IgG control in one experiment.
In a series of three independent control experiments, we compared the effectiveness of IVIG (lot 500 35 311) with that of IVIG from which HI antibodies were removed by immunoabsorption to the HA antigen of the strain A/Scotland/74 (H3N2) and to the commercial HA antigens (Eurobio) of the strain A/Kumamoto/22/76 (H3N2) or B/Kanagawa/3/76 adjusted to 4 HAU in PBS. HA antigens were mixed with 1% chicken erythrocytes in PBS. The mixture was incubated for 1 h at room temperature. The agglutinated erythrocytes were then washed once by centrifugation at 700 g for 10 min and were suspended in 16.3 mg/ml IVIG in PBS. A control IVIG preparation, at the same concentration, was mixed with non-sensitized chicken erythrocytes and incubated for 1 h at room temperature. The erythrocytes were sedimented by centrifugation as above, the supernatants were collected and their protein concentration (biuret automated method, SYS1 BM/Hitachi 704; Boehringer, Mannheim, Germany) and the HI of the corresponding antigen determined. Passive intranasal immunotherapy was assayed 2 h after lethal intranasal challenge with the strain Scotland using 50 μg of IVIG (unbound or preadsorbed onto HA antigen) per mouse.
RESULTS
HI titration
Both lots of IVIG and the F(ab′)2 fragments derived from lot 50035311 adjusted to a concentration of 10 mg/ml had a mean HI titre of 1/16 for the Scotland HA antigen, but no detectable HI for PR8, suggesting that the HA antibodies in IVIG recognize type H3 but not H1. With commercial HA antigens, we measured an HI titre of 1/8 against influenza A and 1/64 for influenza B in both IVIG and F(ab′)2 fragments.
Effectiveness of passive immunotherapy with IVIG administered intravenously or intranasally
We first assayed the protective effects against lethal doses of influenza pneumonia (1000 TCID50) by i.v. injection of 100, 500, 1000 or 5000 μg IVIG/mouse using PBS as a control, 3 h after infection. One thousand and 5000 μg of IVIG were required to give 60% and 70% protection, respectively (Fig. 1a). Forty to 100 times less IVIG (25–50 μg) was required to give 90% protection with intranasal than with i.v. injection 3 h after infection (Fig. 1b). This shows that topical administration of IVIG was very effective against early pulmonary infection with influenza virus.
Fig. 1.
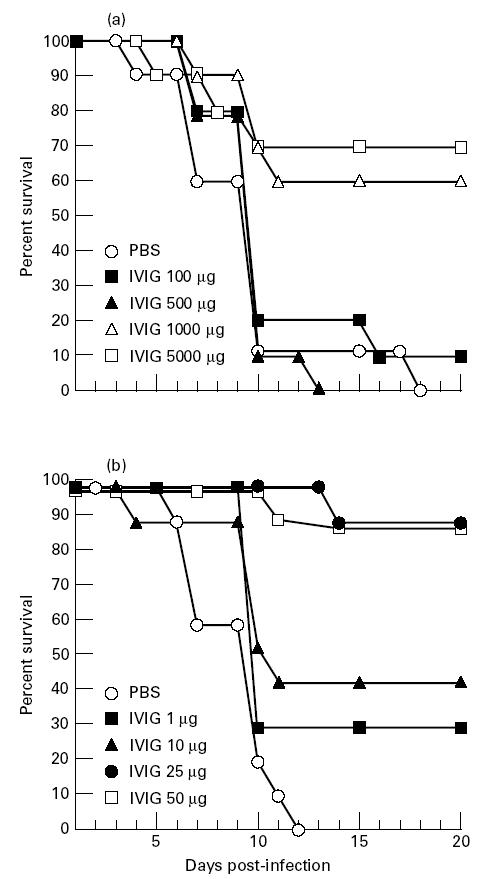
Efficacy of passive immunotherapy of influenza A virus pneumonia by i.v. injection of IVIG at doses of 100, 500, 1000 and 5000 μg/mouse (a) or intranasal administration of IVIG at doses of 1, 10, 25, 50 μg/mouse (b) at 3 h after intranasal challenge with the influenza A strain Scotland. Data are the means of two independent experiments, for each route of IVIG administration, using each lot of IVIG, with 10 mice per group in each experiment.
Efficacy of passive immunotherapy with IVIG or their F(ab′)2 fragments at various stages of infection
Mice were injected intranasally with either 50 μg of intact IVIG or 50 μg of F(ab′)2 2, 8 or 24 h after infection, to check whether topical IVIG neutralizes influenza virus pneumonia at later stages of infection, and whether protection was Fc-dependent. Passive protection against lethal influenza pneumonia was obtained with an intranasal dose of 50 μg IVIG or F(ab′)2 per mouse, but only at 2 h and 8 h after infection (Fig. 2). Infected mice treated 24 h after infection died at the same rate as mice treated with PBS. Thus, topical administration of IVIG or their F(ab′)2 fragments was effective against early influenza virus infection, but did not cure established influenza virus pneumonia. Protective effects were also evaluated by assessing differences in the lung virus titres of groups of mice challenged with a sublethal inoculum about 100 TCID50, and killed by an overdose of pentobarbital on day 3, at the stage when gross lobar pneumonia lesions were forming, or on day 9, corresponding to 90% lethality, in lethal challenges with 1000 TCID50 (Figs 1 and 2). Virus clearance in IVIG- and F(ab′)2-treated mice was highly effective (Table 1), because values were below the threshold of the assay by the third day after day 3 infection and treatment.
Fig. 2.
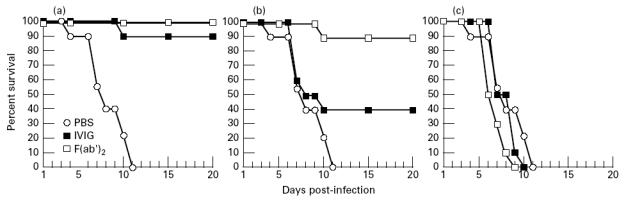
Efficacy of IVIG (lot 500 353 11) or their F(ab′)2 fragments, administered intranasally (50 μg/mouse) against influenza A virus pneumonia, 2 h (a), 8 h (b) or 24 h (c) after intranasal challenge with the influenza A strain Scotland. Data for IVIG- and F(ab′)2-treated mice are the means of two independent experiments with 10 mice per group. Data for PBS controls are the means of four experiments with 10 mice per group.
Table 1.
Effectiveness against influenza A virus of IVIG and F(ab′)2

Control experiments with anti-influenza negative IgG antibodies
To assess the need for antibodies in IVIG directed specifically against influenza A virus, we compared the effectiveness of intranasal administration of 50 μg of IVIG or the unrelated control, anti-RhD human IgG1, against influenza pneumonia 3 h after lethal challenge. Only IVIG-treated mice survived (9/10), whereas all mice treated with anti-RhD human monoclonal IgG1 or PBS died within 12 days (data not shown). Preabsorption of IVIG onto 4 HAU of HA antigen from either the strain Scotland or commercial HA antigen preparations from the strains A/Kumamoto/22/76 (H3N2) or B/Kanagawa/3/76, completely abolished the HI properties of the remaining IgG. Protein concentration was 2–10% lower in these three preabsorption assays, whereas it was < 1% lower for the control preparation of IVIG incubated with non-sensitized erythrocytes. Passive intranasal immunotherapy assays were performed with 50 μg per mouse of the various IVIG preparations. The efficacy of IVIG, preabsorbed onto the HA antigen from the strain Scotland, was compared with that of unbound IVIG in two independent experiments. Lethal intranasal challenges with about 1000 TCID50 of the strain Scotland showed that whereas IVIG simply preincubated with chicken erythrocytes gave effective protection (9/10 and 10/10 survivors in the two experiments), preadsorption with HA antigen-sensitized erythrocytes of the strain A/Scotland significantly reduced or abolished the protective effects of IVIG (with survival scores of 2/10 and 0/10, similar to those observed for PBS-treated controls, with 0/10 survivors in each experiment). Preabsorption of IVIG onto the HA antigen from the influenza A virus strain Kumamoto gave 50% less protection with IVIG (4/8 survivors), but preabsorption of IVIG onto the HA antigen from the strain B/Kanagawa did not significantly remove antibodies protective against the strain Scotland (7/8 survivors), whereas non-preabsorbed IVIG protected 8/8 mice and all eight PBS-treated controls died by day 13 of this experiment.
Prophylactic passive immunotherapy
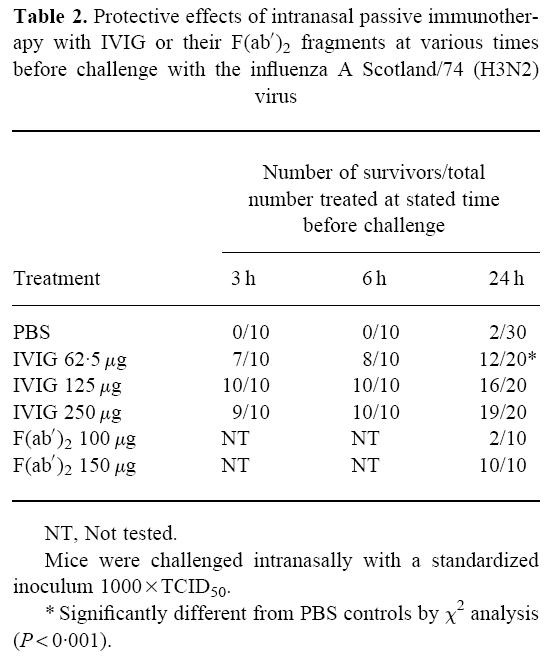
To evaluate the efficacy of IVIG in prophylaxis against influenza pneumonia, 62.5, 125 or 250 μg IVIG per mouse were administered intranasally 3, 6 or 24 h before virus challenge. F(ab′)2 were also tested intranasally for prophylactic effects at doses of 100 and 150 μg given 24 h before challenge. Effective dose-dependent protection was observed with IVIG, whereas F(ab′)2 were only protective at a dose of 150 μg per mouse (Table 2).
Table 2.
Protective effects of intranasal passive immunotherapy with IVIG or their F(ab′)2 fragments at various times before challenge with the influenza A Scotland/74 (H3N2) virus
DISCUSSION
Intranasal challenge with influenza A virus strain Scotland, adapted to BALB/c mice, reproducibly induced pneumonia with a mortality rate of 100% between days 10 and 18 (see data from controls in Figs 1 and 2). The pathogenic properties of the strain Scotland are similar to those of the mouse-adapted strain, PR8, used in several other studies [4–7] and in preliminary assays in this study (data not shown). As strain A/Scotland is of the H3N2 type, it is probably a more accurate model of the strains predominantly involved in recent outbreaks worldwide [16] than the older strain PR8 (type H1N1), for testing IVIG currently in use, which contain influenza HA antibodies specific for current strains from influenza-convalescent and vaccinated donors. Intravenous injection of IVIG was effective against influenza at doses of 1000–5000 μg/mouse (Fig. 1a and Table 1), whereas IVIG administration by the intranasal route gave higher protection, with a dose 40–100 times lower (Fig. 1b and Table 1). The mechanism of protection was Fc-independent, because F(ab′)2 fragments were at least as protective as intact IVIG at the effective dose of 50 μg/mouse (Fig. 2a,b), suggesting that complement or phagocytic or cytotoxic Fcγ receptor-bearing cells were not required, as reported previously [6]. The fact that an unrelated human monoclonal IgG1 (RhD antibody) and IVIG preparations from which influenza A antibodies had been fully (homologous HA antigen) or partially (heterologous HA antigen) removed did not protect mice against lethal doses of the influenza A virus demonstrated that passive protection with IVIG or its F(ab′)2 fragments was due to a specific immunological protective mechanism and not to a non-specific effect of IgG or another IVIG component on the respiratory epithelium. However, topical immunotherapy with IVIG or F(ab′)2 was protective only if given 2–8 h after infection. Topical treatment 24 h after infection with IVIG or F(ab′)2 did not protect the mice (Fig. 2c). This time-dependency suggests that influenza A antibodies in topical IVIG neutralize the influenza A virus at early stages of acute infection, mostly by inhibiting intercellular infection. This led us to check the efficacy of intranasal IVIG and F(ab′)2 in prophylaxis. The potential value of topical administration of IVIG and F(ab′)2 in preventing influenza A pneumonia was demonstrated by the experiment reported in Table 2, in which intranasal administration of antibodies, even 24 h before challenge, gave effective dose-dependent protection, although F(ab′)2 were only protective at the highest dose, 150 μg per mouse. This was probably due to the inability of F(ab′)2 fragments, devoid of the Fc portion of the IgG, to bind to any cellular receptor, whereas intact IgG in IVIG could bind to Fcγ receptors and thus persist at the surface of the respiratory epithelium. It is unknown whether intranasally administered foreign IgG attach to resident macrophages sharing FcγRI and II or to polymeric immunoglobulin receptors [17], or other unknown binding molecules on epithelial cells.
HA antibodies of the IgA and IgG isotypes protect against influenza virus, but by different mechanisms. In vitro studies in polarized monolayers of the epithelial MDCK cell line infected with influenza virus have shown that polymerized anti-HA IgA neutralize intracellular virus by polymeric immunoglobulin receptor-mediated transcytosis, whereas IgG do not [17]. Other studies in vitro have shown that IgG-neutralized influenza virus enters the cells and may inhibit endosomal fusion [18]. These mechanisms of influenza virus neutralization by IgG may account, at least partly, for the protective effects of IVIG in the first 8 h after infection. F(ab′)2-neutralized viruses may be subject to more efficient and prolonged intracellular inhibition than those neutralized with intact IgG (Fig. 2b). Other specific influenza A antibody titres such as those of antibodies against neuraminidase, nucleoprotein, M protein and other potentially protective antigens of the influenza virus [3] were not determined in IVIG in this study. IVIG is polyclonal and polyvalent, so it may contain a wide variety of influenza antibodies to variable or stable influenza virus antigens, and may thus be of value for use in passive immunotherapy for prophylaxis and early treatment of various influenza virus pneumonias. Its efficacy in topical administration, at low doses, even as F(ab′)2 fragments, as previously demonstrated in other models of respiratory infection [10–12], may make it possible to develop new passive prophylactic immunotherapy strategies.
Acknowledgments
This work was supported by a grant from the French Ministry of Defence (convention 93-169/DRET). We thank Jacques Bartholeyns for providing the monoclonal human anti-RhD IgG1, Sylvie Monet, Françoise Ventre and Valérie Morineaux for expert technical assistance, and Pascale Van-Steenkiste for her excellent preparation of the manuscript.
References
- 1.Doherty PC, Allen W, Eichelberger M. Roles of αβ and γδ T cell subsets in viral immunity. Annu Rev Immunol. 1992;10:123–51. doi: 10.1146/annurev.iy.10.040192.001011. [DOI] [PubMed] [Google Scholar]
- 2.Bot A, Reichlin A, Isobe H. Cellular mechanisms involved in protection and recovery from influenza virus infection in immunodeficient mice. J Virol. 1996;70:5668–72. doi: 10.1128/jvi.70.8.5668-5672.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Virelizier JL, Allison AC, Schild GC. Immune responses to influenza virus in the mouse and their role in control of the infection. Brit Med Bull. 1979;35:65–68. doi: 10.1093/oxfordjournals.bmb.a071544. [DOI] [PubMed] [Google Scholar]
- 4.Scherle PA, Palladino G, Gerhard W. Mice can recover from pulmonary influenza virus infection in the absence of class I-restricted cytotoxic T cells. J Immunol. 1992;148:212–7. [PubMed] [Google Scholar]
- 5.Bender A, Bui LK, Feldman MAV. Inactivated influenza virus, when presented on dendritic cells elicits human CD8+ cytolytic T cell responses. J Exp Med. 1995;182:1663–71. doi: 10.1084/jem.182.6.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palladino G, Mozdzanowska K, Washko G. Virus neutralizing antibodies of immunoglobulin G (IgG) but not of IgM or IgA isotypes can cure influenza virus pneumonia in SCID mice. J Virol. 1995;69:2075–81. doi: 10.1128/jvi.69.4.2075-2081.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tamura SI, Funato H, Hirabayashi Y. Cross-protection against influenza A virus infection by passively transferred respiratory tract IgA antibodies to different hemagglutinin molecules. Eur J Immunol. 1991;21:1337–44. doi: 10.1002/eji.1830210602. [DOI] [PubMed] [Google Scholar]
- 8.Stiehm ER. New uses for intravenous immune globulin. N Engl J Med. 1991;325:123–5. doi: 10.1056/NEJM199107113250209. [DOI] [PubMed] [Google Scholar]
- 9.Casadevall A, Scharff MD. Return to the past: the case for antibody-based therapies in infectious diseases. Clin Infect Dis. 1995;21:150–61. doi: 10.1093/clinids/21.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramisse F, Szatanik M, Binder P, Alonso JM. Passive local immunotherapy of experimental staphylococcal pneumonia with human intravenous immunoglobulins. J Infect Dis. 1993;168:1030–3. doi: 10.1093/infdis/168.4.1030. [DOI] [PubMed] [Google Scholar]
- 11.Ottolini MG, Hemming VG, Piazza F. Topical immunoglobulin is an effective therapy for parainfluenza type 3 in a cotton rat model. J Infect Dis. 1995;172:243–5. doi: 10.1093/infdis/172.1.243. [DOI] [PubMed] [Google Scholar]
- 12.Ramisse F, Binder P, Szatanik M, Alonso JM. Passive and active immunotherapy for experimental pneumococcal pneumonia by polyvalent human immunoglobulin or F(ab′)2 fragments administered intranasally. J Infect Dis. 1996;173:1123–8. doi: 10.1093/infdis/173.5.1123. [DOI] [PubMed] [Google Scholar]
- 13.IUIS/WHO notice. Appropriate use of human immunoglobulin in clinical practice. Clin Exp Immunol. 1983;52:417–22. [PMC free article] [PubMed] [Google Scholar]
- 14.Kendal AP, Dowdle WR. Influenza virus. In: Rose NR, Friedman H, Fahey JL, editors. Manual of clinical laboratory immunology. Washington, DC: American Society for Microbiology; 1986. pp. 515–20. [Google Scholar]
- 15.Deramoudt FX, Gilard C, Lepine N, Alonso JM, Romet-Lemonne JL. Bispecific anti-human red blood Rhesus-D antigen x anti-FcγRI targeted antibody-dependent cell-mediated cytotoxicity and phagocytosis by mononuclear leucocytes. Clin Exp Immunol. 1992;89:310–4. doi: 10.1111/j.1365-2249.1992.tb06951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO. Influenza in the world, 1 October 1994–30 September 1995. Overview. Wkly Epidemiol Rec. 1996;71:1–8. [PubMed] [Google Scholar]
- 17.Mazanec MB, Coudret CL, Fletcher DR. Intracellular neutralization of influenza virus by immunoglobulin A anti-hemagglutinin monoclonal antibodies. J Virol. 1995;69:1339–43. doi: 10.1128/jvi.69.2.1339-1343.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Outlaw MC, Dimmock NJ. IgG neutralization of type A influenza viruses and the inhibition of the endosomal fusion stage of the infectious pathway in BHK cells. Virol. 1993;195:413–21. doi: 10.1006/viro.1993.1391. [DOI] [PubMed] [Google Scholar]


