Abstract
Most patients with primary IgA nephropathy (IgAN) have a significantly higher memory repertoire of IgA1-producing B lymphocytes in their bone marrow together with high plasma levels of IgA1. The connection between the mucosal immune system and the bone marrow compartment is probably based on traffic of either antigen-presenting cells (APC) or antigen-specific lymphocytes. Cytokines play an important role in the proliferation and differentiation of lymphoid cells. In order to mimic the in vivo situation as much as possible, we assessed cytokine production profiles ex vivo in 23 IgAN patients and matched controls, using lipopolysaccharide (LPS)- or phytohaemagglutinin (PHA)-stimulated whole blood (WB) cultures. Interferon-gamma (IFN-γ), IL-2, IL-6, IL-10 and tumour necrosis factor-alpha (TNF-α) production in culture supernatants were determined by cytokine-specific ELISAs. Compared with controls, PHA-stimulated cultures resulted in significantly higher IL-10 (P < 0.001), IL-2 (P < 0.005) and IFN-γ (P < 0.001) levels in IgAN patients, but no significant differences in TNF-α or IL-6 levels were found. In LPS-stimulated cultures, the only significant difference (P < 0.001) between the two groups was the increased IL-10 production in IgAN patients. The enhanced cytokine production in stimulated WB cultures suggests altered monocyte-related T cell responses in patients with IgAN. Increased IL-10 production may eventually result in an increased number of IgA-producing B lymphocytes in the bone marrow. In addition, high levels of endogenous IL-10 may down-regulate the effector functions of monocytes, or possibly APC in general, and consequently the IgA response at the mucosal level.
Keywords: IgA nephropathy, whole blood, cytokine production
INTRODUCTION
Elevated plasma levels of IgA1 are thought to play an essential role in the pathogenesis of primary IgA nephropathy (IgAN), and are the consequence of an increased production, in which the bone marrow may be the predominant site [1]. The clinical association of exacerbations of the disease with upper respiratory tract infections suggests that the trigger for the increased IgA1 production is frequently in the nasopharynx. It is still unclear how mucosal infections may result in overproduction of IgA1 in the bone marrow of IgAN patients. The connection between the mucosal immune system and the bone marrow, the so-called mucosa–bone marrow axis, is not based on the overflow of IgA antibodies from the mucosa into the vascular compartment, but is probably based on traffic of either antigen-presenting cells (APC) or antigen-specific lymphocytes from the mucosa to the bone marrow compartment [2, 3]. Studies with peripheral blood mononuclear cells (PBMC) have provided conflicting results with respect to T and B cell abnormalities in patients with IgAN [4].
Cytokines play an important role in the proliferation and differentiation of lymphoid cells. However, due to regulatory networks and redundancy in the immune system, specific cytokine dysregulation may never become apparent in body fluids. Earlier studies in IgAN patients have reported enhanced IL-2 production by PBMC, both in unstimulated and mitogen-induced cultures [5–7]. In addition, increased production of IL-4 and interferon-gamma (IFN-γ) was found after mitogen activation of PBMC [6–8]. Procedures used to isolate PBMC modify lymphocyte/monocyte ratios and may result in the loss of other stimulatory and/or inhibitory mediators [9]. Thus, cytokine secretion in culture supernatants of PBMC may reflect in vivo activation, but it may also reflect skewing of the cytokine response due to removal of these cells from their in vivo environment. The whole blood (WB) culture system mimics the natural cell-to-cell interactions to a greater extent. This system has been shown to be reproducible when stimulated by mitogens [9–11]. When monocyte-stimulating agents are used, cytokine production is modulated in the same manner as in purified monocytes [11].
Current evidence indicates that IL-10 directly affects the proliferation and differentiation of IgA-producing B cells [12]. In addition, IL-10 plays a major role in the regulation of immune responses as an inhibitor of monocytes/macrophages, dendritic cells and T cell effector functions [12]. In the present study we have assessed IL-10 production in patients with IgAN and healthy volunteers using the WB culture system.
SUBJECTS AND METHODS
Human subjects
The study protocol was approved by the Ethical Committee of the Leiden University Medical Centre. The subjects included 23 patients (19 males and four females, mean age 38 years, range 19–56 years) with biopsy-proven IgAN, defined by mesangial deposits of IgA as the dominant isotype. None of the patients had clinical or laboratory evidence of Henoch–Schönlein purpura, systemic lupus erythematosus, or liver disease. None of the patients received immunosuppressive therapy. Kidney function was normal or mildly impaired (creatinine clearance > 80 ml/min). None of the patients had macroscopic haematuria or proteinuria > 2 g/24 h. As controls, 23 age- and sex-matched healthy volunteers were recruited. Neither patients nor controls had symptoms or signs of mucosal infection in the 2 weeks preceding the study.
Assays
Analysis of leucocyte populations. Venous blood was collected in sterile, heparinized (10 U/ml) syringes. The total leucocyte count was obtained for each blood sample using a Technicon H3-RTX counter (Bayer Diagnostics, Tarrytown, NY). The different cell populations present in whole blood were quantified by FACScan immunofluorescence staining performed with anti-CD3, anti-CD4 (T helper lymphocytes), anti-CD8 (cytotoxic and suppressive lymphocytes), anti-CD14 (monocytes), and anti-CD45 (leucocytes) MoAbs (Becton Dickinson, San Jose, CA).
Whole blood culture system. Fresh blood was diluted five-fold in complete culture media consisting of MCDB 302 (Sigma Chemical Co., St Louis, MO) supplemented with 100 U/ml penicillin and 100 μg/ml streptomycin. All cell cultures were performed in 24-well flat-bottomed microtitre plates (Greiner, Alphen a/d Rijn, The Netherlands) in triplicate at 37°C in humidified air containing 5% CO2 in a total volume of 200 μl. Cultures were stimulated using either lipopolysaccharide (LPS) from Salmonella typhosa (Sigma) or purified phytohaemagglutinin (PHA; Murex Diagnostic Ltd, Dartford, UK), both at a final concentration of 10 μg/ml. After various incubation periods, the plates were centrifuged (150 g, 10 min), and the cell-free supernatants were aliquoted, sealed and stored at −20°C until assayed.
ELISA. Culture supernatants were tested for tumour necrosis factor-alpha (TNF-α), IFN-γ, IL-6 and IL-10 content by sandwich ELISA. Primary MoAbs were MoAb 2-179-E11 (ATCC, Rockville, MD; 2 μg/ml), MoAb 45B3 (ATCC; 10 μg/ml), MoAb 5E1 (kindly provided by Dr W. Buurman, University of Maastricht, The Netherlands; 1 μg/ml) and MoAb 9D7 (kindly provided by Dr J. Banchereau, Schering Plough, Dardilly, France; 1 μg/ml), respectively. Maxisorb 96-well ELISA plates (Nunc, Roskilde, Denmark) were coated overnight at room temperature with 100 μl/well of the capturing antibody, appropriately diluted in PBS. After three washings with PBS containing 0.05% Tween 20 (PBS–T), non-specific binding sites were blocked with PBS–T containing 1% bovine serum albumin (BSA; Sigma), 1% fetal calf serum (FCS; Gibco, Breda, The Netherlands) in the IL-10 assay or 2% casein (Sigma) in the IL-6 assay. Appropriate serial dilutions of cell-free culture supernatants were added to triplicate wells and incubated at 37°C. Each assay was standardized using serial two-fold dilutions of human recombinant TNF-α (5000–39 pg/ml), IFN-γ (1000–7.8 U/ml), IL-6 (1000–7.8 pg/ml) or IL-10 (1000–7.8 pg/ml). The standard sera yielded optical density (OD) values in a dose-dependent linear fashion. Bound TNF-α and IL-6 were detected by affinity-purified rabbit IgG directed against TNF-α or IL-6, followed by horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG diluted in PBS/2 mm EDTA/0.5% human serum albumin (HSA)/25% glycerol (Jackson, West Grove, PA). Bound IFN-γ was detected by MoAb MD1 (a kind gift from Dr P. vd Meide, TNO, Rijswijk, The Netherlands), IL-10 by MoAb 12G8 (kindly provided by Dr J. Banchereau), both conjugated to digoxigenin (DIG; Boehringer, Mannheim, Germany) and consecutively incubated with sheep anti-DIG–HRP (Boehringer). Between each step the wells were washed three times with PBS–T. The plates were shaken by rotation and finally enzyme substrate (2,2′-azino-bis [3-ethylbenzthiazoline-6-sulphonic acid]; Sigma) containing 0.0075% H2O2 was added. The reaction was stopped by adding 2% oxalic acid dihydrate (Merck, Darmstadt, Germany). OD was measured at 415 nm on a microplate reader (Bio-Kinetics Reader EL 312e; Biotek Instruments Inc., Winooski, VT). Concentrations were obtained by interpolation on the standard curves using a four-parameter modelling procedure (KinetiCalc; EIA Application Software). The final concentrations in each sample were calculated as the mean of the results at the proper sample dilutions yielding ODs in the linear parts of the calibration curves. The assay for IL-2 (immunoradiometric assay) has been described in detail before [13].
Statistical analysis
All statistical calculations were performed using the SPSS for Windows Release 6.0 software package. Data on the absolute count or relative frequencies of granulocytes, monocytes, major lymphocyte populations and lymphocyte subsets were normally distributed, and analysed by (two-tailed) t-tests and expressed as arithmetic means ± s.d. Data on cytokine production in WB cultures showed a skewed distribution, and were transformed logarithmically prior to analysis. The two-way analysis of variance (anova) for repeated measures was used to study the effect of both group (patient versus control) and time of culture. Post hoc comparisons were made using Scheffe's procedure. P < 0.05 was considered statistically significant. These results were expressed as their geometric means ± s.e.m.
RESULTS
Flow cytometric analysis
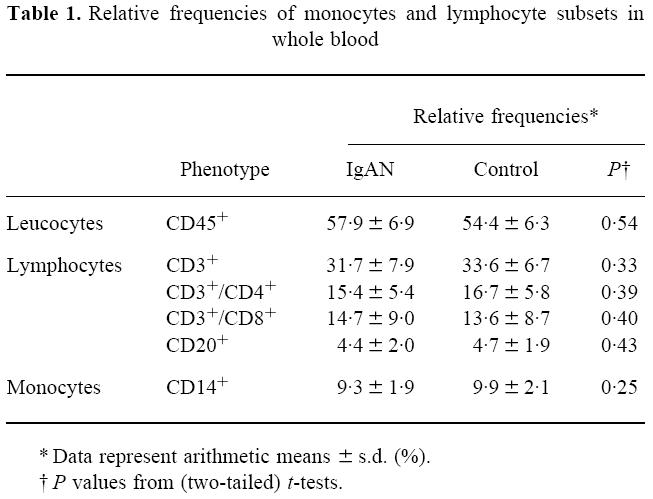
The different cell populations present in whole blood samples from patients and controls were quantified by flow cytometric analysis. No significant differences between patients with IgAN and healthy volunteers were found with respect to absolute count or relative frequencies of granulocytes, monocytes, major lymphocyte populations and lymphocyte subsets. These results are summarized in Table 1.
Table 1.
Relative frequencies of monocytes and lymphocyte subsets in whole blood
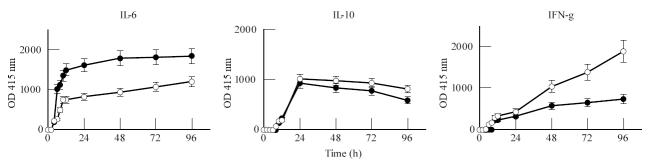
Kinetics of cytokine production in leucocytes
The optimal stimulation conditions for LPS and PHA were assessed in preliminary titration studies and were found to be dose-dependent, with maximal production occurring at 10 μg/ml for both mitogens. Kinetic studies showed three patterns of cytokine production. An example of one subject, representative for the production profiles of IL-6, IL-10 and IFN-γ in WB from patients and controls, is depicted in Fig. 1. First a group of early produced cytokines, including TNF-α and IL-6, were studied. Cytokine levels increased rapidly during the first 8 h of culture, then stabilized or continued to increase slowly up to 96 h (IL-6). The T lymphocyte-related cytokines IL-2 and IFN-γ were produced relatively late, with slowly increasing levels from 24 h up to 96 h. Comparison of the production curves showed a pronounced increase of IFN-γ production upto 96 h in PHA-stimulated cultures, whereas in LPS-stimulated cultures IFN-γ levels stabilized after 24 h. A third pattern was found for IL-10, where levels increased during the first 24 h and then stabilized into a plateau. Comparison of the respective cytokine production curves between LPS- and PHA-stimulated cultures or between patients with IgAN and healthy volunteers showed comparable patterns of secretion.
Fig. 1.
Representative example of the kinetics of IL-6, IL-10 and IFN-γ production in whole blood (WB) cultures. WB (diluted 1:5) was cultured in triplicate in the absence or presence of lipopolysaccharide (LPS; •; 10 μg/ml) and phytohaemagglutinin (PHA; ○; 10 μg/ml), respectively. Production (expressed as optical density (OD)) was determined in the culture supernatants, harvested after 0, 2, 4, 6, 8, 10, 12, 24, 48, 72 and 96 h, by cytokine-specific ELISAs.
Comparison of cytokine production profiles in IgAN and controls
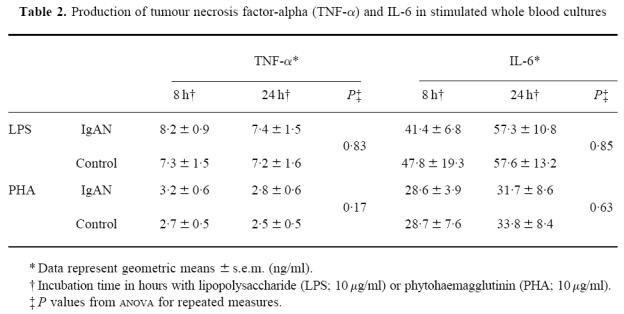
Initially we determined cytokine levels in serum and unstimulated cultures. These levels were below the detection level of the assays employed. On the basis of the kinetic experiments with stimulated WB, culture periods of 0, 8, 24 and 72 h were chosen. After stimulation with LPS or PHA, analysis of variance showed a significant (P < 0.0001) increase in the production of all the cytokines tested. Comparisons between patients with IgAN and healthy volunteers showed the following. No significant differences in the production profiles of the early produced cytokines, TNF-α and IL-6, were found independent of the type of stimulation. These results are summarized in Table 2.
Table 2.
Production of tumour necrosis factor-alpha (TNF-α) and IL-6 in stimulated whole blood cultures
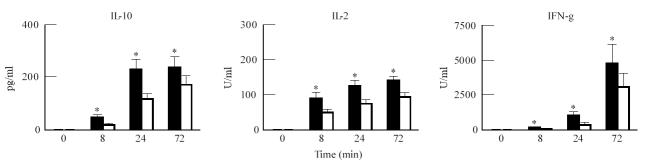
The T cell-related cytokines, IFN-γ (P < 0.001) and IL-2 (P < 0.005) were significantly higher in PHA-stimulated cultures from IgAN patients compared with controls (Fig. 2). IL-2 and IFN-γ levels in LPS-stimulated cultures tended to be higher in patients, though differences did not reach statistical significance (P = 0.08 and 0.15, respectively). LPS-stimulated IFN-γ production in patients and controls at 8 h (P = 0.07), 24 h (P = 0.06) and 72 h (P = 0.38) is plotted in Fig. 3.
Fig. 2.
Comparison of IL-10, IL-2 and IFN-γ production in whole blood (WB) cultures from IgA nephropathy (IgAN) patients (▪) and controls (□). WB (diluted 1:5) was cultured in triplicate in the absence or presence of phytohaemagglutinin (PHA; 10 μg/ml). Production was determined in the culture supernatants, harvested after 0, 8, 24 and 72 h, by cytokine-specific ELISAs. Results represent geometric means ± s.e.m. *Significant differences (Scheffe's procedure) between patients and controls.
Fig. 3.
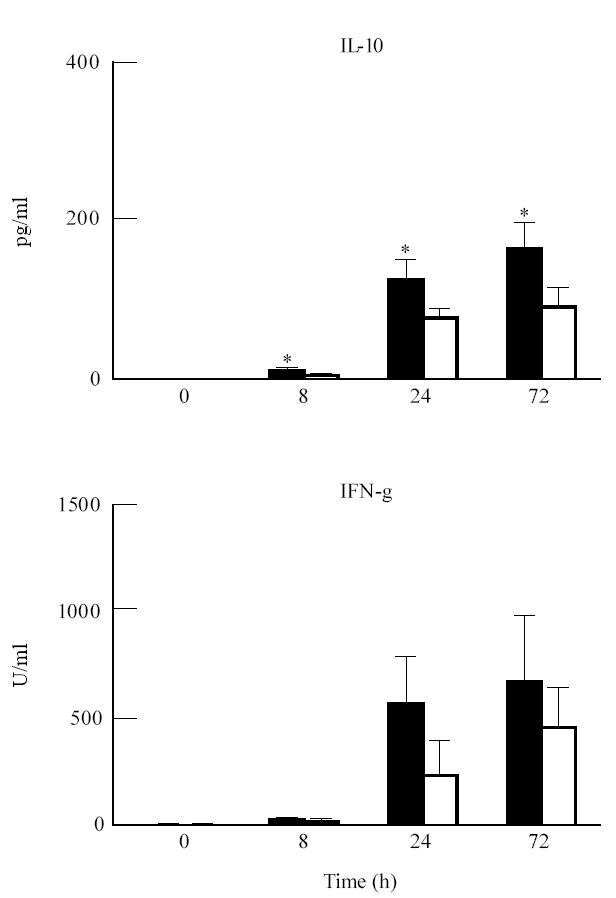
Comparison of IL-10 and IFN-γ production in whole blood (WB) cultures from IgA nephropathy (IgAN) patients (▪) and controls (□). WB (diluted 1:5) was cultured in triplicate in the absence or presence of lipopolysaccharide (LPS; 10 μg/ml). Production was determined in the culture supernatants, harvested after 0, 8, 24 and 72 h, by cytokine-specific ELISAs. Results represent geometric means ± s.e.m. *Significant differences (Scheffe's procedure) between patients and controls.
When studying the production of IL-10 in WB cultures, we found that both PHA and LPS induced an increased IL-10 production. Comparison between IgAN patients and controls showed that, both with LPS (P < 0.001) and PHA (P < 0.001), patients displayed significantly higher production of IL-10. Comparison at the different time points (Scheffe's procedure) showed significant differences at 8 h (LPS P < 0.0005; PHA P < 0.005), 24 h (LPS P < 0.05; PHA P < 0.0001) and 72 h (LPS P < 0.005; PHA P < 0.05) (Figs 2 and 3).
DISCUSSION
Cytokines act mainly in a paracrine or autocrine manner, and are generally not detectable in peripheral blood. To assess the ability of immunocompetent cells to produce cytokines, stimulation with polyclonal activators is required to analyse the patterns of cytokine production [14]. Both with LPS and PHA, the proinflammatory cytokines (TNF-α, IL-6) were not significantly different in stimulated WB cultures from patients with IgAN compared with a group of matched healthy controls. Significantly increased T cell-derived cytokines (IL-2, IFN-γ) were found only in PHA-stimulated WB from patients, confirming previous results obtained with stimulated PBMC [5–8]. Significantly higher levels of IL-10 in activated WB were found in patients with IgAN, independent of the mitogen used. To our knowledge, this is the first study to investigate IL-10 production in patients with IgAN.
IL-10 plays a major role in the regulation of immune responses and has been shown to inhibit both proliferation and cytokine synthesis of Th1 lymphocytes, possibly by blocking the synthesis of IFN-γ and/or antagonizing its activating effects on monocytes [15, 16]. In LPS-stimulated WB cultures, there was a clear trend to higher IL-2 and IFN-γ responses in patients. However, besides IL-10, none of the other cytokines (TNF-α, IL-6, IL-2 or IFN-γ) showed significant differences between patients and controls. Compared with TNF-α and IL-6, IL-10 is produced relatively late by monocytes [17, 18]. This aspect is important for its inhibitory role in monocyte activation, since endogenously produced IL-10 has autoregulatory activities both on monokine (TNF-α, IL-6) production and MHC class II expression by LPS-activated monocytes [15]. Compatible with previous studies using PBMC [5–8], PHA stimulation of WB cultures resulted in significantly higher levels of IL-2 and IFN-γ in IgAN patients. In combination with the previously reported increase of both Th1 and Th2 lymphocyte subsets in the circulation of patients with IgAN [7], these data suggest that IgAN patients may have ongoing antigen-specific T cell responses. The different results obtained for IL-2 and IFN-γ in LPS- or PHA-stimulated cultures may be explained by the specific ability of PHA to stimulate IL-2 synthesis more strongly in IgAN. This additional effect on IL-2 production may overcome the monocyte-dependent inhibitory effect of IL-10 on the synthesis of IL-2 and IFN-γ [15, 16]. This is supported by the fact that TNF-α and IL-6 levels (direct inhibition) were not significantly different in patients and controls after PHA stimulation.
The production of higher levels of IL-10 is compatible with enhanced humoral immune responses [12, 19], and may play a role in the dysregulation of the IgA immune response frequently found in patients with IgAN. In the presence of IL-10, transforming growth factor-beta (TGF-β) is an IgA switch factor. Addition of this factor to CD40-activated naive B cells cultured with IL-10 induces large amounts of IgA, while inhibiting IgM and IgG production [20]. Clearly, since IL-10 can be secreted by monocytes and lymphocytes, WB cultures can not be used to reliably determine its cellular source. An increased percentage of monocytes expressing high levels of class II antigen has been reported in patients with IgAN [21]. Furthermore, class II expression was significantly higher in patients with detectable IFN-γ transcripts [21]. The present data suggest that monocytes may be involved in the increased IL-10 production found in IgAN patients. The kinetics of IL-10 production in the WB culture system was very similar to that found in studies using elutriated monocytes [17]. In accordance with the notion that PHA-activated T lymphocyte proliferation requires the presence of monocytes [22], we found similar kinetics for LPS- or PHA-activated IL-10 production by elutriated monocytes (data not shown). Second, compared with other cytokines, IL-10 is produced relatively late following activation of monocytes/macrophages or T lymphocytes [17, 18, 23]. In addition, activation of WB cultures with a monocyte-specific mitogen (LPS) resulted in a significantly higher production of IL-10 in IgAN patients.
Inhibition of monocyte/macrophage function by endogenous IL-10 would be a disadvantage in the initiation of immune responses to novel antigens. IL-10 has been shown to strongly inhibit antigen-specific T cell proliferation, when monocytes were used as the APC [15, 16]. Recently, IL-10 was found to inhibit antigen presentation by Langerhans cells in a mouse model [24] and to inhibit T cell proliferation induced by human dendritic cells in an alloresponse model [25]. Therefore, increased IL-10 production could possibly contribute to the deficient primary mucosal IgA immune response found in IgAN patients after nasal presentation, despite the use of a potent immunogen [2]. A deficient primary immune response may lead to persistence or recurrence of the antigenic stimulus in patients, whereas healthy individuals succeed in elimination or exclusion of the antigen by a more effective mucosal immune response. The resulting ongoing or repeated stimulation of the immune response in IgAN patients may eventually lead to appropriate protection at the mucosal level. However, as a consequence, overproduction of IgA antibodies occurs in the systemic compartment, accompanied by an increased number of IgA memory cells. The previously reported increased IgA immune response to recall antigens may be the reflection of this increased level of immunological memory [26, 27].
In conclusion, the enhanced cytokine production in PHA-stimulated WB cultures from IgAN patients suggests altered antigen-specific T cell responses that may eventually result in an increased number of IgA-producing B lymphocytes in the bone marrow. The present data suggest that monocytes may be involved in the significantly higher IL-10 levels found in patients with IgAN. The high levels of endogenous IL-10 may down-regulate effector functions of APC. Recently, a direct functional effect of dendritic cells, physiologically the principal cells involved in mucosal antigen presentation, on IgA subclass switch was found [28]. Since IL-10 and/or IFN-γ may influence dendritic cells differently [25, 29], further studies are needed to evaluate their role in the abnormal IgA1 responses in IgAN patients at the mucosal level.
References
- 1.Van den Wall Bake AWL, Daha MR, Haaijman JJ, Radl J, van der Ark A, van Es LA. Elevated production of polymeric and monomeric IgA1 by the bone marrow in patients with IgA nephropathy. Kidney Int. 1989;35:1400–4. doi: 10.1038/ki.1989.139. [DOI] [PubMed] [Google Scholar]
- 2.De Fijter JW, Eijgenraam JW, Braam CA, Holmgren J, Daha MR, van Es LA, van den Wall Bake AWL. Deficient IgA1 immune response to nasal cholera toxin subunit B in primary IgA nephropathy. Kidney Int. 1996;50:952–61. doi: 10.1038/ki.1996.396. [DOI] [PubMed] [Google Scholar]
- 3.Russell MW, Lue C, van den Wall Bake AWL, Moldoveanu Z, Mestecky J. Molecular heterogeneity of human antibodies during an immune response. Clin Exp Immunol. 1992;87:1–6. doi: 10.1111/j.1365-2249.1992.tb06404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson S, Galla JH, Kirk KA, Thorn BT, Julian BA. Epstein–Barr virus transformation of B lymphocytes from IgA nephropathy patients and first-degree relatives results in increased immunoglobulin synthesis not restricted to IgA. Am J Kidney Dis. 1991;17:55–61. doi: 10.1016/s0272-6386(12)80251-4. [DOI] [PubMed] [Google Scholar]
- 5.Schena FP, Mastrolitti G, Jirollo E, Munno I, Pellegrino N, Frascasso AR, Aventaggiato L. Increased production of interleukin-2 and IL-2 receptor in primary IgA nephropathy. Kidney Int. 1989;35:875–9. doi: 10.1038/ki.1989.67. [DOI] [PubMed] [Google Scholar]
- 6.Lai KN, Leung JC, Li PK, Lui SF. Cytokine production by peripheral blood mononuclear cells in IgA nephropathy. Clin Exp Immunol. 1991;85:240–5. doi: 10.1111/j.1365-2249.1991.tb05712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai KH, Ho RT, Lai CK, Chan CH, Li PK. Increase of both circulating Th1 and Th2 T lymphocyte subsets in IgA nephropathy. Clin Exp Immunol. 1994;96:116–21. doi: 10.1111/j.1365-2249.1994.tb06240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scivittaro V, Gesualdo L, Ranieri E, Marfella C, Schewn SA, Emancipator SN, Schena FP. Profiles of immunoregulatory cytokine production in vitro in patients with IgA nephropathy and their kindred. Clin Exp Immunol. 1994;96:311–6. doi: 10.1111/j.1365-2249.1994.tb06559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Groote D, Zangerle PF, Gevaert Y, et al. Direct stimulation of cytokines (IL-1β, TNF-α, IL-6, IL-2, IFN-γ and GM-CSF) in whole blood. Comparison with isolated PBMC stimulation. Cytokine. 1992;4:239–48. doi: 10.1016/1043-4666(92)90062-v. [DOI] [PubMed] [Google Scholar]
- 10.Kirchner H, Kleinicke C, Digel W. A whole blood technique for testing production of human interferon by leucocytes. J Immunol Methods. 1982;48:213–9. doi: 10.1016/0022-1759(82)90195-8. [DOI] [PubMed] [Google Scholar]
- 11.Wilson BMG, Severn A, Rapson NT, Chana J, Hopkins P. A convenient human whole blood culture system for studying the regulation of tumor necrosis factor release by bacterial lipopolysaccharide. J Immunol Methods. 1991;139:233–40. doi: 10.1016/0022-1759(91)90193-j. [DOI] [PubMed] [Google Scholar]
- 12.Moore KW, O'Garra A, de Waal Malefijt R, Vieira P, Mosmann TR. Interleukin-10. Ann Rev Immunol. 1993;11:165–90. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 13.Miltenburg AMM, van Laar JM, de Kuiper R, Daha MR, Breedveld FC. T cells cloned from human rheumatoid synovial membrane functionally represent the Th1 subset. Scand J Immunol. 1992;35:603–10. doi: 10.1111/j.1365-3083.1992.tb03260.x. [DOI] [PubMed] [Google Scholar]
- 14.Cavaillon JM, Fitting C, Haeffner-Cavaillon N, Kirsch SJ, Warren HS. Cytokine response by monocytes/macrophages to free and lipoprotein bound lipopolysaccharide. Infect Immunol. 1990;58:2375–82. doi: 10.1128/iai.58.7.2375-2382.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Waal Malefijt R, Haanen Jabg, Spits H, et al. IL-10 and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II MHC expression. J Exp Med. 1991;174:915–24. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taga K, Tosato G. IL-10 inhibits T cell proliferation and IL-2 production. J Immunol. 1992;148:1143–8. [PubMed] [Google Scholar]
- 17.de Waal Malefijt R, Abrams JS, Bennett B, Figdor CG, de Vries JE. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–20. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiorentino DF, Zlotnik A, Mosmann TR, Howard MH, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–22. [PubMed] [Google Scholar]
- 19.Van Kooten C, Banchereau J. CD40-CD40 ligand: a multifunctional receptor–ligand pair. Adv Immunol. 1996;61:1–77. doi: 10.1016/s0065-2776(08)60865-2. [DOI] [PubMed] [Google Scholar]
- 20.Defrance T, Vanbervliet B, Briere F, Durand I, Rousset F, Banchereau J. Interleukin 10 and transforming growth factor beta cooperate to induce anti-CD40-activated naive human B cells to secrete immunoglobulin A. J Exp Med. 1992;175:671–82. doi: 10.1084/jem.175.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Caestecker MP, Bottomley M, Telfer BA, Hutchinson IV, Vose BM, Ballardie FW. Detection of abnormal peripheral blood mononuclear cell cytokine networks in human IgA nephropathy. Kidney Int. 1993;44:1298–308. doi: 10.1038/ki.1993.382. [DOI] [PubMed] [Google Scholar]
- 22.Thiele DW, Kurosaka M, Lipsky PE. Phenotype of the accessory cell necessary for mitogen-stimulated T and B cell responses in human peripheral blood: delineation by its sensitivity to the lysosomotropic agent, L-leucine methyl ester. J Immunol. 1983;131:2282. [PubMed] [Google Scholar]
- 23.Yssel H, de Waal Malefijt R, Roncarolo MG, Abrams JS, Lahesman R, Spits H, de Vries J. Interleukin 10 is produced by subsets of human CD4+ T cell clones and peripheral blood T cells. J Immunol. 1992;149:2378–84. [PubMed] [Google Scholar]
- 24.Ozawa H, Aiba S, Nakagawa S, Tagami H. Interferon-γ and interleukin-10 inhibit antigen presentation by Langerhans cells for T helper type 1 cells by suppressing their CD80 (B7-1) expression. Eur J Immunol. 1996;26:648–52. doi: 10.1002/eji.1830260321. [DOI] [PubMed] [Google Scholar]
- 25.Caux C, Massacrier C, Vanbervliet B, Barthelemy C, Liu Y-J, Banchereau J. Interleukin 10 inhibits T cell alloreaction induced by human dendritic cells. Int Immunol. 1994;6:1177–85. doi: 10.1093/intimm/6.8.1177. [DOI] [PubMed] [Google Scholar]
- 26.Leinikki PO, Mustonen M, Pasternack A. Immune response to oral polio vaccine in patients with IgA glomerulonephritis. Clin Exp Immunol. 1987;68:33–38. [PMC free article] [PubMed] [Google Scholar]
- 27.Van den Wall Bake AWL, Beyer WEP, Evers-Schouten JH, Hermans J, Daha MR, Masurel N, van Es LA. Humoral immune response to influenza vaccination in patients with primary IgA nephropathy. An analysis of isotype distribution and size of the influenza specific antibodies. J Clin Invest. 1989;84:1070–5. doi: 10.1172/JCI114269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fayette J, Dubois B, Vandenabeele S, et al. Human dendritic cells skew isotype switching of CD40-activated naive B cells toward IgA1 and IgA2. J Exp Med. 1997;185:1909–18. doi: 10.1084/jem.185.11.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ding L, Shevach EM. IL-10 inhibits mitogen-induced T cell proliferation by selectively inhibiting macrophage costimulatory function. J Immunol. 1992;148:3133–9. [PubMed] [Google Scholar]