Abstract
To investigate the effects of Mycobacterium tuberculosis on HIV-1 replication, peripheral blood mononuclear cells (PBMC) of bacille Calmette–Guérin (BCG)-vaccinated donors and non-BCG-vaccinated donors were infected in vitro with a lymphotropic isolate of HIV-1 and cultured in the presence of purified protein derivative (PPD). Addition of PPD resulted in enhanced HIV-1 replication and lymphoproliferation in BCG-vaccinated donor PBMC, while PPD had no such effects in control PBMC. HIV-1 replication increased even more when monocytes were removed from PBMC, while lymphoproliferation was decreased. High percentages of monocytes were associated with a decreased HIV-1 replication and proliferation that could not be reversed by addition of antibodies against the cytokines IL-1, transforming growth factor-beta (TGF-β) or indomethacin. PPD stimulates PBMC to release IL-10, a cytokine known to down-regulate proliferation and HIV-1 replication. PPD-induced effects on proliferation as well as HIV-1 replication could be partially blocked by adding a monoclonal antibody against MHC class II molecules, suggesting that part of the mechanism of PPD-induced enhancement is T memory cell activation.
Keywords: Mycobacterium, purified protein derivative, HIV, monocytes
INTRODUCTION
About one-third of the world population harbours latently Mycobacterium tuberculosis in the body without overt progression to disease. Infection with human HIV-1, however, reactivates latent M. tuberculosis, but also promotes progression of a primary infection to disease. In 1992 there was an estimated 4.4 million people world-wide coinfected with M. tuberculosis and HIV [1]. Infection with M. tuberculosis results in activation of macrophages and T cells that may harbour latent HIV-1. Although such cellular immune activation is needed for the host to fight mycobacterial disease, it may have an opposite effect on HIV-1 [2–5]. In vitro infection of macrophages with M. tuberculosis or stimulation with mycobacterial products induces release of several cytokines, such as tumour necrosis factor-alpha (TNF-α), IL-1, IL-6, IL-8, IL-10 and transforming growth factor-beta (TGF-β) [6–15]. Although TNF-α produced in response to M. tuberculosis is important in granuloma formation [16], it promotes HIV-1 expression in both monocytes and lymphocytes, via activation of nuclear factor κB (NFκB) [17–24]. For IL-1 and IL-6 also, HIV-1-enhancing effects have been described [18, 25]. IL-10 and TGF-β, both immunosuppressive cytokines, are thought to prevent excessive inflammation and tissue damage in tuberculosis (TB) infection [12]. IL-10 in some cases can inhibit HIV-1 replication, while for TGF-β-inhibiting as well as enhancing effects on HIV-1 replication have been described [26–31]. Since TB tends to occur early in the course of HIV disease, its effect on cytokine release may have an effect on viral burden and may influence the progression of HIV-1 disease. It has already been suggested that dual infection might accelerate HIV-1 disease. HIV-1-associated TB enhanced TNF-α expression and elevated β2-microglobulin (β2-M) levels [32]. Blood monocytes from TB patients showed an enhanced susceptibility to productive infection with HIV-1 [33]. Moreover, M. tuberculosis and its secreted products were shown to enhance expression of HIV-1 in monocytic cell lines by binding of NFκB to the enhancer region of the viral long-terminal repeat (LTR) [34, 35]. The present study was performed to examine the effects of proteins of M. tuberculosis, as present in purified protein derivative (PPD), on HIV-1 replication in peripheral blood mononuclear cells (PBMC). It was found that PPD significantly enhanced HIV-1 replication in PBMC from bacille Calmette–Guérin (BCG)-vaccinated donors. High percentages of monocytes mediated suppression of PPD-induced HIV-1 replication. A MoAb directed against MHC class II molecules reduced the PPD-induced enhancement of HIV-1 replication.
SUBJECTS AND METHODS
Human subjects
Venous blood samples were collected from healthy, BCG-vaccinated (Mantoux-positive) and non-BCG-vaccinated (Mantoux-negative), volunteers, who had no history of previous clinical TB. All volunteers were HIV-1−.
Reagents and monoclonal antibodies
Purified protein derivative (PPD-RT 47) was purchased from Statens Seruminstitut (Copenhagen, Denmark). Indomethacin was obtained from Sigma (St Louis, MO). Recombinant human IL-1β was obtained from Genzyme Diagnostics (Cambridge, MA). MoAb PdV5.2 directed against MHC class II molecules (anti-MHCII, IgG1) was a gift from Dr F. Koning (Leiden, The Netherlands). MoAb 60bca (anti-CD14, IgG1) was kindly provided by J. A. G. van Strijp (Department of Medical Microbiology, University of Utrecht). PE-labelled Leu-M3 and Leu-12 (Becton Dickinson, Mountain View, CA) were MoAbs used in FACS analysis on a FACScan flow cytometer (Becton Dickinson) to measure percentages of monocytes and B cells, respectively. Anti-IL-1β MoAb was purchased from R&D Systems, (Minneapolis, MN). Anti-TGF-β MoAb was purchased from Genzyme Diagnostics.
Preparation of lymphocytes
PBMC were isolated from heparinized blood by centrifugation on Ficoll–Histopaque (Pharmacia, Uppsala, Sweden). PBMC were suspended in RPMI 1640, supplemented with 5 mm HEPES, 19 mm sodium bicarbonate, 10 μg/ml gentamycin and 10% heat-inactivated fetal bovine serum (FBS; Hyclone Labs Inc., Logan, UT). For isolation of peripheral blood lymphocytes (PBL), the cells were allowed to adhere for 1 h at 37°C in 5% CO2 in a humidified atmosphere in fibronectin-coated tissue culture dishes (Costar, Cambridge, MA). In some experiments adherent cells (monocytes) were collected by gentle scraping with a rubber policeman. Non-adherent cells were loaded onto a nylon wool column. Nylon wool columns comprised about 1.5-g aliquots of nylon wool packed into 30-ml disposable syringes. The columns were preincubated with medium at 37°C for 30 min and washed before the cells were applied. The cells were incubated on columns for at least 1 h at 37°C and then eluted in a dropwise fashion. Subsequently, the columns were rinsed by addition of 20 ml prewarmed medium. Purified PBL suspensions contained < 1% monocytes as determined by FACScan analysis.
HIV-1 infection
The lymphotropic virus strain HIV-1AT, kindly provided by P. K. Peterson (University of Minneapolis, MN) [36], was grown to high titre in PBMC. TCID50, as determined according to Japour et al. [37], was found to be 1 × 104/ml. PBMC or PBL were infected for 2 h with HIV-1AT (1 × 104 TCID50/2 × 107 cells), washed twice to remove unbound virus, and cultured in 48-well plates (Costar) (1 × 106 cells/well) in culture medium in the presence of 0.1–100 μg/ml PPD. In experiments testing the influence of monocytes, different percentages (0–33%) of uninfected monocytes were seeded before HIV-1-infected PBL were added. In some control experiments with different percentages of monocytes, the cells were stimulated with phytohaemagglutinin (PHA; Sigma; 3 μg/ml) or the recall antigen tetanus toxoid (TT; 5 limit of flocculation (Lf)/ml; RIVM, Bilthoven, The Netherlands). After 7 days supernatants were collected and stored at −20°C until assayed for p24 antigen using an ELISA (Diagnostics Pasteur, Marnes la Coquette, France). In experiments studying kinetic effects of PPD and PHA on HIV-1 replication in PBMC, supernatants were collected subsequently on days 1, 3, 4, 7 and 10. The effect of recombinant human IL-1β (rIL-1β) on HIV-1 replication in PBL was tested in the presence of 5% monocytes using concentrations ranging from 0.1 to 1000 ng/ml. The effects of anti-IL-1β (10 μg/ml) and anti-TGF-β (25 μg/ml) on HIV-1 replication in PBL were tested in the presence of 0%, 5%, 20% and 33% of monocytes. In some experiments the effect of supernatant of PPD-stimulated monocytes was tested on HIV-1 replication. Therefore, monocytes of tuberculin non-reactors were isolated by adherence and incubated in the presence or absence of 10 or 100 μg/ml PPD, with or without additional indomethacin (1 μg/ml), in 24-well plates (Costar). After 24 h supernatants were collected and filtrated through 0.2-μm filters (Schleicher & Schuell, Dassel, Germany). Supernatants were added 1:2 and 1:4 diluted to PBL in the presence of 5% monocytes.
Proliferation assay
PBMC or PBL were suspended in culture medium and cultured in triplicate wells of 96-well flat-bottomed microtitre plates (Nunc, Roskilde, Denmark; 2 × 105 cells/well) in the presence of PPD (0.1–100 μg/ml) in a total volume of 200 μl for 4 days at 37°C in 5% CO2 in a humidified atmosphere. Experiments with different percentages of monocytes were cultured in the presence of 10 μg/ml PPD for 7 days. Eighteen hours before terminating each experiment, 1 μCi 3H-thymidine (3H-TdR), specific activity 5 Ci/mmol (The Radiochemical Centre, Amersham, UK), was added. Cells were collected onto glassfibre filters (printed filtermat A; Pharmacia, Wallac Oy, Turku, Finland) by means of a Titertek Cell Harvester 530 (Flow Labs, Irvine, UK). After harvesting the filters were dried for 1 h at 60°C and placed in betaplate sample bags (Pharmacia). Subsequently, 10 ml of betaplate scintillation fluid were added, whereafter the sample bags were sealed and placed in T-Tray cassettes. Radioactivity was measured by using a 1205 betaplate liquid scintillation counter (Pharmacia).
Statistical analysis
Comparisons between the BCG-vaccinated and non-BCG-vaccinated groups and between PBMC and PBL were done by Student's t-test for independent samples; n refers to the number of subjects used as PBMC donors. P ≥ 0.05 was considered not significant.
RESULTS
Effect of PPD on HIV-1 replication in PBMC
The effects of PPD on HIV-1 replication in PBMC were studied. Using PBMC of random donors, variable effects of PPD on HIV-1 replication were found, ranging from no effect to high effects (data not shown). In order to include activation of antigen-specific T lymphocytes, cells were divided into PBMC from BCG-vaccinated and non-BCG-vaccinated donors. PBMC from BCG-vaccinated donors showed higher PPD-induced HIV-1 replication compared with PBMC from non-BCG-vaccinated donors (Fig. 1). Kinetic experiments, using PHA as a stimulus for T cells, revealed that the optimal effect of PHA on HIV-1 replication (day 3) preceded the optimal effect of PPD on HIV-1 replication (day 7). In some BCG-vaccinated donors PPD showed hardly any effect on HIV-1 replication (data not shown). Because monocytes can suppress PPD-induced blastogenesis of PBMC from healthy tuberculin-positive subjects, monocytes were removed from PBMC as described in Subjects and Methods. FACScan analysis revealed that PBL contained < 1% monocytes. Comparing the effects of PPD on HIV-1 replication in PBL with PBMC, it was found that PPD-induced effects on HIV-1 replication in PBL were significantly higher compared with PPD-induced effects in PBMC (Fig. 2).
Fig. 1.

Purified protein derivative (PPD)-induced HIV-1 replication in peripheral blood mononuclear cells (PBMC) of bacille Calmette–Guérin (BCG)-vaccinated and non-BCG-vaccinated donors. PBMC of BCG-vaccinated (□) and non-BCG-vaccinated donors (▪) were cultured in the presence of increasing amounts of PPD for 7 days. Before the addition of PPD the cells were infected for 2 h with HIV-1. The amount of HIV-1 produced during the total incubation period was assessed by a p24 ELISA. Relative amounts of p24 were determined and expressed as stimulation index (SI), amount of p24 above background level. Mean ± s.d. of five individuals is shown. Each determination was performed in triplicate. *P < 0.05; **P < 0.01; ***P < 0.005.
Fig. 2.
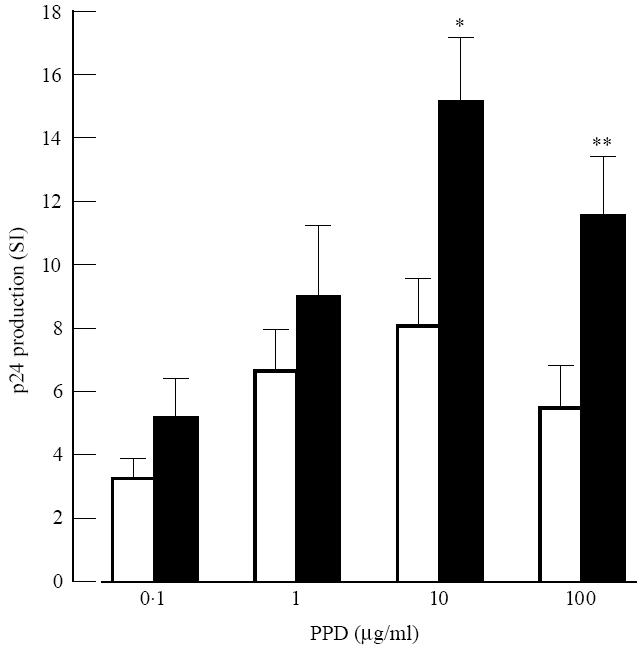
Purified protein derivative (PPD)-induced HIV-1 replication in peripheral blood mononuclear cells (PBMC) and peripheral blood lymphocytes (PBL) of bacille Calmette–Guérin (BCG)-vaccinated donors. Comparison of PBMC (□) and PBL (▪) of BCG-vaccinated donors in their capacity to promote HIV-1 replication after stimulation by increasing amounts of PPD. For details, see Fig. 1.
Effect of PPD on proliferation in PBMC and PBL
As an increase in HIV-1 replication is often associated with cell division of the infected cells, PPD-induced HIV-1 replication in PBMC and PBL were compared, in parallel experiments, with PPD-induced proliferation of these cells. PPD-induced proliferation of PBMC gave comparable results to those of PPD-induced HIV-1 replication (Fig. 3a). However, in PBL from BCG-vaccinated donors the PPD-induced proliferation was significantly lower compared with the PPD-induced effect on HIV-1 replication (Fig. 3b).
Fig. 3.

Purified protein derivative (PPD)-induced HIV-1 replication and proliferation of peripheral blood mononuclear cells (PBMC) and peripheral blood lymphocytes (PBL) of bacille Calmette–Guérin (BCG)-vaccinated donors. The effect of PPD on HIV-1 replication in PBMC (a) and PBL (b) was measured after 7 days of incubation (○). For proliferation assays, 3H-TdR incorporation was measured after 4 days of incubation (•). Relative amounts of p24 and 3H-TdR incorporation were determined and expressed as stimulation index (SI). Mean ± s.d. for HIV-1 replication (n = 5) and 3H-TdR incorporation (n = 3) are shown. Each determination was performed in triplicate. *P < 0.05; **P < 0.01; ***P < 0.005.
Effect of monocytes on PPD-induced HIV-1 replication
As the effects of PPD on HIV-1 replication were higher in PBL from BCG-vaccinated donors compared with PBMC (Fig. 3b), the effects of monocytes on PPD-induced HIV-1 replication were investigated. Therefore, PBL were incubated with different percentages of autologous monocytes, ranging from 0% to 33% monocytes. In order to keep HIV-1 infection of the cells comparable, PBL were first infected with HIV-1AT before different percentages of uninfected monocytes were added. Cells were stimulated with 10 μg/ml PPD. Figure 4 shows an optimal PPD-induced effect when about 5% monocytes were present. The presence of higher percentages of monocytes decreased the PPD-induced effects on HIV-1 replication. Comparable effects were found when lymphoproliferation was studied (Fig. 5). The decreasing effects of monocytes on PPD-induced HIV-1 replication could not be blocked by adding anti-IL-1β, nor was exogenous rIL-1β able to mimic the decreasing effects. Supernatant of monocytes stimulated with PPD without or together with indomethacin, to increase IL-1 activity [38], was also not able to suppress PPD-induced HIV-1 replication in PBL in the presence of 5% monocytes. Anti-TGF-β also had no effect on the suppressive action of monocytes. Prostaglandin E2 (PGE2), which can be produced by monocytes after PPD stimulation [9], can inhibit cell proliferation. However, indomethacin, an inhibitor of PGE2 synthesis, could also not decrease the monocyte-induced suppression (data not shown). Another cytokine that down-regulates antigen-specific T cell proliferation, and hence HIV replication as well, is IL-10 [31, 39, 40]. Therefore, we studied the ability of PPD to induce IL-10 in PBMC. At 1 day after stimulation a five-fold increase (44 ng) of IL-10 was observed in BCG-vaccinated donors versus a two-fold increase in the non-vaccinated group. Experiments performed with PHA or TT did not show decreasing effects on HIV-1 replication when high percentages of monocytes were present (data not shown).
Fig. 4.

Suppression of purified protein derivative (PPD)-induced HIV-1 replication by increasing amounts of monocytes. HIV-1-infected peripheral blood lymphocytes (PBL) were incubated together with different percentages of autologous monocytes in the presence of 10 μg/ml PPD. After 7 days, p24 production was determined. Mean ± s.d. of three individuals is shown. Each determination was performed in triplicate.
Fig. 5.

Suppression of purified protein derivative (PPD)-induced lymphoproliferation by increasing amounts of monocytes. Peripheral blood lymphocytes (PBL) were incubated together with different percentages of autologous monocytes in the presence of 10 μg/ml PPD. Lymphoproliferation was determined after 7 days of incubation. Mean ± s.d. of three individuals is shown. Each determination was performed in triplicate.
Effect of anti-MHC class II MoAb on PPD-induced HIV-1 replication
To investigate whether a MoAb against MHC class II could inhibit the PPD-induced effects on HIV-1 replication, anti-MHC class II MoAb PdV5.2 was tested. Anti-CD14 MoAb 60bca was used as a control MoAb of the same isotype. MoAb PdV5.2 could partially block PPD-induced HIV-1 replication in PBL (Fig. 6), suggesting that the few monocytes present in PBL could still stimulate PPD-specific T lymphocytes by means of antigen presentation. Comparable effects were found when studying lymphoproliferation. The experiments are in agreement with previous findings from our laboratory [40].
Fig. 6.
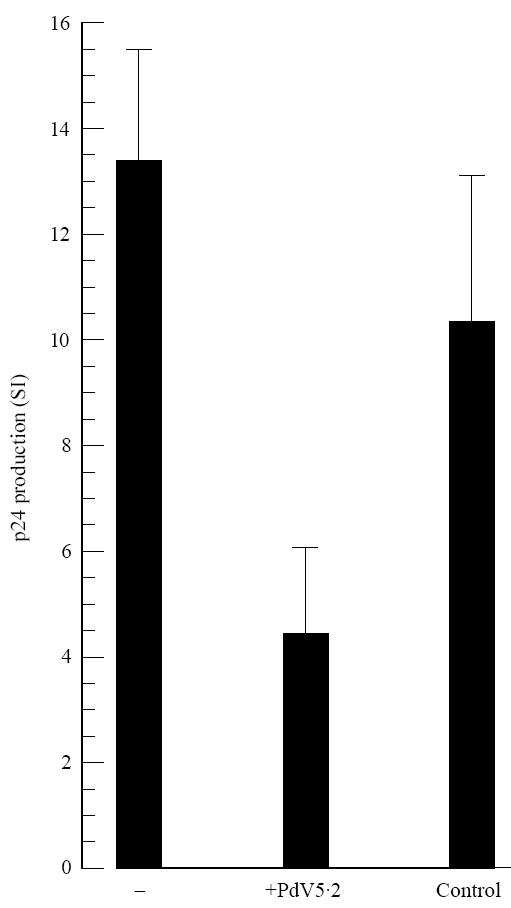
Partial inhibition of purified protein derivative (PPD)-induced HIV-1 replication by anti-MHC class II MoAb. HIV-1-infected peripheral blood lymphocytes (PBL) of bacille Calmette–Guérin (BCG)-vaccinated donors were incubated with 10 μg/ml PPD for 7 days, in the presence (PdV5.2) or absence (−) of anti-MHC class II MoAb PdV5.2, and p24 production was determined. As an isotype control, anti-CD14 MoAb 60bca was used (control). Mean ± s.d. of three individuals is shown. Each determination was performed in triplicate.
DISCUSSION
In the present experiments, the in vitro effects of PPD on HIV-1 replication in PBMC were investigated. We found quite a variation in PPD-induced effects when studying PBMC of random donors.
Because PPD can activate antigen-specific T cells, cells were divided into PBMC from BCG-vaccinated and non-BCG-vaccinated donors. It was then demonstrated that PPD-enhanced replication of a lymphotropic HIV-1 strain occurred in cells from BCG-vaccinated individuals. Similar effects were also found when PPD-induced proliferation was studied. PBMC of some BCG-vaccinated donors hardly showed an effect of PPD on HIV-1 replication. It has already been described that monocytes can act as suppressor cells in response to PPD [41, 42]. In patients with newly diagnosed pulmonary TB, negative tuberculin tests have been associated with depressed PPD-induced blastogenesis in PBMC. These low responders showed an increased proportion of monocytes among the PBMC compared with the high tuberculin responders. Depletion of adherent cells produced a 24-fold increment in PPD-induced blastogenesis in tuberculin low responders compared with a three-fold increase in high responders [41].
To investigate if low PPD-induced effects on HIV-1 replication in some BCG-vaccinated donors was due to the presence of PPD-induced suppressor monocytes, PBMC were depleted of monocytes. While effects of PPD on HIV-1 replication and lymphoproliferation were comparable in PBMC, depletion of monocytes resulted in different effects of PPD on HIV-1 replication and lymphoproliferation. In PBL, we demonstrated that PPD enhanced HIV-1 replication compared with PBMC, while PPD-induced lymphoproliferation was lower in PBL compared with PBMC. Further investigation of the effect of different percentages of monocytes on HIV-1 replication showed that the presence of about 5% of monocytes yielded an optimal PPD-induced HIV-1 replication. Higher percentages of monocytes decreased the PPD-induced effects on HIV-1 replication. Similar effects were observed when lymphoproliferation was studied. The same experiments with PHA or TT showed no monocyte-induced decrease in HIV-1 replication, suggesting that the PPD-induced decreasing effect of high percentages of monocytes on HIV-1 replication was an antigen-specific effect.
Suppression by monocytes in TB patients has been associated with expression of class II MHC products [43] and expression and release of IL-2 receptors by monocytes from TB patients [44]. However, these observations were all made with monocytes of TB patients comparing them with monocytes from healthy tuberculin reactors. In the present study monocyte-induced suppression was found using cells of healthy tuberculin reactors, suggesting that another mechanism must play a role in the monocyte-induced suppression. Suppression by monocytes has also been attributed to increased production of IL-1 by monocytes stimulated with mycobacterial antigens [6, 38]. IL-1 production by monocytes showed a positive correlation with monocyte suppressor activity for PPD-induced blastogenesis. PPD was also able to induce IL-1 release by monocytes of healthy tuberculin non-reactors. Moreover, exogenous IL-1 was capable of suppressing PPD-induced blastogenesis of PBMC of healthy tuberculin reactors, suggesting that PPD-induced IL-1 production by monocytes could be the suppressive factor in our cell system. However, exogenous rIL-1β showed no suppressive effect on PPD-induced HIV-1 replication, and anti-IL-1β was not able to block the PPD-induced monocyte suppression. Also, the supernatant of PPD-stimulated monocytes did not suppress PPD-induced HIV-1 replication. This indicates that IL-1 is not involved as a mediator of monocyte-induced suppression in our cell system.
Another candidate for mediation of suppression by monocytes is TGF-β. TGF-β is present in significant concentrations in the supernatants of unstimulated monocytes from patients with TB. PPD further stimulated these monocytes for expression of TGF-β [15]. Peterson et al. found a dose-dependent alteration of the HIV-1 replication induced by TGF-β. Low concentrations of TGF-β increased HIV-1 replication, while higher concentrations of TGF-β decreased HIV-1 replication [27]. Therefore, it is possible that when more monocytes are present, PPD induces an increase in TGF-β production, influencing HIV-1 expression. However, anti-TGF-β could not block the PPD-induced suppressive action of monocytes. Immunosuppression by monocytes in health is usually attributed to production of prostaglandins [45]. As PPD also induces production of PGE2 by monocytes [9], PGE2 could also be the factor causing the monocyte-induced immunosuppression. However, indomethacin, a cyclo-oxygenase inhibitor, which blocks synthesis of PGE2, could not inhibit the monocyte-induced decreasing effects on HIV-1 replication.
Until now we have not been able to explain the monocyte-induced inhibition of PPD-responses on HIV-1 replication in BCG-vaccinated donors. A role for IL-10 can not be excluded [31, 39, 40]. A five-fold increase of IL-10 was observed 1 day after in vitro stimulation with PPD using PBMC derived from BCG-vaccinated donors. On the other hand, antigen-specific stimulation of PBMC with TT (recall antigen) or non-specific stimulation with PHA had no decreasing effects on HIV-1 replication when high percentages of monocytes were present. A trivial explanation is that higher numbers of monocytes less efficiently present the same amount of antigen to T cells than lower numbers of monocytes do. PPD-induced effects on lymphoproliferation and HIV-1 replication in PBL of BCG-vaccinated donors could partially be inhibited by the anti-MHC class II MoAb PdV5.2. This indicates that part of the PPD-induced effects on HIV-1 replication is activation of PPD-specific memory T cells [40]. The observations are in line with other reports on virus replication after vaccination of HIV-1-infected individuals [2–5], resulting in a higher temporary viral load. This study stresses the role of monocytes in this process.
References
- 1.Snider DE, Jr, La Montagne JR. The neglected global tuberculosis problem: a report of the 1992 world congress on tuberculosis. J Infect Dis. 1994;169:1189–96. doi: 10.1093/infdis/169.6.1189. [DOI] [PubMed] [Google Scholar]
- 2.Staprans SI, Hamilton BL, Follansbee SE, Elbeik T, Barbosa P, Grant RM, Feinberg MB. Activation of virus replication after vaccination of HIV-1-infected individuals. J Exp Med. 1995;182:1727–37. doi: 10.1084/jem.182.6.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Røsok B, Voltersvik P, Bjerknes R, Axelsson M, Haaheim LR, Åsjö B. Dynamics of HIV-1 replication following influenza vaccination of HIV+ individuals. Clin Exp Immunol. 1996;104:203–7. doi: 10.1046/j.1365-2249.1996.25732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stanley SK, Ostrowski MA, Justement JS, et al. Effect of immunization with common recall antigen on viral expression in patients infected with human immunodeficiency virus type 1. New Engl J Med. 1996;334:1222–30. doi: 10.1056/NEJM199605093341903. [DOI] [PubMed] [Google Scholar]
- 5.Goletti D, Weissman D, Jackson RW, et al. Effect of Mycobacterium tuberculosis on HIV replication. Role of immune activation. J Immunol. 1996;157:1271–8. [PubMed] [Google Scholar]
- 6.Wallis RS, Fujiwara H, Ellner JJ. Direct stimulation of monocyte release of interleukin 1 by mycobacterial protein antigens. J Immunol. 1986;136:193–6. [PubMed] [Google Scholar]
- 7.Valone SE, Rich EA, Wallis RS, Ellner JJ. Expression of tumor necrosis factor in vitro by human mononuclear phagocytes stimulated with whole Mycobacterium bovis BCG and mycobacterial antigens. Infect Immun. 1988;56:3313–5. doi: 10.1128/iai.56.12.3313-3315.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallis RS, Amir-Tahmassed M, Ellner JJ. Induction of interleukin 1 and tumor necrosis factor by mycobacterial proteins: the monocyte Western blot. Proc Natl Acad Sci USA. 1990;87:3348–52. doi: 10.1073/pnas.87.9.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cadranel J, Philippe C, Perez J, Milleron B, Akoun G, Ardaillou R, Baud L. In vitro production of tumour necrosis factor and prostaglandin E2 by peripheral blood mononuclear cells from tuberculosis patients. Clin Exp Immunol. 1990;81:319–24. doi: 10.1111/j.1365-2249.1990.tb03338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogawa T, Uchida H, Kusumoto Y, Mori Y, Yamamura Y, Hamada S. Increase in tumor necrosis factor alpha- and interleukin-6-secreting cells in peripheral blood mononuclear cells from subjects infected with Mycobacterium tuberculosis. Infect Immun. 1991;59:3012–25. doi: 10.1128/iai.59.9.3021-3025.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnes PF, Chatterjee D, Abrams JS, Lu S, Wang E, Yamamura M, Brennan PJ, Modlin RL. Cytokine production induced by Mycobacterium tuberculosis lipoarabinomannan. Relationship to chemical structure. J Immunol. 1992;149:541–7. [PubMed] [Google Scholar]
- 12.Maeda J, Ueki N, Ohkawa T, Iwahashi N, Takano T, Hada T, Higashino K. Local production and localization of transforming growth factor-beta in tuberculous pleurisy. Clin Exp Immunol. 1993;92:32–38. doi: 10.1111/j.1365-2249.1993.tb05944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallis RS, Ellner JJ. Cytokines and tuberculosis. J Leukoc Biol. 1994;55:677–81. doi: 10.1002/jlb.55.5.676. [DOI] [PubMed] [Google Scholar]
- 14.Johnson BJ, McMurray DN. Cytokine gene expression by cultures of human lymphocytes with autologous Mycobacterium tuberculosis-infected monocytes. Infect Immun. 1994;62:1444–50. doi: 10.1128/iai.62.4.1444-1450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toossi Z, Young T-G, Averill LE, Hamilton BD, Shiratsuchi H, Ellner JJ. Induction of transforming growth factor β by purified protein derivate of Mycobacterium tuberculosis. Infect Immun. 1995;63:224–8. doi: 10.1128/iai.63.1.224-228.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kindler V, Sappino AP, Grau GE, Piguet PF, Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989;56:731–40. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- 17.Israël N, Hazan U, Alcami J, Munier A, Arenzana-Seisdedos F, Bachelerie F, Israël A, Virelizier JL. Tumor necrosis factor stimulates transcription of HIV-1 in human T lymphocytes, independently and synergistically with mitogens. J Immunol. 1989;143:3956–60. [PubMed] [Google Scholar]
- 18.Osborn L, Kunkel S, Nabel GJ. Tumor necrosis factor α and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of nuclear factor κB. Proc Natl Acad Sci USA. 1989;86:2336–40. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duh EJ, Maury WJ, Folks TM, Fauci AS, Rabson AB. Tumor necrosis factor α activates human immunodeficiency virus type 1 through induction of nuclear factor binding to the NF-κB sites in the long terminal repeat. Proc Natl Acad Sci USA. 1989;86:5974–8. doi: 10.1073/pnas.86.15.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okamoto T, Matsuyama T, Mori S, et al. Augmentation of human immunodeficiency virus type 1 gene expression by tumor necrosis factor α. Aids Res Hum Retrovir. 1989;5:131–8. doi: 10.1089/aid.1989.5.131. [DOI] [PubMed] [Google Scholar]
- 21.Folks TM, Clouse KA, Justement J, Rabson A, Duh E, Kehrl JH, Fauci AS. Tumor necrosis factor α induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc Natl Acad Sci USA. 1989;86:2365–8. doi: 10.1073/pnas.86.7.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vyakarnam A, McKeating J, Meager A, Beverley PC. Tumor necrosis factors (α,β) induced by HIV-1 in peripheral blood mononuclear cells potentiate virus replication. AIDS. 1990;4:21–27. doi: 10.1097/00002030-199001000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Poli G, Kinter A, Justement JS, Kehrl JH, Bressler P, Stanley S, Fauci AS. Tumor necrosis factor α functions in an autocrine manner in the induction of human immunodeficiency virus expression. Proc Natl Acad Sci USA. 1990;87:782–6. doi: 10.1073/pnas.87.2.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuyama T, Kobayashi N, Yamamoto N. Cytokines and HIV infection: is AIDS a tumor necrosis factor disease? AIDS. 1991;5:1405–17. doi: 10.1097/00002030-199112000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi N, Hamamoto Y, Koyanagi Y, Chen ISY, Yamamoto N. Effect of interleukin-1 on the augmentation of human immunodeficiency virus gene expression. Biochem Biophys Res Commun. 1989;165:715–21. doi: 10.1016/s0006-291x(89)80025-7. [DOI] [PubMed] [Google Scholar]
- 26.Poli G, Bressler P, Kinter A, et al. Interleukin 6 induces human immunodeficiency virus expression in infected monocytic cells alone and in synergy with tumor necrosis factor α by transcriptional and post-transcriptional mechanisms. J Exp Med. 1990;172:151–8. doi: 10.1084/jem.172.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peterson PK, Gekker G, Chao CC, Schut R, Molitor TW, Balfour HH., Jr Cocaine potentiates HIV-1 replication in human peripheral blood mononuclear cell cocultures. J Immunol. 1991;146:81–84. [PubMed] [Google Scholar]
- 28.Poli G, Kinter AL, Justement JS, Bressler P, Kehrl JH, Fauci AS. Transforming growth factor β suppresses human immunodeficiency virus expression and replication in infected cells of the monocyte/macrophage lineage. J Exp Med. 1991;173:589–97. doi: 10.1084/jem.173.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lazdins JK, Klimkait T, Woods-Cook K, et al. In vitro effect of transforming growth factor-β on progression of HIV-1 infection in primary mononuclear phagocytes. J Immunol. 1991;147:1201–7. [PubMed] [Google Scholar]
- 30.Lazdins JK, Klimkait T, Woods-Cook K, et al. The replicative restriction of lymphocytotropic isolates of HIV-1 in macrophages is overcome by TGF-β. AIDS Res Hum Retrovir. 1992;8:505–11. doi: 10.1089/aid.1992.8.505. [DOI] [PubMed] [Google Scholar]
- 31.Mackewicz CE, Ortega H, Levy JA. Effect of cytokines on HIV replication in CD4+ lymphocytes: lack of identity with the CD8+ cell antiviral factor. Cell Immunol. 1994;153:329–43. doi: 10.1006/cimm.1994.1032. [DOI] [PubMed] [Google Scholar]
- 32.Wallis RS, Vjecha M, Amir-Tahmasseb M, et al. Influence of tuberculosis on human immunodeficiency virus (HIV-1): enhanced cytokine expression and elevated β2-microglobulin in HIV-1-associated tuberculosis. J Infect Dis. 1993;167:43–48. doi: 10.1093/infdis/167.1.43. [DOI] [PubMed] [Google Scholar]
- 33.Toossi Z, Sierra-Madero JG, Blinkhorn RA, Mettler MA, Rich EA. Enhanced susceptibility of blood monocytes from patients with pulmonary tuberculosis to productive infection with human immunodeficiency virus type 1. J Exp Med. 1993;177:1511–6. doi: 10.1084/jem.177.5.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shattock RJ, Friedland JS, Griffin GE. Phagocytosis of Mycobacterium tuberculosis modulates human immunodeficiency virus replication in human monocytic cells. J Gen Virol. 1994;75:849–56. doi: 10.1099/0022-1317-75-4-849. [DOI] [PubMed] [Google Scholar]
- 35.Lederman MM, Georges DL, Kusner DJ, Mudido P, Giam C-Z, Toossi Z. Mycobacterium tuberculosis and its purified protein derivate activate expression of the human immunodeficiency virus. J Acq Imm Def Syndr. 1994;7:727–33. [PubMed] [Google Scholar]
- 36.Peterson PK, Sharp BM, Gekker G, Portoghese PS, Sannerud K, Balfour HH., Jr Morphine promotes the growth of HIV-1 in human peripheral blood mononuclear cell cocultures. AIDS. 1990;4:869–73. doi: 10.1097/00002030-199009000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Japour AJ, Mayers DL, Johnson VA, et al. Standardized peripheral blood mononuclear cell culture assay for determination of drug susceptibilities of clinical human immunodeficiency virus type 1 isolates. Antimicrob Agents Chemother. 1993;37:1095–101. doi: 10.1128/aac.37.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujiwara H, Kleinhenz ME, Wallis RS, Ellner JJ. Increased interleukin-1 production and monocyte suppressor cell activity associated with human tuberculosis. Am Rev Respir Dis. 1986;133:73–77. doi: 10.1164/arrd.1986.133.1.73. [DOI] [PubMed] [Google Scholar]
- 39.Orendi JM, Verheul AFM, de Vos NM, et al. Mannoproteins of Cryptococcus neoformans induce proliferative response in human peripheral blood mononuclear cells (PBMC) and enhance HIV-1 replication. Clin Exp Immunol. 1997;107:293–9. doi: 10.1111/j.1365-2249.1997.283-ce1169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waal Malefyt R, Haanen J, Spits H, et al. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–24. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ellner JJ. Suppressor adherent cells in human tuberculosis. J Immunol. 1978;121:2573–9. [PubMed] [Google Scholar]
- 42.Ellner JJ. Regulation of the human cellular immune response to Mycobacterium tuberculosis. The mechanism of selective depression of the response to PPD. Bull Int Union Tuberc Lung Dis. 1991;66:129–32. [PubMed] [Google Scholar]
- 43.Tweardy DJ, Schacter BZ, Ellner JJ. Association of altered dynamics of monocyte surface expression of human leukocyte antigen DR with immunosuppression in tuberculosis. J Infect Dis. 1984;149:31–37. doi: 10.1093/infdis/149.1.31. [DOI] [PubMed] [Google Scholar]
- 44.Toossi Z, Sedor JR, Lapurga JP, Ondash RJ, Ellner JJ. Expression of functional interleukin 2 receptors by peripheral blood monocytes from patients with active pulmonary tuberculosis. J Clin Invest. 1990;85:1777–84. doi: 10.1172/JCI114635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ellner JJ, Spagnuolo PJ. Suppression of antigen and mitogen-induced human T lymphocyte DNA synthesis by bacterial lipopolysaccharide: mediation by monocyte activation and production of prostaglandins. J Immunol. 1979;123:2689. [PubMed] [Google Scholar]


