Abstract
The phenotypic changes of T lymphocytes during the reactivation of latent Mycobacterium tuberculosis infection by activation of the hypothalamic–pituitary–adrenal (HPA) axis was monitored using flow cytometric analysis. Subsets of CD4+ and CD8+ lymphocyte populations from the lung, spleen and draining lymph nodes of infected mice were identified based on their differential expression of the cell surface antigens CD44 and CD45RB. Latent infection was characterized by an accumulation of both naive, activated and memory CD4 and CD8 T lymphocytes in the lung and mediastinal lymph nodes. No changes were observed in the spleen of mice with latent infection when compared with uninfected mice. Immediately following the activation of the HPA axis, a reduction in all CD4+ and CD8+ T cells in the lung and mediastinal lymph nodes was observed. This correlated with the reactivation of mycobacterial growth. The decrease was transient for memory and naive CD4 and CD8 T lymphocyte populations in the lung. However, the number of naive CD4 and CD8 T lymphocyte populations in the mediastinal lymph node following reactivation was less than that found in mice with latent infection. These data provide the first characterization of T lymphocyte populations which may be functionally involved in the immunological response to HPA axis-induced reactivation of M. tuberculosis infection.
Keywords: tuberculosis, T cells, stress, hypothalamic–pituitary–adrenal axis
INTRODUCTION
Infection of individuals with Mycobacterium tuberculosis results in the establishment of immunity to the bacterium and the control of Mycobacterial growth [1, 2]. The immunologically mediated inhibition of bacterial growth is thought to be mediated by antigen-specific T cells that result in macrophage activation [3–6]. The development of cell-mediated immunity results in the establishment of a latent infection; that is, viable bacteria are present but they do not increase in number. The latent state can last for the life of an infec\ted individual, who stands a 10% life time chance that the growth of the bacteria will reactivate. Reactivation leads to the growth of large numbers of bacteria, which disseminate into the airways and spread to the general population via droplet aerosol. The reactivation of the growth of the Tubercule bacillus is probably the result of a temporary suppression of the immune response, thus permitting the bacteria to grow [2,7–10].
Studies in our laboratory have shown that the growth of M. tuberculosis can be reactivated by activation of the hypothalamic–pituitary–adrenal (HPA) axis [11–13]. A steady state infection was established following systemic infection with low numbers of Mycobacteria. Reactivation resulted in the re-initiation of growth of the bacterium, especially in the lungs of mice. We show now that intranasal infection of mice, as described by Saunders & Cheers [14], also results in a steady state infection that was initially localized to the lung. Activation of the HPA axis resulted in the reactivation of the growth of the Tubercule bacillus. We monitored the changes in T cells following the establishment of the steady state infection and during reactivation by multi-parameter flow cytometry. Reactivation of mycobacterial growth by HPA axis activation resulted in changes in both CD4 and CD8 memory populations in the lung as well as the mediastinal lymph nodes. The results suggest that CD8 T cells may play an important role in the maintenance of latency.
MATERIALS AND METHODS
Mice
Specific pathogen-free male BALB/c mice were obtained from Charles River Labs (Wilmington, MA). Mice were 6–8 weeks of age at the start of each experiment. The animals were housed under sterile conditions in micro isolator cages (Lab Products, Maywood, NJ) in a BSL-3 facility and given food and water ad libitum.
Bacterial infection
Mycobacterium tuberculosis (Erdman) was obtained from the American Type Culture Collection (ATCC 35801, TMC 107; Rockville, MD) and maintained as previously described by us [11]. Mice were infected intranasally as described by Saunders & Cheers [14]. In preliminary experiments we determined that instillation of 103 colony-forming units (CFU) resulted in the appearance of lung colonies 2 weeks after inoculation. Lower doses did not yield productive infections in all inoculated mice, while higher doses resulted in the progressive growth of the bacillus. The mice were inoculated, following anaesthesia with a ketamine/xylacine mixture, with 25 μl of the bacterial suspension containing 1000 CFU placed on the external nares with a micropipette. The number of microorganisms was confirmed by plate count. Bacterial growth was monitored by determining the number of CFU in the lung and spleen at various times after inoculation. A steady state infection was achieved by week 12 after the initial infection. This is similar to that which we had previously observed following systemic infection using 100 CFU [13].
HPA axis activation
The HPA axis was activated as previously described by us using a restraint paradigm [11–13]. Briefly, mice were placed into well ventilated 50-ml conical centrifuge tubes overnight. The mice were restrained for five daily 15-h cycles, rested for 2 days and then restrained again for five 15-h cycles. The mice were placed in conventional housing at the end of each period of HPA activation. Control mice, with latent infection, were housed without access to food and water.
Lymphocyte preparation
Mice were killed at selected time points following reactivation and the spleen and mediastinal lymph nodes were removed and placed separately into 5 ml of ice cold Dulbecco's modified Eagles' medium (DMEM; Gibco BRL, Grand Island, NY) supplemented with 20% heat-inactivated fetal bovine serum (FBS; Hyclone, Logan, UT) containing glutamine and penicillin/streptomycin. Single-cell suspensions were prepared as previously described [11], resuspended in PBS and stored on ice until analysed.
The lungs were placed into Hanks' balanced salt solution (HBSS) containing 1% bovine serum albumin (BSA; Sigma, St Louis, MO), aspirated to remove blood and then cut into fine pieces. The tissue was digested for 45 min with a pre-screened lot of collagenase (150 U/ml; Worthington Biochemical, Cleveland, OH). The digested tissue was passed through 40 G stainless steel sieves and then through 18 G and 21 G gauge needles. The lymphocytes were purified from the lung cell suspension by centrifugation through a Ficoll–Paque (Pharmacia, Uppsala, Sweden) gradient. Following centrifugation the purified lymphocyte population was washed twice with PBS.
Flow cytometric analysis
Flow cytometric analysis was used to determine the expression of CD3, CD4, CD8, CD44 and CD45 by the T cell populations. The following conjugated antibodies were obtained from Pharmingen (San Diego, CA): anti-CD3, cytochrome-conjugated; anti-CD8, PE-conjugated; anti-CD45RB, FITC-conjugated and biotinylated anti-CD44. Anti-CD4 conjugated to RED 613 was obtained from Gibco BRL and streptavidin conjugated to AMCA was from Vector Technology (Burlingame, CA). Staining was performed by incubating 1–2 × 106 cells with antibody (1 μg/ml) for 25 min at 4°C. After washing twice the strepavidin-conjugated AMCA was added and the samples incubated for an additional 20 min before washing with ice-cold PBS. Five-colour multi-parameter analysis was performed on a Coulter EPICS Elite flow cytometer (Coulter Corp., Hialeah, FL). Optical laser alignment calibration of the flow cytometer was performed using Coulter's DNA-Check EPICS alignment fluoro-sphere beads (Coulter) with coefficient of variations routinely < 2%. Standard Brite (Coulter) fluoro-sphere reference channel beads were used to standardize photomultiplier tube fluorescent intensities. Approximately 100 000 lymphocytes at a rate of 500 events/s were gated from monocytes and debris using linear forward light scatter versus 90° light scatter characteristics. CD3–Cychrome, CD4–PE, CD8–Red 613, and CD45RB–FITC were excited with a 488-nm 15-mW air-cooled Argon laser, and fluorescent light emission was collected through 675-nm, 575-nm, 610-nm and 525-nm bandpass filters, respectively. CD44–AMCA was excited with multiline UV (351–364 nm) 50-mW water-cooled Argon laser and collected with a 460-nm bandpass filter at 40-μs gated amplifier delay. All fluorescent signals were measured in logarithmic mode. Data files were stored in list mode format and extended analyses was performed using Winlist Software (Verity Software House, Topsham, ME). Each single colour-stained control was used to establish an off line compensation curve using Winlist Software. The T cells were identified by gating of the CD3+ cells. Those cells were then analysed for CD4 and CD8 expression and those populations were further analysed for the expression of CD45RB and CD44. The MoAbs to CD44 and CD45RB revealed bimodal staining patterns which resulted in the identification of cells with bright and dim intensities indicating the presence of two populations. The data represent the means of four different experiments using three mice per time point. Data are expressed as cell numbers based on the total yield of cells and the percentage of each population.
Preparation of tissue section for histologic examination and staining
The lungs were perfused via the trachea with HBSS and immersed for at least 24 h with 10% buffered formalin. Specimens were processed, stained with haematoxylin and eosin and sectioned routinely for light microscopic quantitative evaluation.
Statistical analysis
The differences in T lymphocyte populations at each time point was determined by one-way analysis of variance (anova) (StatMost, Salt Lake City, UT). The differences between the T lymphocyte populations from uninfected mice and mice with latent infection were evaluated, as was the effect of time on changes in T cells from latently infected mice. The changes in T cell subpopulations that resulted from activation of the HPA axis were also analysed by anova. The results were significant if they equalled or exceeded the 95% confidence level (P < 0.05).
RESULTS
Reactivation of M. tuberculosis growth by activation of the HPA axis
Activation of the HPA axis resulted in the resumption of growth of M. tuberculosis. The results in Fig. 1 show that the number of microorganisms isolated from the lung following intranasal instillation increased to 46 400 CFU at week 6 after infection and then decreased until it reached a level that varied from 14 000 to 28 000 CFU/lung by weeks 12–14. This level remained relatively constant for the duration of the experiment. Following activation of the HPA axis beginning at week 12, the numbers of bacteria increased to 81 600 CFU/lung by week 20 before declining. Bacteria were also detected in the spleen, and the pattern of growth, while similar, remained 10-fold less than that isolated from the lungs (data not shown).
Fig. 1.
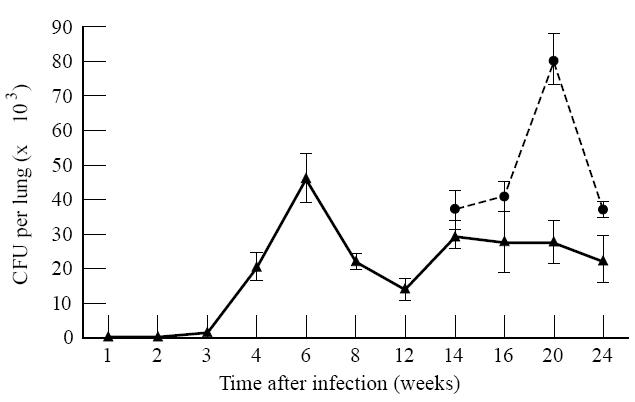
Mycobacterium tuberculosis growth and reactivation. Mice were infected with 103 colony-forming units (CFU) of M. tuberculosis (Erdman) intranasally. Growth in the lung was monitored at the times indicated. Once a latent infection was established, the hypothalamic–pituitary–adrenal (HPA) axis was activated by restraining the mice for 10 15-h sessions. The growth of the Mycobacteria was monitored by plate count. The time represents weeks after initial infection. Values represent means ± s.e.m. of n = 4 individual groups of three mice. The effect of HPA activation and of time was significant as determined by anova. ▴, Control; •, HPA activation.
Analysis of T cell populations in mice with latent tuberculosis
The changes in CD45 and CD44 were used as markers for naive, activated and memory cells [15–17]. Thus, cells expressing higher levels of CD45RB and low levels of CD44 were considered to be naive, those expressing low levels of both CD45RB and CD44 were considered to be activated, and those expressing high levels of CD44 but low levels of CD45RB were classified as memory cells. The total cellularity of all organs was significantly decreased following activation of the HPA axis (week 2) and there was a significant increase in total cellularity at 6 weeks only in the lung. At all other time points there was no change in cellularity compared with the number of cells obtained from animals with latent disease. Therefore, changes in T cell populations were due to alterations in the percentage of distinct T cell subsets.
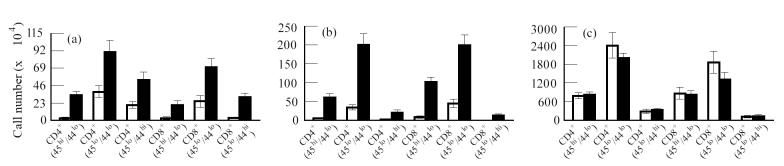
The results in Fig. 2a show that there was an increase in both naive (CD45RBhi), activated (CD45RBlo) and memory (CD44hi) CD4+ and CD8+ cell populations in the lungs of mice with latent disease compared with the T cell populations in the lungs of uninfected mice. The number of CD44hi memory cells isolated from the lungs of mice with latent disease was also greater then the number of memory cells from uninfected mice and greater then CD45RBhi (naive) cells from infected mice. Similarly, there was a significant increase in both naive, activated and memory CD4+ and CD8+ populations in the draining mediastinal lymph nodes (Fig. 2b). In this case, and in contrast to that observed in the lung, high levels of activated (CD45RBlo/CD44lo) and low numbers of memory (CD44hi) CD4+ or CD8+ cells were found in the mediastinal node of infected mice. No changes in T cell populations were associated with latent disease in the spleen (Fig. 2c).
Fig. 2.
Analysis of T lymphocyte populations in uninfected mice and in mice with latent Mycobacterium tuberculosis infection. Mice were infected intranasally with 103 colony-forming units (CFU) of M. tuberculosis. Twelve weeks after the initial infection, when latent infection was established (see Fig. 1), the mice were killed and the T cell populations from these mice and from age-matched uninfected control mice were analysed by flow cytometry. The number of T cells was determined by multiplying the total number of viable cells by the percent of CD3+ T cells in the population isolated from the lung, spleen, and mediastinal lymph nodes. The number of CD4+ and CD8+ cells was calculated by multiplying the percentage of gated CD4+ or CD8+ cells harvested from each organ by the total number of CD3+ cells. Finally, dual expression of CD45RB and CD44 was calculated by multiplying the percentage of gated populations determined to be either high and low expressors of CD45RB and CD44 by the total number of either CD4+ or CD8+. (a) Lung. (b) Mediastinal lymph node. (c) Spleen. Values represent means ± s.e.m. for four groups of n = 3 mice. The effect of infection with M. tuberculosis on T cell populations in lung and mediastinal lymph node was significantly different as determined by anova. □, Uninfected; ▪, latent infection.
Changes in naive, activated and memory T cell populations associated with reactivation of latent M. tuberculosis infection
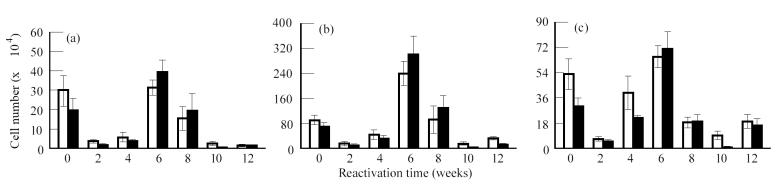
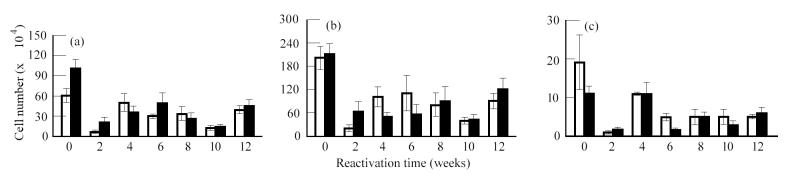
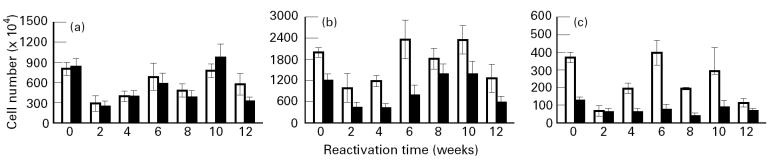
HPA activation resulted in a reduction in the number of naive (CD45RBhi), activated (CD45RBlo) and memory (CD44hi) CD4+ and CD8+ lymphocytes in the lungs, spleen and mediastinal lymph nodes. These changes coincided with a decrease in the cellularity of these organs as a result of HPA activation (data not shown). Figure 3 shows the changes in T cell populations in the lung following reactivation. It should be noted that an analysis of T cell populations in animals with latent disease was also done at each of the time points indicated. Since these did not change during the course of these studies as determined by anova, they were treated as one group and the collapsed data are found at time 0, i.e. before activation of the HPA axis. Activated (Fig. 3b) (CD45RBlo/CD44lo) CD4 and CD8 populations recovered quickly, as did memory (Fig. 3c) (CD44hi) CD4 and CD8 populations, the lungs reaching a peak in cell number by week 6 following reactivation. These cell numbers declined thereafter, which coincided with a decline in the numbers of Tubercle bacilli isolated from the lung. The recovery of the naive (Fig. 3a) (CD45RBhi) populations of T cells in the lung mimicked that observed for memory cells, except the cell numbers that were recovered remained depressed compared with that of the memory population.
Fig. 3.
Analysis of T lymphocyte populations in the lung following reactivation of latent Mycobacterium tuberculosis infection. Mice with latent infection were restrained to activate the hypothalamic–pituitary–adrenal (HPA) axis. Immediately following HPA activation and at the times indicated the T lymphocyte populations in the lung were analysed using multi-parameter flow cytometry. Mice with latent disease served as controls. No differences in the cell populations from mice with latent disease were observed throughout the time course. The data are therefore collapsed and expressed as time 0, i.e. before HPA activation. The number of cells in each subpopulation was calculated as described in the legend for Fig. 2. (a) CD45hi/CD44lo. (b) CD45lo/CD44lo. (c) CD45lo/CD44hi. Values represent means ± s.e.m. for four groups of n = 3 mice. The effect of HPA activation and of time was significant on the naive, memory and activated populations as determined by anova. □, CD4; ▪, CD8.
The CD4 and CD8 naive, activated and memory cell populations in the mediastinal lymph node were also depressed initially (Fig. 4). However, the CD4 and CD8 naive and activated populations (Fig. 4a,b) remained depressed throughout the 12 weeks of observation. In contrast, the levels of CD8 memory cells recovered to levels found in animals with latent disease and then declined (Fig. 4c). The increase in CD8 memory cells occurred prior to the increase of memory cells in the lung, and their decline was associated with an increase of this T cell population in the lung. The CD4 population of memory cells, however, remained depressed following HPA activation.
Fig. 4.
Analysis of T lymphocyte populations in the mediastinal lymph node following reactivation of latent Mycobacterium tuberculosis. See legend for Fig. 2 for an explanation of methodology. (a) CD45hi/CD44lo. (b) CD45lo/CD44lo. (c) CD45lo/CD44hi. Values represent means ± s.e.m. for four groups of n = 3 mice. The effect of hypothalamic–pituitary–adrenal (HPA) activation was significant on all populations tested. The effect of time on the naive and memory populations was also significant. □, CD4; ▪, CD8.
The naive, activated and memory CD4 cell populations in the spleen also recovered following HPA activation (Fig. 5). In contrast, while the number of naive and activated CD8 T cells in the spleen recovered, the number of memory CD8 T cells remained depressed. No changes were observed in any population of cells obtained from the superficial cervical lymph node (data not shown).
Fig. 5.
Analysis of T lymphocyte populations in the spleen following reactivation of latent Mycobacterium tuberculosis. See legend for Fig. 2 for an explanation of methodology. (a) CD45hi/CD44lo. (b) CD45lo/CD44lo. (c) CD45lo/CD44hi. Values represent means ± s.e.m. for four groups of n = 3 mice. The effect of hypothalamic–pituitary–adrenal (HPA) activation was significant by anova. The effect of time following HPA activation was not significant. □, CD4; ▪, CD8.
Reactivation of M. tuberculosis results in an increase of inflammation in the lung
The lungs of mice with latent disease had multifocal, mild perivascular and peribronchiolar lymphoid infiltrates and minimal granulomatous pneumonia (Fig. 6a). Immediately following activation of the HPA axis, no inflammation was observed in the lungs, which appeared microscopically normal (Fig. 6b). In contrast, 6 weeks after reactivation the lungs contained many foci of severe granulomatous pneumonia and prominent perivascular and peribronchiolar lymphoid infiltrates (Fig. 6c). There were numerous areas of macrophages containing a significant amount of acid-fast debris that was not found in the lungs during latency or immediately after activation of the HPA axis (not shown).
Fig. 6.
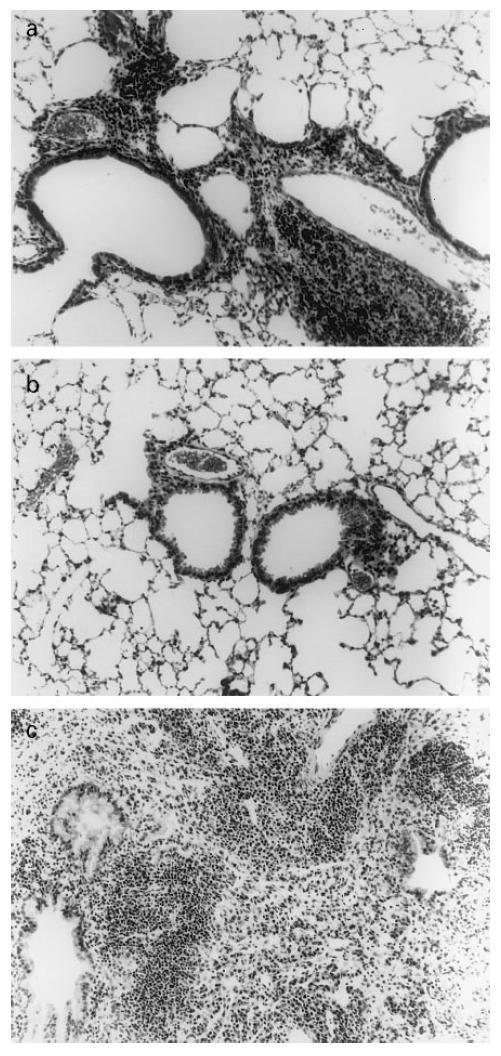
Histology of the lung following reactivation of Mycobacterium tuberculosis. Haematoxylin and eosin staining under light microscope. (a) Twelve weeks post-infection (latency). (b) Immediately following hypothalamic–pituitary–adrenal (HPA) activation. (c) Six weeks later (week 20).
DISCUSSION
Most people who are infected with M. tuberculosis do not develop disease. Infected individuals are assumed to mount an effective immune response to the initial infection that limits proliferation of the bacteria. Thus, infection is likely to be asymptomatic. Infected people can only be identified by a positive skin test reaction. The host factors that are responsible for the development of clinical disease have not been clearly described, but are thought to involve a temporary suppression of the immune response and re-initiation of Mycobacterial growth [1, 2]. A key factor in studying the changes that result in clinical disease is the establishment of a ‘latent’ infection followed by re-initiation of Mycobacterial growth resulting from a temporary suppression of immunity. Previously, we have shown that systemic infection of mice with a low dose of M. tuberculosis (Erdman) resulted in a steady state infection in which the numbers of bacteria isolated from the lung and spleen did not increase for several months [13]. The results of this investigation, using intranasal instillation of low doses of microorganisms, also resulted in the establishment of a steady state infection in the lungs. Our results confirm our previous observations that activation of the HPA axis by restraint resulted in the re-initiation of growth of the Tubercule bacillus [13]. The reactivation was not the result of changes in the nutritional status of the mice, since the control mice, those with latent disease, were housed in cages without food or water during the same time that other mice were being stressed. The restraint stress paradigm, as previously described by us, results in a sustained increase in plasma corticosterone as well as a decreased cellularity of the lymphoid organs and the lung. While this model may not strictly represent clinical disease in man, it does provide an opportunity to monitor the changes in T cell populations that may be responsible for controlling mycobacterial growth during a steady state infection and during an increase in Mycobacterial growth following a temporary suppression of immunity that results from HPA activation [18–23].
Activation-associated alterations of T cell surface phenotypes have been reported to occur following stimulation with antigen [24, 25]. Expression of CD44 increases coincident with the frequency of antigen-specific reactivity. After antigenic challenge, there is also an increase in the expression of VLA-4, LFA-1 and CD54 cell adhesion molecules and a decrease in CD62L and CD45RB. Thus, T cell activation has been shown to correlate with a shift from the CD44lo to CD44hi and from CD45RBhi to the CD45RBlo phenotype.
Based on these phenotypic changes, we found that there was an accumulation of both naive, activated and memory CD4 and CD8 populations in the lung and mediastinal lymph node of mice during latent M. tuberculosis infection compared with uninfected control mice. The populations decreased as a result of HPA activation, which resulted in resumption of the growth of M. tuberculosis. This suggests that increased levels of corticosterone that occur during activation of the HPA axis [11–13] resulted in the breakdown of immunological control of tuberculosis (TB). Interestingly, the cell populations found in the spleen of mice with latent disease resembled the phenotype of those from uninfected mice. The initial immune response to the Mycobacterium, therefore, was probably limited to the lungs because the mice were infected intranasally. Alterations in the T cell population in the spleen were not observed until Mycobacterial growth was reinitiated following HPA activation.
The changes in T cell populations that followed reactivation also indicate that the immune response was largely localized to the lung and draining lymph nodes. The populations in the lung that changed following HPA activation indicated that both CD4 and CD8 activated and memory populations were stimulated. The response in the mediastinal lymph node was different from that observed in the lung; only the activated population increased in response to the increased numbers of microorganisms. Increases in memory cells were not observed.
Our results are similar to those observed by Griffin & Orme [24] and by Andersen et al. [26], who showed that there was a preferential accumulation of CD44hi/CD45RBlo memory subsets in the spleen of mice injected intravenously with M. tuberculosis. Coulson & Wilson also reported increases in CD44hi memory CD4 cells in the lungs of mice following immunization with attenuated Schistosoma mansoni cercariae [27]. We too found that memory cells accumulated in the lungs of mice infected intranasally. Our observations, while similar, were made after allowing the mice to control the growth of low numbers of microorganisms and then temporarily suppressing the immune response by activation of the HPA axis. This allowed the bacteria to resume growth. Recovery of immunocompetence was associated with a decrease in bacterial load. This is analogous to human disease in the pre-antibiotic era. Latent TB is reactivated due to a temporary suppression of immunity; patients would experience episodes of reactivation followed by latent disease during their lifetime [28].
While it is generally agreed that CD4 cells are important cytokine producers that result in macrophage activation, the role of CD8 T cells in mediating protective immunity to TB is not clearly understood. One study by Saunders & Cheers [14] examined the changes in CD4 and CD8 T lymphocytes in response to intranasal infection with M. avium. They found that depletion of CD8+ T cells had no effect on the growth of M. avium in the lung and that CD8+ cells from M. avium-infected mice did not produce interferon-gamma (IFN-γ) in vitro. In contrast, mice that were depleted of CD4+ T cells were more susceptible to the growth of M. avium. We showed that there was an increase in both CD8 and CD4 T cells in response to intranasal infection with M. tuberculosis. The decrease in both T cell populations as a result of HPA activation correlated with an increase in bacterial growth. These results suggest that CD8+ cells may be an important effector cell during an infection with M. tuberculosis. This possibility has been reinforced by recent studies that show that β2-microglobulin knock-out mice, that lack functional MHC class I molecules and consequently are deficient in CD8 T lymphocytes, are more susceptible to the growth of M. tuberculosis [29]. Also, both CD4+ and CD8+ T lymphocytes confer a high degree of protective immunity against TB in mice after vaccination with the Mycobacterial protein heat shock protein (hsp)65 [30].
The results of this study indicate that memory immune T cells play a significant role in controlling M. tuberculosis following reactivation of latent disease. These cells may also be more resistant to the suppressive effects of corticosteroids [19–23]. Both populations of CD4 and CD8 memory cells may mediate cytolysis of infected macrophages releasing bacteria into the environment [31–38]. The release of cytokines, especially IFN-γ produced by both populations, may result in the activation of macrophages that can control the growth of the Mycobacterium. The stimulation of memory immune T cells following reactivation of Mycobacterial growth coincided with the decrease in Mycobacteria in the lungs. The role of the memory immune populations in re-establishing control of the growth of M. tuberculosis is currently under investigation.
Acknowledgments
Supported by NIMH grants MH45679, MH54966 and by Cancer Centre support grant CA16058.
References
- 1.Hopewell PC. Tuberculosis: pathogenesis, protection and control. Washington DC: ASM Press; 1994. Overview of clinical tuberculosis; pp. 25–46. [Google Scholar]
- 2.Smith PG, Moss AR. Bloom BR. Tuberculosis: pathogenesis, protection and control. Washington DC: ASM Press; 1994. Epidemiology of tuberculosis; pp. 47–59. [Google Scholar]
- 3.Kaufmann SHE. Tuberculosis. The role of the immune response. Immunologist. 1993;1:109–14. [Google Scholar]
- 4.McDonough KA, Kress Y, Bloom BR. Pathogenesis of tuberculosis: interaction of Mycobacterium tuberculosis with macrophages. Infect Immun. 1993;61:2763–72. doi: 10.1128/iai.61.7.2763-2773.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rook GAW. Macrophages and Mycobacterium tuberculosis. The key to pathogenesis. In: Zwilling BS, Eisenstein TK, editors. Macrophage pathogen interactions. New York: Marcel Dekker; 1994. pp. 249–61. [Google Scholar]
- 6.Rook GAW, Bloom BR. Bloom BR. Tuberculosis: pathogenesis, protection and control. Washington DC: ASM Press; 1994. Mechanisms of pathogenesis in tuberculosis; pp. 485–501. [Google Scholar]
- 7.Collins FM. Mycobacterial disease, immunosuppression and acquired immunodeficiency syndrome. Clin Microbiol Rev. 1989;2:360–77. doi: 10.1128/cmr.2.4.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wayne LG. Dormancy of Mycobacterium tuberculosis and latency of disease. Eur J Clin Microbiol Infect Dis. 1994;13:908–14. doi: 10.1007/BF02111491. [DOI] [PubMed] [Google Scholar]
- 9.Wiegeshaus E, Balasubramanian V, Smith DW. Immunity to tuberculosis from the perspective of pathogenesis. Infect Immun. 1989;57:3671–6. doi: 10.1128/iai.57.12.3671-3676.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith DW, Wiegeshaus EH, Edwards ML. The protective effects of BCG vaccination against tuberculosis. In: Bendinelli M, Friedman H, editors. Mycobacterium tuberculosis: interactions with the immune system. New York: Plenum Press; 1988. pp. 341–70. [Google Scholar]
- 11.Brown DH, Sheridan JF, Pearl D, et al. Regulation of mycobacterial growth by the hypothalamus–pituitary–adrenal axis: differential responses of Mycobacterium bovis Bcg-resistant and susceptible mice. Infect Immun. 1993;61:4793–800. doi: 10.1128/iai.61.11.4793-4800.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown DH, Zwilling BS. Activation of the hypothalamic–pituitary–adrenal axis differentially affects the anti-mycobacterial activity of macrophages from Bcg resistant and susceptible mice. J Neuroimmunol. 1994;53:181–7. doi: 10.1016/0165-5728(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 13.Brown DH, Miles BA, Zwilling BS. Growth of Mycobacterium tuberculosis in BCG resistant and susceptible mice: establishment of latency and reactivation. Infect Immun. 1995;63:2243–7. doi: 10.1128/iai.63.6.2243-2247.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saunders BM, Cheers C. Inflammatory response following intranasal infection with Mycobacterium avium complex: role of T-cell subsets and gamma interferon. Infect Immun. 1995;63:2282–7. doi: 10.1128/iai.63.6.2282-2287.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Croft M, Duncan DD, Swain SL. Response of naive antigen specific CD4+ T cells in vitro: characteristics and antigen presenting cell requirements. J Exp Med. 1994;176:1431–7. doi: 10.1084/jem.176.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Croft M, Bradley LM, Swain SL. Naive versus memory CD4 T cells response to antigen. Memory cells are less dependent on accessory cell costimulation and can respond to any antigen presenting cell types including resting B cells. J Immunol. 1994;152:2675–85. [PubMed] [Google Scholar]
- 17.Powrie F, Correa-Oliveira R, Mauze S, et al. Regulatory interactions between CD45RBhigh and CD45RBlow CD4+ T cells are important for balance between protective and pathogenic cell mediated immunity. J Exp Med. 1994;179:589–600. doi: 10.1084/jem.179.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnes PJ. Anti-inflammatory mechanisms of glucocorticoids. Biochem Soc Transactions. 1995;23:940–5. doi: 10.1042/bst0230940. [DOI] [PubMed] [Google Scholar]
- 19.Brinkmann V, Kristofic C. Regulation by corticosteroids of Th1 and Th2 cytokine production in human CD4+ effector T cells generated from CD45RO−and CD45RO+ subsets. J Immunol. 1995;155:3322–8. [PubMed] [Google Scholar]
- 20.Chung H-T, Samlowski WE, Daynes RA. Modification of the murine immune system by glucocorticosteroids: alterations of the tissue localization properties of circulating lymphocytes. Cell Immun. 1986;101:571–85. doi: 10.1016/0008-8749(86)90167-x. [DOI] [PubMed] [Google Scholar]
- 21.Daynes RA, Meikle AW, Araneo BA. Locally active steroid hormones may facilitate compartmentalization of immunity by regulating the types of lymphokines produced by helper T cells. Res Immunol. 1991;142:40–45. doi: 10.1016/0923-2494(91)90010-g. [DOI] [PubMed] [Google Scholar]
- 22.Dhabhar F, Miller AH, McEwen BS, et al. Effects of stress on immune cell distribution: dynamics and hormonal mechanisms. J Immunol. 1995;154:5511–27. [PubMed] [Google Scholar]
- 23.Spry CJF. Inhibition of lymphocyte recirculation by stress and corticotropin. Cell Immunol. 1972;4:86–92. doi: 10.1016/0008-8749(72)90007-x. [DOI] [PubMed] [Google Scholar]
- 24.Griffin JP, Orme IM. Evolution of CD4 T cell subsets following infection of naive and memory immune mice with Mycobacterium tuberculosis. Infect Immun. 1994;62:1683–90. doi: 10.1128/iai.62.5.1683-1690.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker PR, Ohteki T, Lopez JA, MacDonald HR, Maryanski JL. Distinct phenotypes of antigen-selected CD8 T cells emerge at different stages of an in vivo immune response. J Immunol. 1995;155:3443–52. [PubMed] [Google Scholar]
- 26.Anderson P, Andersen AB, Sorensen AL, et al. Recall of longed lived immunity to Mycobacterium tuberculosis. J Immunol. 1995;154:3359–72. [PubMed] [Google Scholar]
- 27.Coulson PS, Wilson RA. Pulmonary T helper lymphocytes are CD44hi, CD45RB− effector/memory cells in mice vaccinated with attenuated cercariae of Schistosoma mansoni. J Immunol. 1993;151:3663–71. [PubMed] [Google Scholar]
- 28.Dubos R, Dubos J. Boston: Little Brown and Co.; 1952. The white plague: tuberculosis, man and society. [Google Scholar]
- 29.Flynn JL, Goldstein MM, Triebold KJ, et al. Major histocompatibility complex class I restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA. 1992;89:12013–7. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silva CL, Silva MF, Pietro Rclr, et al. Characterization of T cells that confer a high degree of protective immunity against tuberculosis in mice after vaccination with tumor cells expressing Mycobacterial hsp65. Infect Immun. 1996;64:2400–7. doi: 10.1128/iai.64.7.2400-2407.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boom WH, Wallis RS, Chrevenak KA. Human Mycobacterium tuberculosis-reactive CD4+ T-cell clones: heterogeneity in antigen recognition, cytokine production, and cytotoxicity for mononuclear phagocytes. Infect Immun. 1991;59:2737–43. doi: 10.1128/iai.59.8.2737-2743.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dalton DK, Pitts-Meek S, Keshav S, et al. Multiple defects of cell function in mice with disrupted interferon-γ genes. Science. 1993;259:1739–45. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 33.Kamijo R, Le J, Shapiro D, et al. Mice that lack the interferon-γ receptor have profoundly altered responses to infection with Bacillus Calmette–Guérin and subsequent challenge with lipopolysaccharide. J Exp Med. 1993;178:1435–40. doi: 10.1084/jem.178.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orme IM, Miller ES, Roberts AD, et al. T lymphocytes mediating protection and cellular cytolysis during the course of Mycobacterium tuberculosis infection. Evidence for different kinetics and recognition of a wide spectrum of protein antigens. J Immunol. 1992;148:189–96. [PubMed] [Google Scholar]
- 35.Orme IM, Andersen P, Boom WH. T cell response to Mycobacterium tuberculosis. J Infect Dis. 1993;167:1481–97. doi: 10.1093/infdis/167.6.1481. [DOI] [PubMed] [Google Scholar]
- 36.Orme I. Protective immunity mediated by T cells: protective and memory immunity in mice infected with Mycobacterium tuberculosis. Immunobiology. 1994;191:503–8. doi: 10.1016/S0171-2985(11)80456-0. [DOI] [PubMed] [Google Scholar]
- 37.Walker PR, Ohteki T, Lopez JA, et al. Distinct phenotypes of antigen-selected CD8 T cells emerge at different stages of an in vivo immune response. J Immunol. 1995;155:3443–52. [PubMed] [Google Scholar]
- 38.Zhang M, Lin Y, Iyer DV, et al. T-cell cytokine response in human infection with Mycobacterium tuberculosis. Infect Immun. 1995;63:3231–4. doi: 10.1128/iai.63.8.3231-3234.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]