Abstract
IgA subclass distribution of antibodies against capsular polysaccharide (PS) of Haemophilus influenzae type b (Hib) was studied in saliva and serum samples of children vaccinated with two (n = 58) or three doses (n = 53) of Hib vaccine. One month after the second dose of Hib conjugate vaccine, at 7 months old, 40% of the children had IgA1 and 41% had IgA2 anti-Hib PS antibodies in saliva. One month after the third dose, at 15–25 months old, IgA1 was the predominating subclass; 72% of the children had IgA1, 26% had IgA2 anti-Hib PS in saliva. The mean concentration of IgA1 anti-Hib PS, expressed as optical density (OD) values, was significantly higher after three doses (OD 80.7) than after two doses (OD 18.9). The mean concentration of IgA2 did not change significantly after the third dose (OD 23.8 after two doses, OD 18.1 after three doses). In serum, IgA1 anti-Hib PS predominated both after two (17% had IgA1, none had IgA2) and three doses (72% had IgA1, 4% had IgA2) of Hib vaccine. In conclusion, both IgA1 and IgA2 anti-Hib PS were found in saliva of immunized children after two doses of Hib conjugate vaccine, whereas the third vaccine dose induced a shift towards IgA1 anti-Hib PS dominance in saliva.
Keywords: IgA subclasses, Haemophilus influenzae type b
INTRODUCTION
Human IgA is present in various forms in serum and secretions. In serum IgA is mostly monomeric, whereas dimeric and higher polymeric forms predominate in secretions. In polymeric IgA the subunits are linked by the J chain. Most secretory IgA (sIgA) is produced locally in mucosal tissues. IgA acquires the secretory component (SC) during its transport through epithelium into mucosal surfaces [1].
IgA exists as two subclasses, IgA1 and IgA2, which differ both in their primary amino acid sequences and carbohydrate structures [2]. In serum, 75–93% of IgA is IgA1, whereas in secretions the relative proportion of IgA1 is lower. The distribution of the two subclasses in secretions is dependent on the mucosal site: IgA1-secreting cells predominate in the respiratory tract, in the upper gastrointestinal tract and in mammary glands (55–96%), whereas IgA2-secreting cells predominate in the lower gastrointestinal and in the female reproductive tracts [3–8].
IgA subclass distribution in secretions is also influenced by the nature of the antigen: IgA antibodies against protein antigens are predominantly IgA1, but IgA against polysaccharide (PS), lipopolysaccharide (LPS) of Gram-negative, and lipoteichoic acid of Gram-positive bacteria has been reported to be more often IgA2 [9–11]. In serum, IgA1 predominates, regardless of the nature of the antigen [3, 6].
Functional differences between the IgA subclasses are beginning to be revealed. IgA2 is resistant to IgA1 proteases produced by several pathogenic bacteria, including Haemophilus influenzae type b (Hib), Streptococcus pneumoniae and Neisseria meningitidis [12, 13]. Hence, high concentrations of specific IgA2 on the mucosa may be beneficial in defence against these pathogens.
IgA antibodies may be important in vaccine-induced immunity. Hib PS protein conjugate vaccines are immunogenic and protective in young infants [14] and have been shown to reduce oropharyngeal Hib carriage [15–19]. We suggested earlier that mucosal anti-Hib PS antibodies have a role in reducing Hib carriage. Hib conjugate vaccines induced sIgA anti-Hib PS in saliva of immunized children already at the age of 7 months, after the primary vaccination series. At 15 or 19 months old, after the booster dose, sIgA was more commonly detected in saliva and the concentrations were higher, and also serum-derived IgG anti-Hib PS was found in saliva [20]. Both IgA and IgG anti-Hib PS antibodies decreased nasopharyngeal colonization by Hib in an infant rat model [21, 22].
To characterize further the nature of mucosal IgA response to Hib PS protein conjugate vaccine we analysed IgA subclass distribution in saliva of immunized children and compared it with the IgA response in serum. To our knowledge, this is the first study of the subclass distribution of specific IgA in secretions of children after parenteral immunization.
MATERIALS AND METHODS
Saliva and serum samples
Saliva and serum samples were obtained from the following groups of infants and children enrolled in our immunogenicity studies with Hib conjugates (Table 1): (i) saliva and serum samples of 58 children who had received two doses of PRP-T vaccine (Hib PS conjugated to tetanus toxoid; ActHIB, Pasteur Merieux Serums & Vaccines, Marnes La Coquette, France). Forty-two infants received the vaccine at 4 and 6 months, and 16 at 2 and 6 months old. Samples were taken at 7 months old; (ii) saliva and serum samples of 53 children who had received three doses of Hib vaccine. Twenty-eight children had been immunized with PRP-OMP (Hib PS conjugated to N. meningitidis outer membrane complex; PedVAXHib, Merck Sharp and Dohme Research Labs, West Point, PA) at 4 and 6 months old and boosted either with PRP (Hib PS; Pasteur Merieux Serums & Vaccines) (n = 23) or with PRP-OMP (n = 5) at 14 months old. Seven children were immunized with PRP-T (Pasteur Merieux Serums & Vaccines) at 2, 6, and 18 months old. Five of them were included in the group of 16 children mentioned in (i). Eighteen children were immunized with PRP-T (SmithKline Beecham Biologicals, Rixensaart, Belgium) at 4, 6, and 24 months old [23]. Samples were taken 1 month after the third vaccine dose.
Table 1.
Schemes for Hib vaccinations and sampling

All children received measles-mumps-rubella vaccine (Merck Sharp and Dohme Research Labs) at 14 months old. Inactivated polio virus vaccine was given at 6 and 12 months old (Rijksinstituut vor Volksgezondheit, Bilthoven, The Netherlands), or at 4, 6 and 24 months old (SmithKline Beecham). Diphtheria-tetanus-pertussis vaccine (National Public Health Institute, KTL, Helsinki, Finland) was given at 3, 4 and 5 or at 2 and 6 months old, or at 4, 6 and 24 months old (SmithKline Beecham) [23].
The study protocol was approved by the Ethics Committee of KTL, Helsinki, and written consent was received from the parents. The samples were collected, stored and treated as described previously [20].
Serological assays
Enzyme immunoassay (EIA) was used to determine the concentrations of IgA, IgA1, and IgA2 anti-Hib PS in saliva and serum. The analyses were performed as described before [20]. All saliva samples were analysed with peroxidase-conjugated anti-human IgA (P216; Dakopatts, Glostrup, Denmark) and MoAbs against IgA (M26012; Oxoid, Unipath, Bedford, UK), IgA1 and IgA2 (3763 and 3764; Nordic Immunological Labs, Tilburg, The Netherlands).
Serum samples were first screened for IgA anti-Hib PS by EIA using peroxidase-conjugated anti-human IgA (Dakopatts). The samples containing IgA anti-Hib PS were further analysed with MoAbs against IgA (Oxoid), IgA1, and IgA2 (Nordic Immunological Labs). A peroxidase-conjugated anti-mouse immunoglobulin conjugate (P260; Dakopatts) was used after monoclonal reagents.
Absorbance readings of the saliva samples on the PBS blank plate were subtracted from those on the Hib PS antigen plate, and the means of the triplicates were calculated. A value of 0.03 (2 × s.d. of 10 parallel measurements of three samples containing low concentrations of anti-Hib PS) or higher was considered positive. The results for IgA, IgA1 and IgA2 in saliva were expressed as 1000 × optical density (OD values). Samples with a mean OD value < 0.03 were assigned an OD value of 10 (1000 × 0.01). The sum of OD values of IgA1 and IgA2 was assigned 10 when both were negative, and only the positive result was used if the other was negative.
The results for IgA, IgA1 and IgA2 anti-Hib PS in serum were expressed as titres (reciprocals of sample dilutions giving an absorbance reading of 0.3). A value of 25 (half of the lowest dilution) or higher was considered positive.
Statistical analysis
Statistical comparisons between the groups of children were carried out with analysis of variance (anova) and Student's t-test from log-transformed data (when comparing geometric means) or with Yates-corrected χ2test/Fisher's two-tailed exact test (when comparing proportions).
RESULTS
IgA1 and IgA2 anti-Hib PS antibodies were determined in saliva and serum samples of immunized children. The samples were taken 1 month after the second dose of Hib conjugate vaccine, at 7 months old, or 1 month after the third vaccine dose, at 15, 19 or 25 months old (Table 1).
IgA1 and IgA2 anti-Hib PS in saliva
After two doses of Hib vaccine 23 (40%) of the 58 children had IgA1 and 24 (41%) had IgA2 anti-Hib PS in saliva. Sixteen (28%) samples contained only IgA1, 17 (29%) only IgA2. Seven (12%) of the 58 samples contained both IgA1 and IgA2 (Table 2). There were no significant differences between the groups that had received the vaccine at 2 and 6 months or at 4 and 6 months old.
Table 2.
IgA1 and IgA2 anti-Haemophilus influenzae type b (Hib) capsular polysaccharide (PS) antibodies in saliva and serum after two or three doses of Hib vaccine

After three doses of Hib vaccine 38 (72%) of the 53 children had IgA1, 14 (26%) had IgA2 anti-Hib PS in saliva. Twenty-five (47%) samples contained only IgA1, one (2%) contained only IgA2, and 13 (25%) contained both subclasses (Table 2). There were no significant differences between the groups with different vaccines and vaccination schedules.
To be able to compare the OD values of IgA1 and IgA2 the sum of the OD values of IgA1 and IgA2 anti-Hib PS of each sample was compared with the corresponding OD value of IgA anti-Hib PS (Fig. 1). A significant correlation was found (r = 0.78, P < 0.001), and the correlation was significant irrespective of the dominating IgA subclass in saliva (r = 0.89, P < 0.001 for IgA1 dominating samples, r = 0.59, P < 0.01 for IgA2 dominating samples). Thus, it was considered justified to use OD values semiquantitatively to compare OD values of IgA1 and IgA2 and calculate their ratios in individual samples.
Fig. 1.
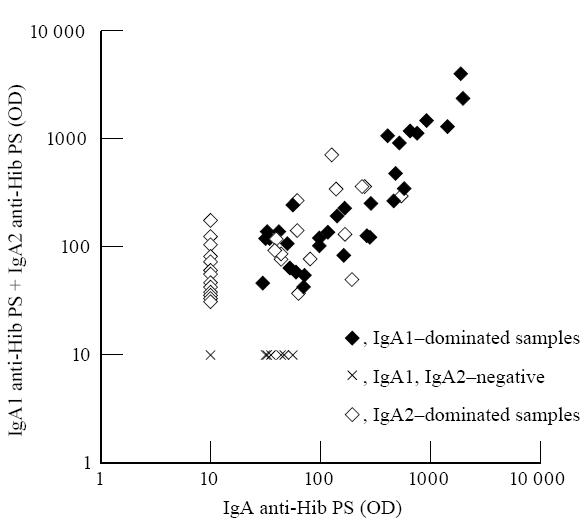
Correlation of the optical density (OD) values of IgA and the sum of OD values of IgA1 and IgA2 antibodies against capsular polysaccharide of Haemophilus influenzae type b (Hib) in saliva samples of children immunized with two or three doses of Hib vaccine.
After two doses of Hib vaccine the geometric mean (GM) OD value of all saliva samples for IgA1 was 18.9 (s.d. 8.1–44.4) and for IgA2 23.8 (s.d. 19.0–75.0) (Fig. 2). The mean ratio of IgA1 and IgA2 anti-Hib PS was 1.8 (Fig. 3).
Fig. 2.

Optical density (OD) values of IgA1 and IgA2 antibodies against capsular polysaccharide of Haemophilus influenzae type b (Hib) in individual saliva samples after two or three doses of Hib vaccine. Horizontal lines indicate geometric mean values.
Fig. 3.

Ratios of the optical density (OD) values of IgA1 and IgA2 antibodies against capsular polysaccharide of Haemophilus influenzae type b (Hib) in saliva samples of individual children immunized with two or three doses of Hib vaccine. Horizontal lines indicate mean values of the ratios.
IgA1 anti-Hib PS accounted for the rise of IgA concentrations in saliva after the booster dose of Hib vaccine. The GM OD value was 80.7 (s.d. 15.0–433.5) for IgA1 and 18.1 (s.d. 6.1–53.6) for IgA2 at 15–25 months old (Fig. 2). GM OD of IgA1 was significantly higher after the booster dose than after the primary series of Hib vaccine (P < 0.001), whereas the GM OD of IgA2 did not change significantly. The mean OD ratio of IgA1 and IgA2 anti-Hib PS after the booster dose was 10.9 (Fig. 3), which was significantly higher than after two doses (P < 0.001).
Consecutive samples were obtained from five children who had received PRP-T at 2, 6, and 18 months old. In all of them OD values of IgA1 in saliva were higher after the booster dose than after the primary doses. OD values of IgA2 rose in saliva of two children and declined in saliva of three children after the booster dose.
IgA1 and IgA2 anti-Hib PS in serum
After two doses of Hib vaccine, 10 (17%) serum samples were positive for IgA anti-Hib PS (Table 2). In every case only IgA1 was detected. The group of children that had received Hib conjugate at 4 and 6 months old had more often anti-Hib PS IgA (IgA1) in serum than the group vaccinated at 2 and 6 months old (10/42 versus 0/16, P < 0.05).
There was no correlation between the IgA1 anti-Hib PS concentrations in serum and saliva after two doses of Hib conjugate (r = – 0.017, P > 0.05). Thirty-two (55%) children had no anti-Hib PS IgA in serum but had IgA1 and/or IgA2 in saliva. Thirteen of these 32 saliva samples contained only IgA1, 14 only IgA2 and five contained both subclasses.
Also after the booster dose of Hib vaccine IgA1 was the dominating subclass in serum. Thirty-six (68%) samples contained only IgA1 anti-Hib PS; two (4%) contained also IgA2 (Table 2). There was an almost significant correlation between the concentrations of salivary and serum IgA1 anti-Hib PS (r = 0.28, P < 0.05).
The group of five children that had received PRP-OMP at 4, 6, and 14 months old had significantly less often IgA anti-Hib PS antibodies in serum than the other groups that had received three doses of Hib vaccine (PRP-OMP and PRP or PRP-T) (0% versus 70–100%). Otherwise, there were no statistically significant differences between the groups of children with different Hib vaccines and vaccination schedules after three doses of Hib vaccine.
DISCUSSION
IgA1 and IgA2 anti-Hib PS concentrations were analysed from saliva and serum samples taken after two or three doses of Hib vaccine. The samples were from children enrolled in different immunogenicity studies with Hib conjugate vaccine. Consecutive samples were obtained only from five children; all the other 101 samples were taken from different individuals after the second or after the booster dose of Hib vaccine.
After two doses of Hib conjugate vaccine, IgA2 was detected in saliva as often as IgA1 (40% of the children had IgA1, 41% IgA2). After the third dose IgA1 predominated (72% of the children had IgA1, 26% IgA2) and the concentration of IgA1 was higher than after two doses. As expected, serum anti-Hib PS IgA was exclusively of subclass IgA1 after two and three doses of vaccine. Only 4% of the children had IgA2 anti-Hib PS in serum.
The different distribution of IgA subclasses in saliva and serum after Hib immunization strengthens the view of independence of mucosal and systemic immune responses. Mucosal antibodies against Hib PS have been reported to develop at an earlier age than the serum response, both after Hib infection and after vaccination with Hib PS vaccine [24–26]. It has been speculated that to some extent the early mucosal response compensates for the poor serum antibody response of young children to PS antigens.
IgA anti-Hib PS in saliva induced by the primary series of Hib conjugate vaccine existed both as IgA1 and IgA2, whereas IgA1 was the predominant subclass after the subsequent booster dose. The shift towards IgA1 dominance in saliva after the booster dose could be explained by the ontogeny of the mucosal immune system as the children grow older. However, subclasses of total IgA have been reported to be present in equal proportions in saliva of 1- to 4-month-old infants and in saliva of adults, and the IgA subclass distribution has not been shown to change in saliva of children between the ages 4 and 18 months [27, 28]. Hence, it seems that there is a specific qualitative difference between the primary and booster IgA anti-Hib PS antibody responses on mucosa after vaccinations with Hib conjugate vaccines.
IgA1 protease, produced also by Hib, cleaves IgA1 molecule to Fab and Fc fragments [12, 13]. IgA1 protease is considered important for colonization of Hib on mucosa because it eliminates Fc-mediated functions of IgA1 anti-Hib antibodies [29]. In addition, Fab fragments retain the antigen-binding ability and may thus, by coating the bacterial surface, block the access of other isotypes of anti-Hib antibodies [29]. In the present study IgA1 anti-Hib PS antibodies predominated in saliva of children after the booster dose. This indicates that IgA1 protease produced by Hib may impair the function of anti-Hib PS IgA antibodies in 1- to 2-year-old children. However, as we have discussed earlier [20, 21], serum-derived IgG anti-Hib PS antibodies are probable to contribute to defence on mucosa when the concentration of IgG rises in serum after the booster dose of Hib vaccine.
Several groups have analysed IgA1- and IgA2-secreting cells after parenteral immunization with either PS or PS–protein conjugate vaccine. Parenteral immunizations with PS antigens of Hib, S. pneumoniae or N. meningitidis and Hib PS conjugated to a carrier protein have been reported to induce antigen-specific IgA-secreting cells in the peripheral blood of young adults, the majority producing IgA2 antibodies [30–33]. Nieminen and co-workers (to be published) analysed antibody-secreting cells from peripheral blood of adults after one dose of parenteral pneumococcal PS or PS–protein conjugate vaccine. Most of the IgA anti-PS-secreting cells produced IgA2 after the PS vaccine, whereas after the conjugate vaccine the proportion of IgA1-producing cells increased.
Barington and co-workers [33] analysed circulating antibody-secreting cells induced by Hib conjugate vaccines in adults and infants. Both IgA1 and IgA2 anti-Hib PS-producing cells were detected in adults after immunization with PRP-T or PRP-D (Hib PS conjugated to diphtheria toxoid), the proportion of IgA2 cells decreasing after the second dose. Contrary to our results, in infants (vaccinated at 5, 6, and 12 months old) IgA1-producing cells dominated both after two and three doses of PRP-T, IgA2 comprising only 10% of the cells on average. However, they were analysing antibody-secreting cells 1 week after immunization, whereas we measured specific antibodies in saliva 1 month after vaccinations.
Few studies include determinations of specific antibody concentrations in secretions. All of them have been done in adults and only the PS vaccine has been used. Thus, the results are not directly comparable to our study. Lue and co-workers [30] analysed saliva samples of adults after immunization with pneumococcal PS vaccine. Both IgA1 and IgA2 concentrations in saliva increased significantly from preimmunization levels. In the study by Tarkowski and co-workers [32] only minor increases of specific IgA1 and IgA2 concentrations were observed after parenteral immunization with Hib PS vaccine in saliva of adults.
Systemic antibody response to PS antigens is T cell-independent (TI), but conjugation of PS to protein carrier converts it into T cell-dependent (TD). If the association of proteins with IgA1 and carbohydrates with IgA2 is true [9–11], the results of the IgA1 anti-Hib PS dominance after the booster dose of Hib conjugate vaccine indicate that Hib conjugate vaccine behaves like a protein (TD) antigen also in the mucosal immune system. However, this raises the question, where would the antigen presentation take place? Parenterally injected Hib PS is known to be dispersed in the body [34, 35]. It can be speculated that PS antigen leaks, for example, to the tonsils and stimulates local antibody-secreting cells there. However, at this stage the protein part of the antigen is likely to have been processed and only the PS is presented. This would lead to a TI response on the mucosa. Alternatively, B cells can be stimulated in lymph nodes near the vaccine injection site and migrate to the mucosa to produce sIgA into secretions [6, 36]. The migration of antigen-specific lymphocytes to secrete antibodies on mucosa could explain the TD type response on the mucosa.
Acknowledgments
We are grateful to parents and the personnel in the study centres for collaboration, to Pirjo-Riitta Saranpää, Kaisa Jousimies, and Jukka Nenonen for technical assistance, and to Professor P. Helena Mäkelä and Jussi Kantele, MSc, for critical reading of the manuscript. This study was presented in part at the 33rd Interscience Conference on Antimicrobial Agents and Chemotherapy, New Orleans, Louisiana, October 1993. It received financial support from The Finnish Cultural Foundation.
References
- 1.Russell MW, Lue C, van den Wall Bake AWL, Moldoveanu Z, Mestecky J. Molecular heterogeneity of human IgA antibodies during an immune response. Clin Exp Immunol. 1992;87:1–6. doi: 10.1111/j.1365-2249.1992.tb06404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grey HM, Abel CA, Yount WJ, Kunkel HG. A subclass of human γA-globulin (γA2) which lacks the disulphide bonds linking heavy and light chains. J Exp Med. 1968;128:1223–36. doi: 10.1084/jem.128.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delacroix DL, Dive C, Rambaud JC, Vaerman JP. IgA subclasses in various secretions and serum. Immunol. 1982;47:383–5. [PMC free article] [PubMed] [Google Scholar]
- 4.Crago SS, Kutteh WH, Moro I, Allansmith MR, Radl J, Haaijman JJ, Mestecky J. Distribution of IgA1-, IgA2-, and J chain-containing cells in human tissues. J Immunol. 1984;132:16–18. [PubMed] [Google Scholar]
- 5.Brandtzaeg P, Kett K, Rognum TO, Söderström R, Björkander J, Söderström T, Petrusson B, Hanson LÅ. Distribution of mucosal IgA and IgG subclass-producing immunocytes and alterations in various disorders. Monogr Allergy. 1986;20:170–94. [PubMed] [Google Scholar]
- 6.Mestecky J, Russell MW. IgA subclasses. Monogr Allergy. 1986;19:277–301. [PubMed] [Google Scholar]
- 7.Kett K, Brandtzaeg P, Radl J, Haaijman JJ. Different subclass distribution of IgA-producing cells in human lymphoid organs and various secretory tissues. J Immunol. 1986;136:3631–5. [PubMed] [Google Scholar]
- 8.Kutteh WH, Hatch KD, Blackwell RE, Mestecky J. Secretory immune system of the female reproductive tract: I. Immunoglobulin and secretory component-containing cells. Obstet Gynecol. 1988;71:56–60. [PubMed] [Google Scholar]
- 9.Brown TA, Mestecky J. Immunoglobulin A subclass distribution of naturally occurring salivary antibodies to microbial antigens. Infect Immun. 1985;49:459–61. doi: 10.1128/iai.49.2.459-462.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown TA, Murphy BR, Radl J, Haaijman JJ, Mestecky J. Subclass distribution and molecular form of immunoglobulin A hemagglutinin antibodies in sera and nasal secretions after experimental secondary infection with influenza A virus in humans. J Clin Microbiol. 1985;22:259–64. doi: 10.1128/jcm.22.2.259-264.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ladjeva I, Peterman JH, Mestecky J. IgA subclasses of human colostral antibodies specific for microbial and food antigens. Clin Exp Immunol. 1989;78:85–90. [PMC free article] [PubMed] [Google Scholar]
- 12.Plaut AG. The IgA1 proteases of pathogenic bacteria. Annu Rev Microbiol. 1983;37:603–22. doi: 10.1146/annurev.mi.37.100183.003131. [DOI] [PubMed] [Google Scholar]
- 13.Kilian M, Mestecky J, Russell MW. Defence mechanisms involving Fc-dependent functions of immunoglobulin A and their subversion by bacterial immunoglobulin A proteases. Mircobiol Rev. 1988;52:296–303. doi: 10.1128/mr.52.2.296-303.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eskola J, Käyhty H, Takala AK, et al. A randomized, prospective field trial of a conjugate vaccine in the protection of infants and young children against invasive Haemophilus influenzae type b disease. N Engl J Med. 1990;323:1381–7. doi: 10.1056/NEJM199011153232004. [DOI] [PubMed] [Google Scholar]
- 15.Takala AK, Eskola J, Leinonen M, et al. Reduction of oropharyngeal carriage of Haemophilus influenzae type b (Hib) in children immunized with an Hib conjugate vaccine. J Infect Dis. 1991;164:982–6. doi: 10.1093/infdis/164.5.982. [DOI] [PubMed] [Google Scholar]
- 16.Murphy TV, Pastor P, Medley F, Osterholm MT, Granoff DM. Decreased Haemophilus colonization in children vaccinated with Haemophilus influenzae type b conjugate vaccine. J Pediatr. 1993;122:517–23. doi: 10.1016/s0022-3476(05)83529-2. [DOI] [PubMed] [Google Scholar]
- 17.Mohle-Boetani JC, Ajello G, Breneman E, et al. Carriage of Haemophilus influenzae type b in children after widespread vaccination with conjugate Haemophilus influenzae type b vaccines. Pediatr Infect Dis J. 1993;12:589–93. doi: 10.1097/00006454-199307000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Takala AK, Santosham M, Almeido-Hill J, et al. Vaccination with Haemophilus influenzae type b meningococcal protein conjugate vaccine reduces oropharyngeal carriage of Haemophilus influenzae type b among American Indian children. Pediatr Infect Dis J. 1993;12:593–9. doi: 10.1097/00006454-199307000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Barbour ML, Mayon-White RT, Coles C, Crook DWM, Moxon ER. The impact of conjugate vaccine on carriage of Haemophilus influenzae type b. J Infect Dis. 1995;171:93–98. doi: 10.1093/infdis/171.1.93. [DOI] [PubMed] [Google Scholar]
- 20.Kauppi M, Eskola J, Käyhty H. Anti-capsular polysaccharide antibody concentrations in saliva after immunization with Haemophilus influenzae type b conjugate vaccines. Pediatr Infect Dis J. 1995;14:286–94. doi: 10.1097/00006454-199504000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Kauppi M, Saarinen L, Käyhty H. Anti-capsular polysaccharide antibodies reduce nasopharyngeal colonization by Haemophilus influenzae type b in infant rats. J Inf Dis. 1993;167:365–71. doi: 10.1093/infdis/167.2.365. [DOI] [PubMed] [Google Scholar]
- 22.Kauppi-Korkeila M, van Alphen L, Madore D, Saarinen L, Käyhty H. Mechanism of antibody-mediated reduction of nasopharyngeal colonization by Haemophilus influenzae type b studied in an infant rat model. J Infect Dis. 1996;174:1337–40. doi: 10.1093/infdis/174.6.1337. [DOI] [PubMed] [Google Scholar]
- 23.Eskola J, Ölander R-M, Hovi T, Litmanen L, Peltola S, Käyhty H. Randomized trial of the effect of co-administration with acellular pertussis DTP vaccine on immunogenicity of Haemophilus influenzae type b conjugate vaccine. Lancet. 1996;348:1688–92. doi: 10.1016/S0140-6736(96)04356-5. [DOI] [PubMed] [Google Scholar]
- 24.Pichichero ME, Hall CB, Insel RA. A mucosal antibody response following systemic Haemophilus influenzae type b infection in children. J Clin Invest. 1981;67:1482–9. doi: 10.1172/JCI110178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pichichero ME, Insel RA. Mucosal antibody response to parental vaccination with Haemophilus influenzae type b capsule. J Allergy Clin Immunol. 1983;72:481–6. doi: 10.1016/0091-6749(83)90585-7. [DOI] [PubMed] [Google Scholar]
- 26.Gilsdorf JR, McDonnell WM. Mucosal antibodies to Haemophilus influenzae type b capsular polysaccharide. Pediatr Res. 1991;29:420–3. doi: 10.1203/00006450-199105010-00002. [DOI] [PubMed] [Google Scholar]
- 27.Smith DJ, Taubman MA. Ontogeny of immunity to oral microbiota in humans. Crit Rev Oral Biol Med. 1992;3:109–33. doi: 10.1177/10454411920030010201. [DOI] [PubMed] [Google Scholar]
- 28.Tappuni AR, Challacombe SJ. A comparison of salivary immunoglobulin A (IgA) and IgA subclass concentrations in predentate and dentate children and adults. Oral Microbiol Immunol. 1994;9:142–5. doi: 10.1111/j.1399-302x.1994.tb00050.x. [DOI] [PubMed] [Google Scholar]
- 29.Kilian M, Reinhodt J, Lomholt H, Poulsen K, Frandsen EVG. Biological significance of IgA1 proteases in bacterial colonization and pathogenesis: critical evaluation of experimental evidence. APMIS. 1996;104:321–38. doi: 10.1111/j.1699-0463.1996.tb00724.x. [DOI] [PubMed] [Google Scholar]
- 30.Lue C, Tarkowski A, Mestecky J. Systemic immunization with pneumococcal polysaccharide vaccine induces a predominant IgA2 response of peripheral blood lymphocytes and increases of both serum and secretory anti-pneumococcal antibodies. J Immunol. 1988;140:3793–800. [PubMed] [Google Scholar]
- 31.Heilmann C, Barington T, Sigsgaard T. Subclass of individual IgA-secreting lymphocytes. Investigation of in vivo pneumococcal polysaccharide-induced and in vitro mitogen-induced blood B cells by monolayer plaque-forming cell assays. J Immunol. 1988;140:1496–9. [PubMed] [Google Scholar]
- 32.Tarkowski A, Lue C, Moldoveanu Z, Kiyono H, McGhee JR, Mestecky J. Immunization of humans with polysaccharide vaccines induces systemic, predominantly polymeric IgA2-subclass antibody responses. J Immunol. 1990;144:3770–8. [PubMed] [Google Scholar]
- 33.Barington T, Juul L, Gyhrs A, Heilmann C. Heavy-chain isotype patterns of human antibody-secreting cells induced by Haemophilus influenzae type b conjugate vaccines in relation to age and preimmunity. Infect Immun. 1994;62:3066–74. doi: 10.1128/iai.62.8.3066-3074.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spinola ST, Sheaffer CI, Philbrick KB, Gilligan PH. Antigenuria after Haemophilus influenzae type b polysaccharide immunization: a prospective study. J Pediatr. 1986;109:835–8. doi: 10.1016/s0022-3476(86)80705-3. [DOI] [PubMed] [Google Scholar]
- 35.Darville T, Jacobs R, Lucas RA, Caldwell B. Detection of Haemophilus influenzae type b antigen in cerebrospinal fluid after immunization. Pediatr Infect Dis J. 1992;11:243–4. doi: 10.1097/00006454-199203000-00017. [DOI] [PubMed] [Google Scholar]
- 36.Brokstadt KA, Cox RJ, Olofsson J, Jonsson R, Haaheim LR. Parenteral influenza vaccination induces a rapid systemic and local immune response. J Infect Dis. 1995;171:198–203. doi: 10.1093/infdis/171.1.198. [DOI] [PubMed] [Google Scholar]


