Abstract
In a previous study, we reported that the high mobility group (HMG) non-histone chromosomal proteins HMG1 and HMG2 were novel target antigens of P-ANCA. In this study, we determined the immunodiagnostic value of anti-HMG1/HMG2 antibodies in patients with UC. Sixty sera from patients with UC were tested for reactivity with HMG1 and HMG2 by means of ELISA. Anti-HMG1 antibody was detected in 32% of patients (40% of P-ANCA+ patients). Anti-HMG2 antibody was detected in 33% (40% of P-ANCA+ patients). Thirty-five percent of sera were positive for antibody to either HMG1 or HMG2 (43% of P-ANCA+ patients). P-ANCA+ patients expressed anti-HMG1/HMG2 antibodies with significantly greater frequency compared with P-ANCA− patients. Furthermore, the anti-HMG1/HMG2 antibodies were significantly related to disease activity in UC. Sixteen of the 18 UC patients, who had high titres of anti-HMG1 or -HMG2 antibody during the active phase, showed lower titres in the inactive phase. Anti-HMG1/HMG2 antibodies appear to be useful as a marker for disease activity in UC.
Keywords: anti-neutrophil cytoplasmic antibodies, HMG1, HMG2, ulcerative colitis
INTRODUCTION
ANCA are divided into the cytoplasmic (C-ANCA) and the perinuclear (P-ANCA) type by indirect immunofluorescence (IIF). Proteinase 3 (PR3) is the major C-ANCA antigen and has proved a useful clinical tool in supporting the diagnosis and monitoring of disease activity in Wegener's granulomatosis (WG) [1–3]. Myeloperoxidase (MPO) is the major P-ANCA antigen in microscopic polyangiitis and has clinical significance [4]. ANCA are also associated with non-vasculitic diseases such as inflammatory bowel disease (IBD) [5–9], chronic liver diseases [10, 11], and, less frequently, various infections [12–14]. The occurrence of P-ANCA in IBD, particularly in UC, has raised the possibility of P-ANCA as a marker of UC. The prevalence of P-ANCA in UC is estimated as 30–83% [5–9]. These P-ANCA do not react with MPO, and some of the antigens have been shown to be cathepsin G and lactoferrin [15, 16]. However, the target antigens useful for differential diagnosis and monitoring disease activity are not yet known.
Recently, we have found that high mobility group (HMG) non-histone chromosomal proteins, HMG1 and HMG2, are target antigens of P-ANCA [17]. Alignment of the HMG1 and the HMG2 family reveals a remarkable conservation in the primary sequence of the proteins [18–22]. There are differences in only two amino acid residues between human and porcine HMG1/HMG2. Because of this high degree of conservation in primary sequences, anti-human HMG1/HMG2 antisera cross-reacted with porcine HMG1 and HMG2 [17]. Therefore, we determined the frequency of antibodies against HMG1/HMG2 in patients with UC by ELISA, using HMG1/HMG2 purified from pig thymus as antigens. In the previous study, we reported that the detection of anti-HMG1/HMG2 antibody was significantly correlated with anti-cathepsin G positivity and these antibodies were correlated with a refractory type of UC [23]. To assess these points, we investigated the relationships between antibodies to HMG1/HMG2 on the one hand and antibodies to cathepsin G and lactoferrin on the other, and analysed the clinical significance of these antibodies.
PATIENTS AND METHODS
Patients and normal controls
Sixty patients, 27 men aged 6–77 years (mean 35 years) and 33 women aged 20–75 years (mean 35 years), participated in this study. Thirty-two of the patients had active disease, that is, at least five loose stools with visible blood (all had visible inflammation at endoscopy). The remaining 28 patients were in clinical and endoscopic remission. Seventeen patients had proctitis, 24 patients had left-sided colitis, and 19 had more extensive or total colitis. The mean duration of the disease was 5.8 years (range 0.1–22 years). Ten patients were being treated with corticosteroids at the time of blood sampling. Seven of 60 patients suffered from refractory type of UC. The refractory type was defined as the UC meeting any one of the following criteria despite the administration of rigorous conventional therapy: (i) active stage even 6 months or more after the first attack (chronic continuous type); (ii) active stage even 6 months or more after a relapse; and (iii) frequent relapsing within 3 months. None of the patients had any evidence of liver disease. The numbers of normal control subjects examined for anti-HMG1/HMG2, anti-cathepsin G, and anti-lactoferrin antibodies were 37 (mean age 30 years; range 19–48 years), 36 (mean age 29 years; range 19–41 years) and 42 (mean age 31 years; range 19–48 years), respectively.
Materials
Porcine HMG1 and HMG2 were purified from pig thymus as described elsewhere [24]. Human cathepsin G (ICN Biomedicals, Cleveland, OH) and human lactoferrin (Sigma, St Louis, MO) were purchased. Their purity was confirmed by SDS–PAGE. Neutrophils were isolated from peripheral blood obtained from normal human volunteers by Ficoll density gradient centrifugation.
IIF assay
ANCA activity was determined by IIF assay reported by Wiik [25]. Briefly, ethanol-fixed cytospin preparations of human neutrophils were incubated with sera diluted 1:10 at room temperature for 20 min and stained with FITC-conjugated rabbit anti-human IgG F(ab′)2 antibodies (Serotec, Oxford, UK). The slides were evaluated by fluorescence microscopy. All samples positive for perinuclear staining were subjected to formalin fixation, instead of ethanol fixation, to distinguish ANCA activity from antinuclear antibodies [26].
ELISA
Antibodies to HMG1/HMG2, cathepsin G and lactoferrin were assayed by ELISA. Fifty microlitres of 5 μg/ml of HMG1/HMG2 in PBS, pH 7.4, cathepsin G in 500 mm sodium acetate with 150 mm NaCl, pH 5.5, and lactoferrin in bicarbonate buffer pH 9.6 were put onto microtitre plates (Immuno-plate Maxisorp; Nunc, Roskilde, Denmark). The plates were blocked with 5% bovine serum albumin (BSA) in PBS. Serum samples were diluted 1:40 in PBS containing 5% BSA, and the secondary antibody used was alkaline phosphatase-conjugated F(ab′)2 goat anti-human IgG (Immunotech, Marseilles, France). All incubations were performed at room temperature for 2 h, and the plates were washed five times with PBS containing 0.05% Tween 20. Antibody titres were expressed as U/ml by reference to the dilution curves for positive control sera, the titres of which were arbitrarily set at 100 U/ml. A level > 3 s.d. above the mean for normal sera was regarded as positive. Competitive inhibition ELISA using HMG1, HMG2, cathepsin G and lactoferrin was performed to determine the specificity of activity to those proteins. HMG1 and/or HMG2 (100 μg/ml), cathepsin G (10 μg/ml) and lactoferrin (100 μg/ml) were incubated with an equal volume of varying dilutions of corresponding antibody-positive sera for 30 min at room temperature. Ovalbumin (100 μg/ml or 10 μg/ml) was used as a control. The inhibition was considered significant if the optical density (OD) value was reduced by 30% or more compared with controls.
Western blotting
SDS–PAGE was performed as described by Laemmli [27]. Briefly, samples were boiled in SDS sample buffer (2% SDS, 50 mm Tris–HCl pH 6.8, 5% 2-mercaptoethanol (2-ME), 5% glycerol and 0.002% bromophenol blue) for 10 min at 85°C before electrophoresis on 3% stacking and 12.5% separating polyacrylamide gels. For immunoblotting, the samples were transferred to Immobilon sheets (Millipore, Bedford, MA). The sheets were then blocked with 5% skimmed milk in PBS for 1.5 h at room temperature, and incubated with patient serum diluted 1:100 with 5% skimmed milk in PBS overnight at 4°C. The secondary antibody used was horseradish peroxidase (HRP)-conjugated antibodies specific for human IgG (Cappel, Durham, NC). The reaction was visualized by incubation with ECL detection reagents (Amersham, Aylesbury, UK).
Statistical analysis
The results were analysed by the χ2 test, Fisher's exact test, Mann–Whitney U-test and Wilcoxon signed rank test.
RESULTS
Antibodies to HMG1, HMG2, cathepsin G and lactoferrin assayed by ELISA
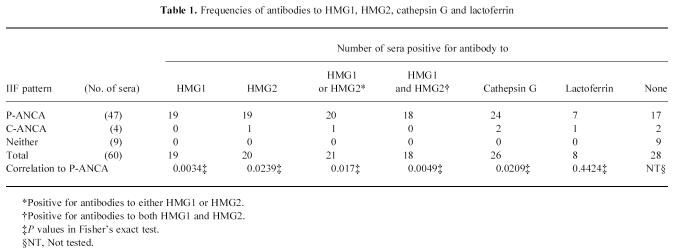
The titres of antibodies to HMG1, HMG2, cathepsin G and lactoferrin are shown in Fig. 1. The frequencies of antibodies to these proteins are summarized in Table 1. Anti-HMG1 antibody was detected in 19 (32%) of 60 patients. Anti-HMG2 antibody was detected in 20 (33%) of 60 patients. Twenty-one (35%) of 60 sera were positive for antibody to either HMG1 or HMG2. Eighteen (30%) of 60 sera were positive for antibodies to both HMG1 and HMG2. Anti-cathepsin G antibody was detected in 26 (43%) of 60 patients. Anti-lactoferrin antibody was detected in eight (13%) of 60 patients. These positive sera seemed to be specific, since preincubation of the sera with the corresponding antigens abrogated activity in the subsequent ELISA (data not shown).
Fig. 1.
Titres of antibodies to HMG1 (a), HMG2 (b), cathepsin G (c) and lactoferrin (d) determined by ELISA. N, Normal controls. The solid lines represent median values of antibody titres. P values by Mann–Whitney U-test.
Table 1.
Frequencies of antibodies to HMG1, HMG2, cathepsin G and lactoferrin
Relationships between P-ANCA and antibodies to HMG1/HMG2, cathepsin G and lactoferrin
P-ANCA were detected in 47 (78%) of 60 patients and C-ANCA were detected in four (7%) of 60 patients (Table 1). All of the 19 sera positive for anti-HMG1 antibody were positive for P-ANCA. Nineteen of the 20 sera positive for anti-HMG2 antibody were positive for P-ANCA and the remainder was positive for C-ANCA. P-ANCA+ patients expressed anti-HMG1/HMG2 antibodies with significantly greater frequency compared with P-ANCA− patients.
Of the 26 sera positive for anti-cathepsin G antibody, 24 sera were positive for P-ANCA, and the remaining two sera were positive for C-ANCA. P-ANCA+ patients expressed anti-cathepsin G antibody with significantly greater frequency than P-ANCA− patients.
Of the eight sera positive for anti-lactoferrin antibody, seven sera were positive for P-ANCA, and the remainder was positive for C-ANCA. No significant correlation was found between anti-lactoferrin antibody positivity and P-ANCA positivity.
Relationships between antibodies to HMG1/HMG2, cathepsin G and lactoferrin
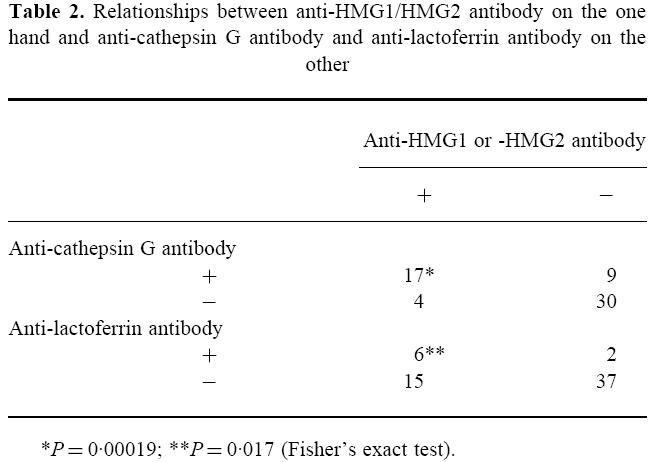
Seventeen of the 21 sera positive for anti-HMG1 or -HMG2 antibody were also positive for anti-cathepsin G antibody (Table 2). There was a significant correlation between the positivities for antibodies to HMG1/HMG2 and cathepsin G. Six of the 21 sera positive for anti-HMG1 or -HMG2 antibody were positive for anti-lactoferrin antibody (Table 2). There was also a significant correlation between the positivities for antibodies to HMG1/HMG2 and lactoferrin. In contrast, there was no correlation between the positivities for antibodies to cathepsin G and lactoferrin (data not shown).
Table 2.
Relationships between anti-HMG1/HMG2 antibody on the one hand and anti-cathepsin G antibody and anti-lactoferrin antibody on the other
Detection of antibodies to HMG1/HMG2 by ELISA and Western blotting
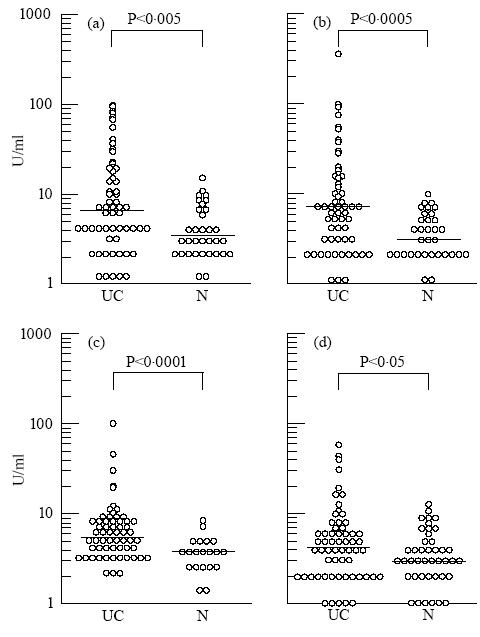
To determine the sensitivity of ELISA and Western blotting in detecting antibodies to HMG1/HMG2, the 21 sera positive for anti-HMG1 or -HMG2 antibody by ELISA were tested by Western blotting. As shown in Fig. 2, most sera positive for anti-HMG1 and -HMG2 antibody by ELISA exhibited 29-kD and 28-kD bands, respectively. Most sera negative for the 28/29-kD bands tended to show lower titres in ELISA. Anti-HMG1 or anti-HMG2 antibody-negative patients did not exhibit the 28/29-kD band. The representative results are shown in Fig. 2c. The serum used in lanes 7 and 8, although positive for anti-HMG1/HMG2 antibodies, showed no bands.
Fig. 2.
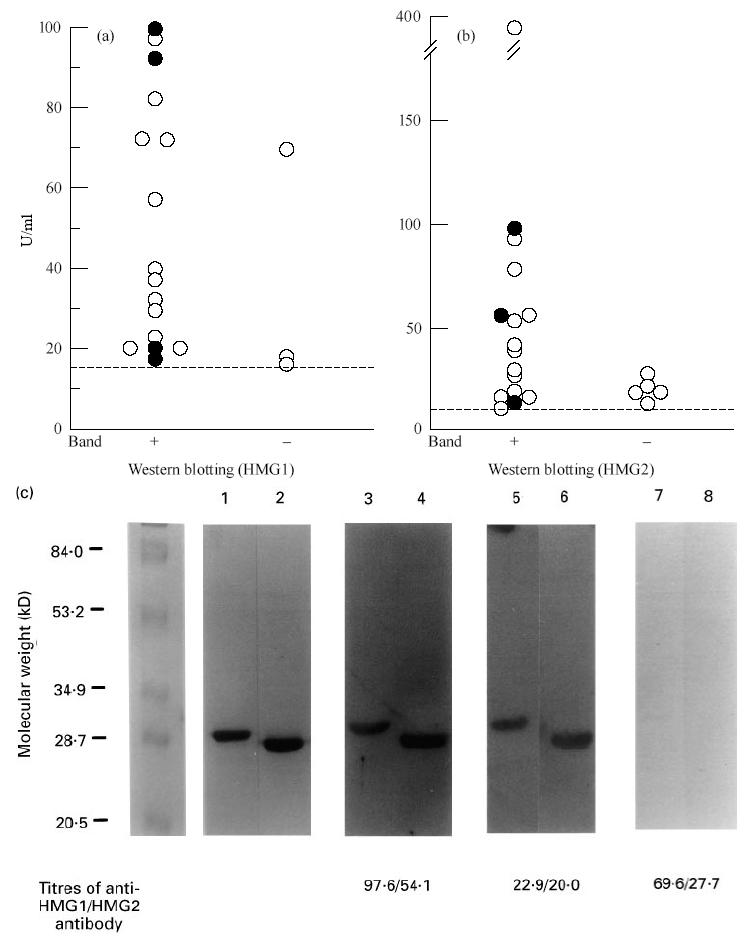
Detection of antibodies to HMG1/HMG2 by ELISA and Western blotting. (a) The 19 sera positive for anti-HMG1 antibody by ELISA were tested by Western blotting using HMG1 as antigen. (b) The 20 sera positive for anti-HMG2 antibody by ELISA were tested by Western blotting using HMG2 as antigen. Closed and open circles represent individual patients with refractory and non-refractory types of UC, respectively. (c) Western blotting analysis of anti-HMG1/HMG2 antibody. Antigenic substrates were 1 μg porcine HMG1 (lanes 1, 3, 5 and 7) or HMG2 (lanes 2, 4, 6 and 8). Lanes 1 and 2, SDS–PAGE stained by Fast stain (Zoion, Allestone, MA); lanes 3–8, sera of UC patients. The titres of anti-HMG1/HMG2 antibody of these sera are indicated below the lanes.
Relationships between antibodies to HMG1/HMG2 and disease activity
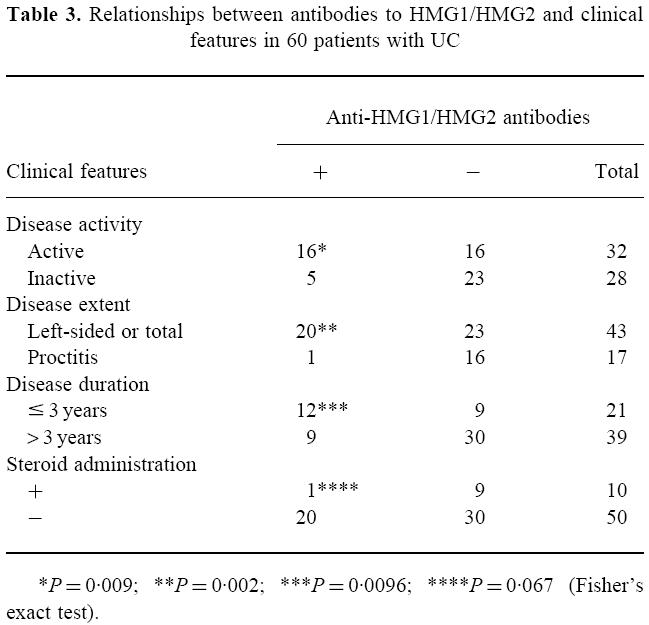
To determine whether the presence of antibodies to HMG1/HMG2 reflected the clinical state, we investigated the relationships between the presence of antibodies to HMG1/HMG2 and various variables, i.e. the duration, extent, activity and drugs (Table 3). We found significant relationships with the duration (≤ 3 years or > 3 years), extent (left-sided/total or proctitis), and activity (active or inactive).
Table 3.
Relationships between antibodies to HMG1/HMG2 and clinical features in 60 patients with UC
Changes in the titres of antibodies to HMG1 and HMG2 in UC following conventional therapy
Among the UC patients examined, 30 patients were followed up from active to inactive phase. Eighteen of them were positive for anti-HMG1 or -HMG2 antibody in the active phase. Among them, 12 patients received salazosulfapyridine and six patients received corticosteroids. Sixteen of these 18 patients showed lower titres in the inactive phase (Fig. 3). The interval of time of blood sampling between active and inactive phase was 17.3 months (range 2–39 months). As for anti-HMG1/HMG2 antibodies, the anti-cathepsin G antibody tended to show lower titres during the inactive phase (data not shown).
Fig. 3.
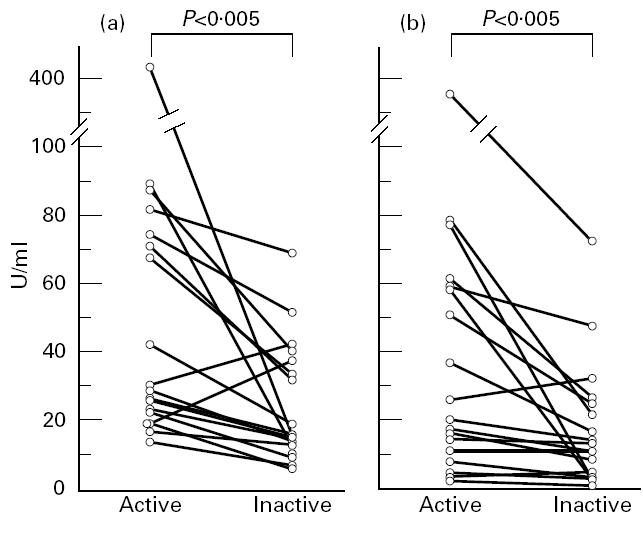
Anti-HMG1/HMG2 antibody titres in individual patients between active and inactive phases. (a) Anti-HMG1 antibody. (b) Anti-HMG2 antibody. Fifteen of the 17 sera positive for anti-HMG1 antibody and 13 of the 14 sera positive for anti-HMG2 antibody showed lower titres in the inactive phase. P values by Wilcoxon signed rank test.
DISCUSSION
Here we use a term HMG-ANCA to express antibodies against either HMG1 or HMG2, since most sera positive for anti-HMG1 antibody were simultaneously positive for anti-HMG2 antibody in UC patients.
HMG-ANCA were detected in 43% of P-ANCA+ patients by ELISA. Cathepsin G, lactoferrin and bactericidal/permeability-increasing protein were reported as target antigens of ANCA in UC [15, 16, 28, 29]. However, none of those proteins, including HMG1/HMG2, is the main target antigen of ANCA in UC patients. It seems that unknown disease-specific antigens of P-ANCA exist, and multiple antibodies against different antigens may contribute to the staining pattern of ANCA in UC.
We previously reported that the 28-kD band positivity by Western blotting using neutrophils as antigen was significantly correlated with anti-cathepsin G antibodies in UC [23]. Here we confirmed a significant association between HMG-ANCA and anti-cathepsin G antibodies. HMG-ANCA were also detected in some patients with systemic rheumatic disease, in whom there was no correlation between the two autoantibodies (unpublished data). It is therefore interesting that HMG-ANCA and anti-cathepsin G antibodies develop simultaneously in patients with UC.
We also reported that positivity for the 28-kD band by Western blotting using neutrophils was significantly correlated with a refractory type of UC [23]. Here we found HMG-ANCA not only in refractory UC but also in non-refractory UC by ELISA. Some sera with refractory type of UC that showed low titres of HMG-ANCA in ELISA exhibited 28/29-kD bands, and some of the high-titre sera with non-refractory type of UC did not exhibit 28/29-kD bands (Fig. 2). Furthermore, some of the low-titre sera with refractory UC more frequently exhibited a 28-kD band in Western blotting using neutrophils, instead of HMG1 and HMG2 (data not shown). These results suggested that individual sera recognize different epitopes on HMG1/HMG2 proteins. In other words, differences in antigenic epitopes might exist between denatured HMG in Western blotting and native HMG in ELISA, and between purified HMG and crude proteins in neutrophil lysates.
The present study revealed that a significant amount of HMG-ANCA appeared in the active stage. This was also true for anti-cathepsin G antibodies. The frequency of anti-cathepsin G antibodies in UC patients was higher in this study than in the previous one, presumably because patients in the active phase were more numerous in this study. PR3 is known to be a marker for relapse in WG [2]. It has been difficult to predict the relapse of UC clinically. We will further investigate whether HMG-ANCA and anti-cathepsin G antibodies can be used for predicting relapse in UC.
HMG1 and HMG2 are distributed in the nuclei and cytoplasm of eukaryotic cells and act as transcription factors [30, 31]. Their localization in the nuclei suggests that anti-HMG1/HMG2 antibodies belong to the category of antinuclear antibody. We reported, however, that anti-HMG1/HMG2 antibodies did not stain the nuclei of ethanol- or formalin-fixed neutrophils, and showed a P-ANCA staining pattern of ethanol-fixed neutrophils on IIF [17]. Furthermore, the affinity-purified antibodies from UC patients' sera and MoAbs to HMG1/HMG2 produced a P-ANCA staining pattern, and did not stain the nuclei or cytoplasm of HEp-2 cells and lymphocytes (unpublished observation). These results indicate that the antibodies to HMG1/HMG2 can fulfil the criteria of P-ANCA.
Amphoterin, a protein identical to HMG1, is known to exist on the membrane of nerve cells and have a role in enhancing neurite outgrowth [32, 33]. These findings raise the possibility that HMG-ANCA binds to HMG1/amphoterin on the cell membrane, thereby inhibiting its action. Alternatively, it is possible that production of HMG-ANCA is a secondary phenomenon, the initiating event being T cell-mediated cytotoxicity directed against endogenous HMG-derived peptides coexisting with class I (occasionally class II) MHC molecules on target cells. To evaluate the pathogenic significance of HMG-ANCA, it will be necessary to clarify the localization and expression of HMG1 and HMG2 on intestinal epithelia and inflammatory cells.
Acknowledgments
This work was supported in part by Grants-in-aid from the Ministry of Education, Science, and Culture of Japan, by research grants from the Ministry of Health and Welfare of Japan, and by grants from the Japan Rheumatism Association.
References
- 1.Lüdemann J, Utecht B, Gross WL. Anti-neutrophil cytoplasm antibodies in Wegener's granulomatosis recognize an elastinolytic enzyme. J Exp Med. 1990;171:357–62. doi: 10.1084/jem.171.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen Tervaert JW, van der Woude FJ, Fauci AS, et al. Association between active Wegener's granulomatosis and anticytoplasmic antibodies. Arch Intern Med. 1989;149:2461–5. doi: 10.1001/archinte.149.11.2461. [DOI] [PubMed] [Google Scholar]
- 3.Nölle B, Specks U, Lüdemann J, et al. Anticytoplasmic autoantibodies: their immunodiagnostic value in Wegener's granulomatosis. Ann Intern Med. 1989;111:28–40. doi: 10.7326/0003-4819-111-1-28. [DOI] [PubMed] [Google Scholar]
- 4.Falk RJ, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med. 1988;318:1651–7. doi: 10.1056/NEJM198806233182504. [DOI] [PubMed] [Google Scholar]
- 5.Saxon A, Shanahan F, Landers C, et al. A distinct subset of antineutrophil cytoplasmic antibodies is associated with inflammatory bowel disease. J Allergy Clin Immunol. 1990;86:202–10. doi: 10.1016/s0091-6749(05)80067-3. [DOI] [PubMed] [Google Scholar]
- 6.Duerr RH, Targan SR, Landers C, et al. Anti-neutrophil cytoplasmic antibodies in ulcerative colitis: comparison with other colitides/diarrheal illnesses. Gastroenterol. 1991;100:1590–6. doi: 10.1016/0016-5085(91)90657-7. [DOI] [PubMed] [Google Scholar]
- 7.Seibold F, Weber P, Klein R, et al. Clinical significance of antibodies against neutrophils in patients with inflammatory bowel disease and primary sclerosing cholangitis. Gut. 1992;33:657–62. doi: 10.1136/gut.33.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cambridge G, Rampton DS, Stevens TRJ, et al. Anti-neutrophil antibodies in inflammatory bowel disease: prevalence and diagnostic role. Gut. 1992;33:668–74. doi: 10.1136/gut.33.5.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falk RJ, Sartor RB, Jones DA, et al. Anti-neutrophil cytoplasmic autoantibodies (ANCA) in ulcerative colitis (UC) Clin Res. 1990;38:387. (Abstr.). [Google Scholar]
- 10.Klein R, Eisenburg J, Weber P, et al. Significance and specificity of antibodies to neutrophils detected by Western blotting for the serological diagnosis of primary sclerosing cholangitis. Hepatol. 1991;14:1147–52. [PubMed] [Google Scholar]
- 11.Targan SR, Landers C, Vidrich A, et al. High-titer antineutrophil cytoplasmic antibodies in type-1 autoimmune hepatitis. Gastroenterol. 1995;108:1159–66. doi: 10.1016/0016-5085(95)90215-5. [DOI] [PubMed] [Google Scholar]
- 12.Koderisch J, Andrassy K, Rasmussen M, et al. “False-positive” anti-neutrophil cytoplasmic antibodies in HIV infection. Lancet. 1990;335:1227–8. doi: 10.1016/0140-6736(90)92755-7. [DOI] [PubMed] [Google Scholar]
- 13.Klaassen RJL, Goldschmeding R, Dolman KM, et al. Anti-neutrophil cytoplasmic autoantibodies in patients with symptomatic HIV infection. Clin Exp Immunol. 1992;87:24–30. doi: 10.1111/j.1365-2249.1992.tb06408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagner J, Andrassy K, Ritz E. Is vasculitis in subacute bacterial endocarditis associated with ANCA? Lancet. 1991;337:799–800. doi: 10.1016/0140-6736(91)91427-v. [DOI] [PubMed] [Google Scholar]
- 15.Halbwachs-Mecarelli L, Nusbaum P, Noël LH, et al. Antineutrophil cytoplasmic antibodies (ANCA) directed against cathepsin G in ulcerative colitis, Crohn's disease and primary sclerosing cholangitis. Clin Exp Immunol. 1992;90:79–84. doi: 10.1111/j.1365-2249.1992.tb05835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peen E, Almer S, Bodemar G, et al. Anti-lactoferrin antibodies and other types of ANCA in ulcerative colitis, primary sclerosing cholangitis, and Crohn's disease. Gut. 1993;34:56–62. doi: 10.1136/gut.34.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sobajima J, Ozaki S, Osakada F, et al. Novel autoantigens of perinuclear anti-neutrophil cytoplasmic antibodies (P-ANCA) in ulcerative colitis: non-histone chromosomal proteins, HMG1 and HMG2. Clin Exp Immunol. 1997;107:135–40. doi: 10.1046/j.1365-2249.1997.d01-907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Majumdar A, Brown D, Kerby S, et al. Sequence of human HMG2 cDNA. Nuc Acids Res. 1991;19:6643. doi: 10.1093/nar/19.23.6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wen L, Huang JK, Johnson BH, et al. A human placental cDNA clone that encodes nonhistone chromosomal protein HMG-1. Nuc Acids Res. 1989;17:1197–214. doi: 10.1093/nar/17.3.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shirakawa H, Yoshida M. Structure of a gene coding for human HMG2 protein. J Biol Chem. 1992;267:6641–5. [PubMed] [Google Scholar]
- 21.Shirakawa H, Tsuda K, Yoshida M. Primary structure of non-histone chromosomal protein HMG2 revealed by the nucleotide sequence. Biochem. 1990;29:4419–23. doi: 10.1021/bi00470a022. [DOI] [PubMed] [Google Scholar]
- 22.Bustin M, Lehn DA, Landsman D. Structural features of the HMG chromosomal proteins and their genes. Biochim Biophys Acta. 1990;1049:231–43. doi: 10.1016/0167-4781(90)90092-g. [DOI] [PubMed] [Google Scholar]
- 23.Sobajima J, Ozaki S, Okazaki T, et al. Anti-neutrophil cytoplasmic antibodies (ANCA) in ulcerative colitis: anti-cathepsin G and a novel antibody correlate with a refractory type. Clin Exp Immunol. 1996;105:120–5. doi: 10.1046/j.1365-2249.1996.d01-724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshida M, Shimura K. Unwinding of DNA by nonhistone chromosomal protein HMG (1+2) from pig thymus as determined with endonuclease. J Biochem. 1984;95:117–24. doi: 10.1093/oxfordjournals.jbchem.a134573. [DOI] [PubMed] [Google Scholar]
- 25.Wiik A. Delineation of a standard procedure for indirect immunofluorescence detection of ANCA. Acta Pathol Microbiol Immunol Scand. 1989;97:12–13. [PubMed] [Google Scholar]
- 26.Charles LA, Falk RJ, Jennette JC. Reactivity of anti-neutrophil cytoplasmic autoantibodies with HL-60 cells. Clin Immunol Immunopathol. 1989;53:243–53. doi: 10.1016/0090-1229(89)90054-8. [DOI] [PubMed] [Google Scholar]
- 27.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Zhao MH, Jones SJ, Lockwood CM. Bactericidal/permeability-increasing protein (BPI) is an important antigen for anti-neutrophil cytoplasmic autoantibodies (ANCA) in vasculitis. Clin Exp Immunol. 1995;99:49–56. doi: 10.1111/j.1365-2249.1995.tb03471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stoffel MP, Csernok E, Herzberg C, et al. Anti-neutrophil cytoplasmic antibodies (ANCA) directed against bactericidal/permeability increasing protein (BPI): a new seromarker for inflammatory bowel disease and associated disorders. Clin Exp Immunol. 1996;104:54–59. doi: 10.1046/j.1365-2249.1996.d01-654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aizawa S, Nishino H, Saito K, et al. Stimulation of transcription in cultured cells by high mobility group protein 1: essential role of the acidic carboxyl-terminal region. Biochem. 1994;33:14690–5. doi: 10.1021/bi00253a006. [DOI] [PubMed] [Google Scholar]
- 31.Ogawa Y, Aizawa S, Shirakawa H, et al. Stimulation of transcription accompanying relaxation of chromatin structure in cells overexpressing high mobility group 1 protein. J Biol Chem. 1995;270:9272–80. doi: 10.1074/jbc.270.16.9272. [DOI] [PubMed] [Google Scholar]
- 32.Merenmies J, Pihlaskari R, Laitinen J, et al. 30-kDa heparin-binding protein of brain (amphoterin) involved in neurite outgrowth: amino acid sequence and localization in the filopodia of the advancing plasma membrane. J Biol Chem. 1991;266:16722–9. [PubMed] [Google Scholar]
- 33.Parkkinen J, Raulo E, Merenmies J, et al. Amphoterin, the 30-kDa protein in a family of HMG1-type polypeptides: enhanced expression in transformed cells, leading edge localization, and interactions with plasminogen activation. J Biol Chem. 1993;268:19726–38. [PubMed] [Google Scholar]