Abstract
Aminopeptidase (AP) A is a transmembrane type II molecule widely distributed in mammalian tissues. Since APA expression may be absent in renal cell carcinoma (RCC), it is possible that there is an altered regulation or other defect of APA upon malignant transformation of proximal tubular cells. However, investigations into the regulation of APA on tumour cells are rare. We report, for the first time, that both transforming growth factor-beta 1 (TGF-β1) and tumour necrosis factor-alpha (TNF-α) down-regulate APA mRNA as well as protein expression in renal tubular epithelial cells and RCC cells in culture. In addition to this, both cytokines decrease dipeptidylpeptidase (DP) IV/CD26 mRNA, but not APN/CD13 mRNA expression. Otherwise, IL-4 and IL-13 increase CD13 as well as CD26 expression, but do not alter APA expression. Interferon-alpha (IFN-α), IFN-β and IFN-γ increase mRNA expression of all the three membrane ectopeptidases, whereas IL-1, IL-6, IL-7, IL-12 and granulocyte-macrophage colony-stimulating factor (GM-CSF) have been found to be without any significant effect. Treatment of cultured cells with cAMP-increasing agents, such as 8-bromo-cAMP or A23187, results in an increase in APA and DPIV/CD26, but no change in APN/CD13 mRNA expression or even a decrease in it. Furthermore, AP inhibitors can influence APA mRNA expression, since bestatin causes an increase in APA expression in a time- and dose-dependent manner, whereas bestatin does not change CD13 or CD26 expression. No difference could be found with respect to the modulation by different mediators between RCC cells and renal epithelial cells, though permanent tumour cell lines such as Caki-1 and Caki-2 may have lost some of the normally expressed peptidases.
Keywords: aminopeptidase A, bestatin, renal cell carcinoma, CD26, CD13, quantitative RT-PCR
INTRODUCTION
Cell surface peptidases play an important role in signal transduction and communication between cells. With respect to cancer cells, first experimental evidence indicates that membrane peptidases play a role in tumour cell growth and metastasis. The brush border membrane of renal tubules contains a variety of proteases that take part in the luminal hydrolysis of oligopeptides filtered across the glomerular membrane. Aminopeptidase (AP) A (glutamyl aminopeptidase, angiotensinase, EC 3.4.11.7) is a homodimeric type II cell membrane-bound hydrolase, which specifically cleaves the aminoterminal glutamyl or aspartyl residue from peptide substrates, such as angiotensin II or cholecystokinin-8 (for review see [1]). APA contains the consensus sequence HEXXH (385–389) found in zinc metallopeptidases such as thermolysin [2, 3] and is located in the brush borders of the renal or intestinal epithelial cells as well as in the vasculature [4], stromal cells derived from bone marrow and early B lineage cells (BP-1) [5]. An APA subtype is a constituent of activated pericytes in angiogenesis [6]. APA is assumed to be involved in diverse biological processes, such as peptide degradation [7] or B cell growth and differentiation [5]. The enzyme has been implicated in renal cell carcinoma (RCC), as an expression of APA on RCC cell lines correlated with resistance to a treatment with interferon-alpha (IFN-α) [8]. The observation that ≈ 20% of RCC lack APA expression indicates an altered regulation or other defect of this glycoprotein upon malignant transformation of proximal tubule cells [8].
APA, CD26/dipeptidylpeptidase (DP) IV (EC 3.4.14.5) and CD13/APN (neutral AP, EC 3.4.11.2) tend to be co-localized in the cortex of human kidneys [7, 9]. CD13 catalyses the removal from small peptides of N-terminal, preferentially neutral, amino acid residues (for review see [10]). The serin-dependent enzyme CD26 releases the N-terminal dipeptides from oligopeptides with proline or alanine as the penultimate amino acid (for review, see [10]). CD26 and CD13 are—as APA—ubiquitously occurring transmembrane type II molecules.
Renal cell tumours of the clear cell type demonstrate antigenic properties of the proximal tubule [11], and thus as tubular epithelial cells they can express APA, CD13 and CD26 [12]. Since membrane peptidases are considered to be involved in cell adhesion processes and metastasis, we were interested in their regulation. In recent investigations, we found that T cell-derived cytokines such as IL-4, IL-13 or IFN-γ can increase protein expression and/or enzymatic activity of CD13 and CD26 in RCC [13]. In this study, we investigate the regulation of APA mRNA and protein expression in renal tubular epithelial cells and RCC cells by various mediators. We compare the regulation of APA expression with the regulation of CD13 and CD26. Evidence is presented to show that (on RCC cells as well as on renal tubular epithelial cells) the expression of different APs is regulated by various mediators in concert.
MATERIALS AND METHODS
Cell culture
Fresh kidney specimens were obtained from the Urological Department of the Martin Luther University in Halle from patients undergoing nephrectomy. Sterile tumour specimens as well as obviously uninvolved cortical tissue of some kidneys were prepared as described [13]. In brief, tissues were minced into small pieces and dissociated by treatment with 0.1% collagenase, type IV (Sigma, Deisenhofen, Germany) and 0.015% DNase, type I (Boehringer, Mannheim, Germany) at 37°C for 1–2 h. After passing through a steel-mesh filter, cells were separated by Ficoll–Hypaque density gradient centrifugation and a two-stage washing procedure. Cells were cultured in Parker medium, supplemented with 10% fetal calf serum (FCS), 2 mm glutamine and antibiotics. Human tubular epithelial cells reached confluence after 7–15 days, RCC cells a few days later. Cells were identified by immunofluorescence techniques revealing co-expression of vimentin (Vim 3B4) and cytokeratin (MNF116). Cells were not positive for anti-von Willebrand factor and anti-CD68 (all MoAbs purchased from Dako, Hamburg, Germany). All cell lines could be grown for three to four passages. Experiments were performed using confluent cell lines of the second to third passage. Renal cancer cell lines Caki-1 and Caki-2 were purchased from DSMZ (Braunschweig, Germany) and grown in RPMI 1640 with 10% FCS.
Cytofluorimetric analysis
Cells were detached from six-well plates using gentle EDTA/trypsin treatment. After two cold washes, standard surface membrane immunofluorescence techniques were used. Cells were stained with the MoAb BP-1 (kindly provided by Dr M. D. Cooper, Howard Hughes Medical Institute, University of Birmingham, AL). After two cold washings, cells were labelled with the PE-conjugated secondary antibody and washed and fixed using 1% paraformaldehyde. The PE-labelled anti-CD13 (IgG1, Leu-M7) and the PE-labelled IgG1 isotype control were purchased from Becton Dickinson (Heidelberg, Germany) and the anti-CD26 (IgG1, M-A261) was obtained from PharMingen (San Diego, CA). Fluorescence was analysed in a Becton Dickinson FACScan. Five thousand cells per sample were counted. Differences of mean fluorescence intensity (MFI) were calculated as sample MFI — control antibody MFI.
Cytokines and reagents
Cells were treated for varying periods of time (4 h to 7 days) with the following recombinant cytokines or other mediators: tumour necrosis factor-alpha (TNF-α; kindly supplied by Knoll AG, Ludwigshafen, Germany; specific activity 8.74 × 106 U/mg), IFN-β (Betaseron; kindly provided by M. Schirner, Schering AG, Berlin, Germany), IFN-α2b (Intron A; Essex Pharma GmbH, München, Germany), IL-1β (> 2 × 108 U/mg), transforming growth factor-beta 1 (TGF-β1; 1–2 × 107 U/mg), IFN-γ (2 × 107 U/mg), IL-4 (1 × 108 U/mg), IL-7 (2–4 × 108 U/mg), granulocyte-macrophage colony-stimulating factor (GM-CSF; 100 U/ml) (all from PBH, Hannover, Germany), IL-6 (2–4 × 108 U/mg, the generous gift of P. Heinrich, RWTH, Aachen, Germany), IL-8 (Genzyme, Cambridge, MA), IL-12 (R&D Systems GmbH, Wiesbaden-Nordenstadt, Germany), IL-13 (ICC, Ismaning, Germany), A23187 from Streptomyces chartreusis (Calbiochem, Bad Soden, Germany), 8-bromoadenosine 3′5′cyclic monophosphate, cholera toxin from Vibrio cholerae, prostaglandin E2(PGE2), bestatin, and forskolin (all from Sigma, Deisenhofen, Germany).
RNA isolation and cDNA synthesis
Total cellular RNA was isolated according to Chomczynski & Sacchi [14]. The first strand of DNA was synthesized (after a 10-min incubation at 20°C) at 42°C for 50 min using 1 μg total RNA in the presence of dilutions of internal competitive standard RNA (5000 pg, 1000 pg, 500 pg, 100 pg, 50 pg, 10 pg, 5 pg) in 5.5 μl DEPC water, 2 μl 5 × first strand buffer (250 mm Tris–HCl pH 8.3, 375 mm KCl, 15 mm MgCl2), 0.5 μl dNTP mix (10 mm each of dATP, dCTP, dGTP, dTTP), 1 μl 0.1 m DTT, 0.5 μl (50 pmol) random primer and 0.5 μl (200 U/μl) Superscript II-RT (Gibco BRL, Eggenstein, Germany).
Construction of the internal standard
For the construction of the internal competitive standard RNA, composite primers were synthesized (see Table 1 for primer sequences). Primer 1 was composed of two specific primers complementary to the coding strand of the appropriate peptidase. Primer 2 contained a sequence for the SP6 RNA polymerase and also one of the specific sequences of primer 1. The purified product of the first polymerase chain reaction (PCR) amplification with primers 1 and 3 was template for the second amplification using primers 2 and 3. The amplified DNA was gel purified (QIA quick Gel Extraction Kit; Qiagen, Hilden, Germany) following an in vitro transcription by the SP6 promotor using the transcription system of Boehringer. The recombinant RNA was quantified by adsorbance at 260 nm and was used as an internal standard in the cDNA synthesis and the competitive PCR reaction.
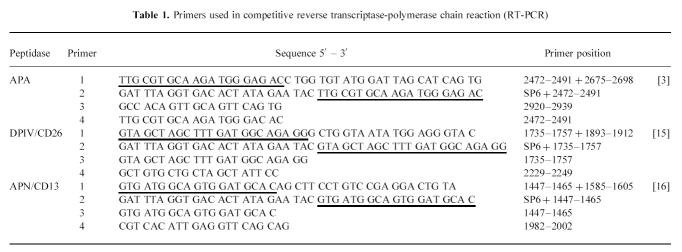
Table 1.
PCR analysis
cDNA (2 μl synthesized as described above) was diluted in 50 μl of the following solution: 50 mm KCl, 10 mm Tris–HCl pH 9.0, 1.5 mm MgCl2, 0.1% Triton X-100, 0.1 mm of each dNTP, 1 U Taq polymerase (AGS, Heidelberg, Germany) and 15 pmol each of primer 4 and primer 3. PCR was conducted in a thermal cycler (Biometra, Göttingen, Germany) for 35 cycles of 60 s at 94°C, 60 s at 60°C and 60 s at 72°C. Ten microlitres of each reaction product were run on a 1.5% agarose gel containing 0.1 μg/ml ethidium bromide in TAE buffer. The relative intensities of the bands corresponding to target and standard PCR products were visualized in UV light. The relative amounts of target and standard products were calculated after densitometric analysis using the Scan Pack 2.0 software (Biometra, Göttingen, Germany).
Statistical analysis
Data are expressed as range or as mean ± s.d. (unless indicated otherwise). The Wilcoxon rank sum test was used to determine whether two experimental values were significantly different, *P < 0.05, **P < 0.01.
RESULTS
Eleven cell lines of renal tubular epithelium with a typical epithelial morphology could be maintained in culture. Additionally, 10 cell lines from RCC (all of clear cell type) could be established. Tumour cells differed in size and exhibited either a spindle-shaped or a polygonal epithelium-like appearance. Immunohistochemically, RCC cell lines showed a uniform cytoplasmic staining with antibodies against vimentin and cytokeratin.
Comparison of the absolute amounts of mRNA of APA, APN/CD13 and DPIV/CD26
Figure 1 illustrates the comparison of the absolute amounts of the mRNA of APA, APN/CD13 and DPIV/CD26 in RCC and renal tubular epithelial cells. RCC cells and tubular epithelial cells propagated by us had comparable values of AP mRNA: APA mRNA concentrations were approximately one hundredth of the mRNA levels of APN/CD13 and DPIV/CD26. DPIV/CD26 mRNA ranged between 1 ng and 5.3 ng mRNA/μg total RNA (median 2.4 ng/μg total RNA). The amount of APN/CD13 mRNA was 0.74–8.2 ng/μg total RNA (median 2.3 ng/μg total RNA) and of APA mRNA 1.2–40 pg/μg total RNA (median 13.9 pg mRNA/μg total RNA). We did not observe a lack of APA expression in all 10 RCC lines established by us. Passaging of cells resulted in decreasing values of mRNA for all three APs. APA mRNA often could not be found in or after the 4th passage of cells when cell growth stopped, therefore only cells of the second and third passages were used for all experiments. Otherwise, commercially available RCC lines had down-regulated or even lost single APs, e.g. the Caki-2 cell line expressed 445 ± 160 pg DPIV/CD26 mRNA/μg total RNA, no quantifiable APN/CD13 mRNA and 48 ± 9 pg APA mRNA/μg total RNA, whereas Caki-1 cells expressed 23 ± 3 pg DPIV/CD26 mRNA, 846 ± 190 pg APN/CD13 mRNA and 1.4 ± 0.6 pg APA mRNA/μg total RNA. The lack of CD13 protein expression in Caki-2 and the down-regulation of CD26 in Caki-1 could be confirmed in immunofluorescence experiments (data not shown).
Fig. 1.
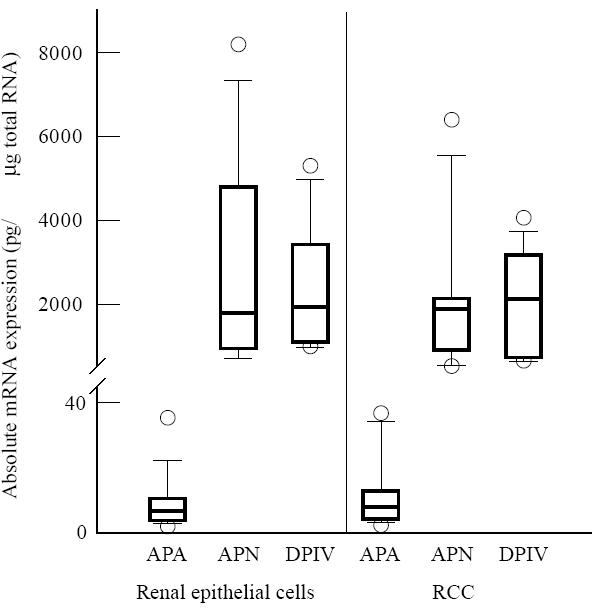
Comparison of the absolute amounts of the mRNA of aminopeptidase (AP) A, APN/CD13 and dipeptidylpeptidase (DP) IV/CD26 in renal tubular epithelial cells (n = 11) and renal cell carcinoma (RCC) cells (n = 10) in culture. Results of competitive reverse transcriptase-polymerase chain reaction (RT-PCR) after scanning and calculation as described in Materials and Methods. Data are given as box plots with the median. The box encompasses the 25th to 75th percentiles. The 5th and 95th percentiles are displayed as error bars.
Effect of different cytokines and bestatin on expression of APA, APN/CD13 and DPIV/CD26
Figure 2 shows the influence of various cytokines on the mRNA expression of the three peptidases investigated. Since there was no systematic difference in the results of RCC cells and tubular epithelial cells, all results were combined in one figure. TGF-β1 as well as TNF-α down-regulated APA mRNA expression. Using flow cytometry, we found that both cytokines also decreased APA protein expression: a 4-day incubation with TGF-β1 decreased APA protein to 93–64% of the control (76 ± 13%, control = 100%), whereas with TNF-α we found an APA expression of 98–33% of the control (64 ± 21%). DPIV/CD26 mRNA expression was also down-regulated by both cytokines, whereas no decrease could be observed with respect to APN/CD13, as already described by us [13]. IL-4 or IL-13, which both increase APN/CD13 as well as DPIV/CD26 expression, had no influence on APA mRNA and protein expression. IFN-α2b, IFN-β and IFN-γ increased mRNA expression of APA, APN/CD13 as well as DPIV/CD26 (Fig. 2). In immunofluorescence analyses, we observed a significant increase in APA protein after treatment of cells with IFN-α2b (133 ± 19%) or IFN-γ (130 ± 21% of control). Contrasting with the results on APA mRNA, we got no significant increase in APA protein after IFN-β treatment of cells, a result that needs further investigation. IL-1, IL-6, IL-7, IL-12 and GM-CSF were without any significant effect on the three peptidases at the concentrations tested (data not shown).
Fig. 2.
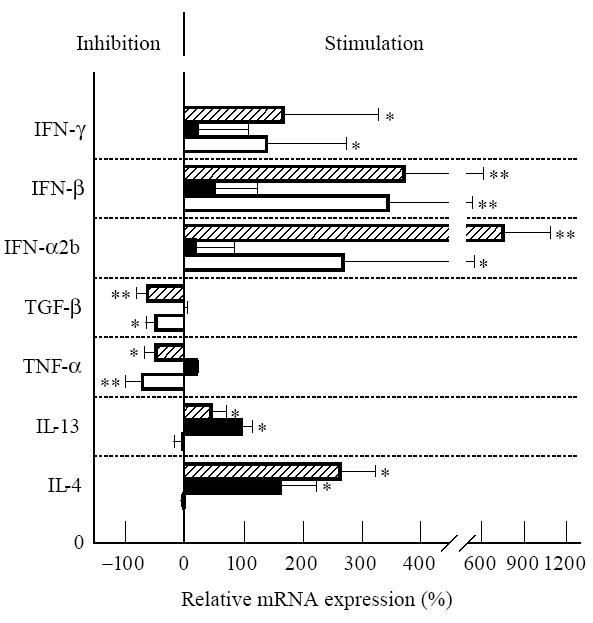
Regulation of the mRNA expression of aminopeptidase (AP) A (□), APN/CD13 (▪) and dipeptidylpeptidase (DP) IV/CD26 ( ) by different cytokines. Renal cell carcinoma (RCC) cells or renal epithelial cells were cultured for 24 h with: (i) IL-4 (200 U/ml); (ii) IL-13 (10 ng/ml); (iii) tumour necrosis factor-alpha (TNF-α) (100 U/ml); (iv) transforming growth factor-beta 1 (TGF-β1) (10 ng/ml); (v) IFN-α2b (1000 U/ml); (vi) IFN-β (1000 U/ml); (vii) IFN-γ (200 U/ml). Results of competitive reverse transcriptase-polymerase chain reaction (RT-PCR) are given as a percentage of the basal control (culture without cytokine = 0%, n = 6). Statistically significant differences from control: *P < 0.05; **P < 0.01.
) by different cytokines. Renal cell carcinoma (RCC) cells or renal epithelial cells were cultured for 24 h with: (i) IL-4 (200 U/ml); (ii) IL-13 (10 ng/ml); (iii) tumour necrosis factor-alpha (TNF-α) (100 U/ml); (iv) transforming growth factor-beta 1 (TGF-β1) (10 ng/ml); (v) IFN-α2b (1000 U/ml); (vi) IFN-β (1000 U/ml); (vii) IFN-γ (200 U/ml). Results of competitive reverse transcriptase-polymerase chain reaction (RT-PCR) are given as a percentage of the basal control (culture without cytokine = 0%, n = 6). Statistically significant differences from control: *P < 0.05; **P < 0.01.
Since CD13 has been reported to inactivate the chemotactic cytokine IL-8 [17], we combined IL-8 with bestatin to prevent IL-8 cleavage. We found that bestatin alone induces APA mRNA expression in a dose-dependent manner (Fig. 3). The stimulatory effect of bestatin on APA mRNA expression was confirmed by immunofluorescence experiments. Bestatin increased APA protein expression to 106–169% of the control (137 ± 32%). The bestatin effect was maximal with respect to APA mRNA after a 24-h incubation with the mediator. IL-8 caused a small, non-significant elevation of the bestatin-induced increase in mRNA expression. Bestatin had no effect on the expression of CD13 or CD26 (data not shown).
Fig. 3.
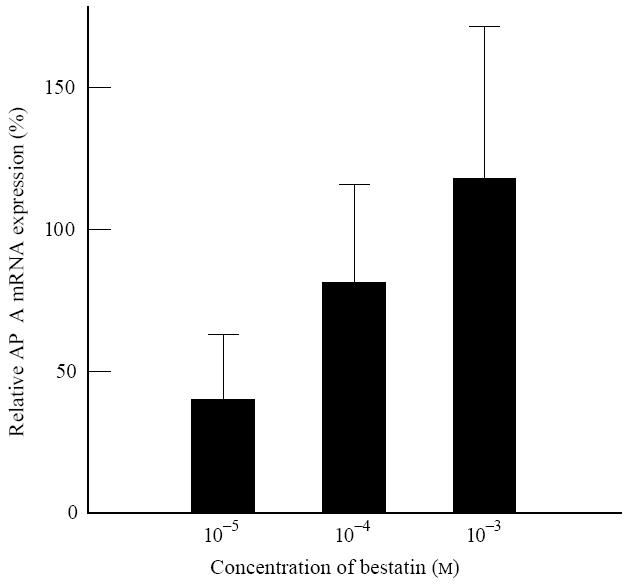
Effect of different concentrations of bestatin on aminopeptidase (AP) A mRNA expression of renal cell carcinoma (RCC) cells and renal epithelial cells after a 24-h incubation by use of competitive reverse transcriptase-polymerase chain reaction (RT-PCR). On the ordinate the relative APA mRNA expression is given as a percentage of the control (culture without bestatin = 0%, n = 5).
Effect of A23187, cAMP, forskolin, cholera toxin and PGE2 on AP mRNA expression
We treated RCC cells as well as renal tubular epithelial cells with the membrane-permeable cAMP analogue 8-bromoadenosine 3′5′cyclic monophosphate (8-bromo-cAMP), to investigate the direct effects of intracellular cAMP elevations on AP mRNA expression. In addition, we investigated the effect of various mediators known to elevate cAMP expression. Our results of the competitive reverse transcriptase (RT)-PCR demonstrate the enhancing effect of cAMP on APA mRNA, showing an increase after treatment of cells with 8-bromo-cAMP, A23187, forskolin, cholera toxin or PGE2 (Fig. 4). Whereas DPIV/CD26 mRNA expression was similarly enhanced after treatment of cells with 8-bromo-cAMP, A23187 or PGE2, no significant effect could be observed with forskolin or cholera toxin. APN/CD13 mRNA expression was rather decreased by cAMP-elevating mediators (significant decrease with 8-bromo-cAMP and forskolin, no significant change with A23187 and cholera toxin). Only with PGE2 was a small increase in APN/CD13 mRNA found.
Fig. 4.
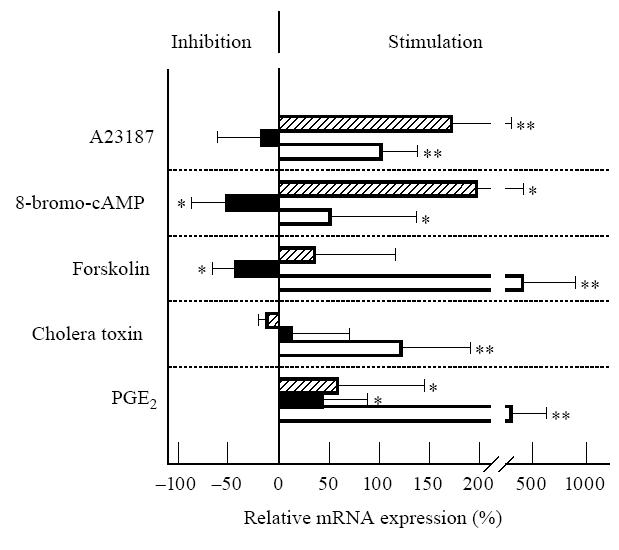
Effects of different mediators on mRNA expression of aminopeptidase (AP) A (□), APN/CD13 (▪) and dipeptidylpeptidase (DP) IV/CD26 ( ). Renal tubular epithelial cells or renal cell carcinoma (RCC) cells were cultured with A23187 (200 ng/ml), 8-bromoadenosine 3′5′monophosphate (100 μ m), forskolin (10 μ m), cholera toxin (1 μg/ml), or prostaglandin E2 (PGE2) (1 μ m) for 24 h. Results of competitive reverse transcriptase-polymerase chain reaction (RT-PCR) are shown as a percentage of the control (culture without mediator = 0%, n = 6). Statistically significant differences from control: *P < 0.05; **P < 0.01.
). Renal tubular epithelial cells or renal cell carcinoma (RCC) cells were cultured with A23187 (200 ng/ml), 8-bromoadenosine 3′5′monophosphate (100 μ m), forskolin (10 μ m), cholera toxin (1 μg/ml), or prostaglandin E2 (PGE2) (1 μ m) for 24 h. Results of competitive reverse transcriptase-polymerase chain reaction (RT-PCR) are shown as a percentage of the control (culture without mediator = 0%, n = 6). Statistically significant differences from control: *P < 0.05; **P < 0.01.
DISCUSSION
Using cell cultures of human RCC cells, we report for the first time the different regulation of APA in comparison with CD13 and/or CD26. Furthermore, we examine for the first time absolute mRNA expression for APA as well as DPIV/CD26 in human renal tubular epithelial cells and RCC cells by means of quantitative RT-PCR assays with competitive internal standards. We found that mRNA expression of APN/CD13 and DPIV/CD26 in tumour cells and epithelial cells was hundreds of times higher than APA mRNA expression. Additionally, we show that the regulation of these three ectopeptidases is a complex phenomenon, since at least seven cytokines influence mRNA and protein expression. No difference could be found with respect to the modulation by different mediators between renal cancer cells and renal epithelial cells, though permanent tumour cell lines such as Caki-1 and Caki-2 may have lost some of the membrane peptidases.
We confirm the presence of CD13 and CD26 on cultured RCC cells, as already described [12]. Since APA is detectable only in 80% of RCC [8], we had expected to find some RCC cells to be negative for APA expression. Though we did not find APA-negative RCC, we observed a decrease in APA as well as CD26 expression after incubation of cells with TGF-β1 or TNF-α, whereas both cytokines had no influence on CD13 expression. We are not aware of similar results from literature on the effect of TGF-β1 or TNF-α on APA expression. TGF-β1 is a multifunctional homodimeric polypeptide with potent action upon many target cells. TGF-β inhibits the growth of RCC in vitro [18] and could modulate a putative tumour-related immune response. Increased levels of TGF-β1 are common in the plasma of patients with RCC [19]. Similarly, an anti-proliferative activity of TNF-α on RCC has been described [20]. The finding that TNF-α down-regulates CD26 mRNA expression in RCC is in contrast to the results of experiments on human luteinizing granulosa cells, where TNF-α and IL-1α stimulate CD26 expression in vitro [21]. Otherwise, IL-4 or IL-13, cytokines which increase CD13 as well as CD26 expression in RCC cells [13], are without any significant effect on APA expression. We confirm the results of Stefanovic et al. with respect to an increase of CD26 expression in glomerular epithelial cells induced by IFN-γ [22]. IFN-γ as well as IFN-α2b increased APA expression in RCC cells, a result which is especially interesting with respect to the IFN-α treatment practised in patients with metastatic RCC. The mode of action of IFN-α is presumed to involve both direct cytotoxic and anti-proliferative effects on tumour cells and indirect effects that facilitate immune detection by the host [23]. Our findings add a new aspect to observations on the influence of IFN on RCC: if APA expression really does correlate with resistance to an IFN-α treatment—as discussed by Nanus et al. [8]—the IFN-α-induced up-regulation of APA expression on RCC tissues could counteract the success of an IFN-α therapy.
Recently it has been reported that control of the expression of CD13 and APA in mesangial or glomerular epithelial cells seems to vary. Whereas CD13 is sensitive to mitogens and T cell-derived cytokines, such as IFN-γ and IL-4 [13, 24], APA is sensitive to cAMP and glucocorticoids [24, 25]. We confirm the APA-enhancing effects of cAMP also in RCC cells and describe an increase in DPIV/CD26 and a decrease in APN/CD13 mRNA expression after treatment of cells with various cAMP-elevating agents. The antibiotic ionophore A23187, which has been discussed as being capable of elevating intracellular cAMP levels [26], shows a similar regulation pattern of APA to the membrane permeable cAMP analogue 8-bromo-cAMP or to forskolin. We cannot explain why forskolin, cholera toxin and PGE2 especially increased APA mRNA, whereas A23187 and 8-bromo-cAMP particularly elevated DPIV/CD26 mRNA expression. Forskolin is a diterpene which activates the catalytic moiety of adenyl cyclase [27]. Cholera toxin is known to ADP-ribosylate the α subunit of the Gs in an active configuration with consecutive activation of adenyl cyclase [28]. PGE2 appears to activate cells through binding to specific receptors on the cell surface, which also activate the α subunit of stimulatory GTP-binding protein [29]. It has to be considered, however, that these mediators have pleiotropic effects. Forskolin, as one example, may stimulate apical exocytosis or apical endocytosis in Caco-2 cells, and thus can induce a decrease in the brush border appearance of intestinal hydrolases, such as CD26 [30].
Surprisingly, we found an APA-enhancing effect of bestatin, an inhibitor of various APs, among them CD13 and basic AP (APB) (but rarely APA) [31]. We combined IL-8 treatment with bestatin, because CD13 has been reported to inactivate IL-8 [17], and we wanted to prevent IL-8 cleavage. Whereas with IL-8 alone we got rather scattered results for IL-8-induced APA regulation, bestatin alone elevated APA expression. IL-8 caused a further small increase of the bestatin effect. The significance of our observation is still unclear. Up to now there has been no information on an interactive regulation between APA and CD13 or a possible interaction between the chemotactic peptide IL-8 and APA activity. Bestatin (Ubenimex) has been shown to display immunomodulating properties [32] and to act as an anti-tumour agent [33]. These actions are believed to be, in part, due to its inhibition of cell surface CD13. However, in the light of the present results, these actions may either/also be due to the up-regulation of APA expression. Since bradykinin and substance P have been described to be endogenous CD13 inhibitors [34], it would be interesting to investigate the possible influence of these substances on APA activity.
Whether membrane-bound peptidases play a role in tumourigenesis remains unclear. First experimental evidence, however, indicates that membrane peptidases do play a role in tumour metastasis. Furthermore, their enzyme activity alters the biological activity of molecules that regulate tumour cell growth. A high level of neutral AP activity has been detected on the plasma membrane of several tumour cells: thyroid carcinomas express aberrant CD26, therefore staining for CD26 is a useful tool to aid in distinguishing benign from malignant thyroid neoplasms [35]. CD26 has been shown to bind to collagen and fibronectin [36] and has recently been implicated in the attachment of cancer cells to lung endothelium [37]. CD13 could play a role in tumour cell invasion and extracellular matrix degradation by human melanoma cells and RCC cells [38, 39], therefore up-regulation of CD13 expression on RCC cells could correlate with a greater risk of metastasis. Otherwise, inhibition of CD13 on choriocarcinoma cells has been reported to induce tumour growth suppression [40]. Nevertheless, over-expression of APs is not a uniform feature of cancer cells: CD26 is expressed on normal melanocytes, whereas its expression is lost as melanoma growth becomes independent of exogenous growth factors [41]. Similarly, CD10/neutral endopeptidase 24.11, a membrane-associated peptidase that cleaves potential autocrine growth factors, including bombesin-like peptides, is down-regulated in small-cell lung cancers [42]. Investigations into the function of membrane peptidases could be of help in gaining deeper insights into tumour cell survival.
In conclusion, we have investigated the regulation of the three membrane-associated ectopeptidases APA, CD13 and CD26 in RCC cells and renal tubular epithelial cells in culture. The precise role of membrane peptidases in tumour growth and metastasis still needs to be defined. However, a deeper understanding of the many pathways regulating their expression will be necessary not only for a better comprehension of the many facets of tumour cell growth and metastasis, but also for the development of therapeutic approaches.
Acknowledgments
We are grateful to co-workers from the Department of Urology of the Martin Luther University for supplying kidney probes. We acknowledge the technical assistance of Karin Bornschein, Rosemarie Meinicke and Karin Jung, as well as the expert secretarial assistance of Christel Walcker. We thank Matthias Löhn for helpful comments and Katja Thiele for critical reading the manuscript. This work was supported by the Deutsche Forschungsgemeinschaft (DFG Rie 799/1-1).
References
- 1.Wilk S, Healy DP. Glutamyl aminopeptidase (aminopeptidase A), the BP-1/6C3 antigen. Adv Neuroimmunol. 1993;3:195–207. [Google Scholar]
- 2.Nanus DM, Engelstein D, Gastl GA, et al. Molecular cloning of the human kidney differentiation antigen gp160: human aminopeptidase A. Proc Natl Acad Sci USA. 1993;90:7069–73. doi: 10.1073/pnas.90.15.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li L, Wang J, Cooper MD, et al. cDNA cloning and expression of human glutamyl aminopeptidase (aminopeptidase A) Genomics. 1993;17:657–64. doi: 10.1006/geno.1993.1386. [DOI] [PubMed] [Google Scholar]
- 4.Lojda Z, Gossrau R. Study of aminopeptidase A. Histochem. 1980;67:267–90. doi: 10.1007/BF00692761. [DOI] [PubMed] [Google Scholar]
- 5.Li L, Wu Q, Wang J, et al. Widespread tissue distribution of aminopeptidase A, an evolutionarily conserved ectoenzyme recognized by the BP-1 antibody. Tissue Antigens. 1993;42:488–96. doi: 10.1111/j.1399-0039.1993.tb02193.x. [DOI] [PubMed] [Google Scholar]
- 6.Schlingemann RD, Oosterwijk E, Wesseling P, et al. Aminopeptidase A is a constituent of activated pericytes in angiogenesis. J Pathol. 1996;179:436–42. doi: 10.1002/(SICI)1096-9896(199608)179:4<436::AID-PATH611>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 7.Kenny AJ, Stephenson SL, Turner AJ. Cell surface peptidases. In: Kenny AJ, Turner AJ, editors. Mammalian ectoenzymes. Amsterdam: Elsevier Science Publishers; 1987. pp. 169–210. [Google Scholar]
- 8.Nanus DM, Pfeffer LM, Bander NH, et al. Antiproliferative and antitumor effects of α-interferon in renal cell carcinomas: correlation with the expression of a kidney-associated differentiation glycoprotein. Cancer Res. 1990;50:4190–4. [PubMed] [Google Scholar]
- 9.Mentzel S, Dijkman HBP, VanSan Jphf, et al. Organ distribution of aminopeptidase A and dipeptidyl peptidase IV in normal mice. J Histochem Cytochem. 1996;44:445–61. doi: 10.1177/44.5.8627002. [DOI] [PubMed] [Google Scholar]
- 10.Shipp MA, Look AT. Hematopoietic differentiation antigens that are membrane-associated enzymes: cutting is the key! Blood. 1989;82:1052–70. [PubMed] [Google Scholar]
- 11.Holthöfer H, Miettinen A, Paasivuo R, et al. Cellular origin and differentiation of renal cell carcinoma. A fluorescence microscopic study with kidney-specific antibodies, anti-intermediate filament antibodies, and lectins. Lab Invest. 1983;49:319–26. [PubMed] [Google Scholar]
- 12.Ebert T, Bander NH, Finstad CL, et al. Establishment and characterization of human renal cancer and normal kidney cell lines. Cancer Res. 1990;50:5531–6. [PubMed] [Google Scholar]
- 13.Riemann D, Kehlen A, Langner J. Stimulation of the expression and the enzyme activity of aminopeptidase N/CD13 and dipeptidylpeptidase IV/CD26 on human renal cell carcinoma cells and renal tubular epithelial cells by T cell-derived cytokines, such as IL-4 and IL-13. Clin Exp Immunol. 1995;100:277–83. doi: 10.1111/j.1365-2249.1995.tb03665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chomczynski P, Sacchi N. Single step method of RNA isolation by acid guanidinium thiocyanate phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 15.Tanaica T, Camerini D, Seed B, et al. Cloning and functional expression of the T cell activation antigen CD26. J Immunol. 1992;149:481–6. [PubMed] [Google Scholar]
- 16.Look AT, Ashmun RA, Shapiro LH, et al. Human myeloid plasma membrane glycoprotein CD13 (gp150) is identical to aminopeptidase N. J Clin Invest. 1989;83:1299–307. doi: 10.1172/JCI114015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanayama N, Kajiwana Y, Goto J, et al. Inactivation of interleukin-8 by aminopeptidase N (CD13) J Leukocyte Biol. 1995;57:129–34. doi: 10.1002/jlb.57.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomella LG, Sargent ER, Linehan WM, et al. Transforming growth factor beta inhibits the growth of renal cell carcinoma in vitro. J Urol. 1989;141:1240–4. doi: 10.1016/s0022-5347(17)41230-4. [DOI] [PubMed] [Google Scholar]
- 19.Wunderlich H, Steiner T, Junker U, et al. Serum transforming growth factor-β1 in patients with renal cell carcinoma. J Urol. 1997;157:1602–3. [PubMed] [Google Scholar]
- 20.Heicappell R, Naito S, Ichinose Y, et al. Cytostatic and cytolytic effects of human recombinant tumor necrosis factor on renal carcinoma cell lines derived from a single surgical specimen. J Immunol. 1987;138:1634–40. [PubMed] [Google Scholar]
- 21.Fujiwara H, Fukuoka M, Yasuda K, et al. Cytokines stimulate dipeptidyl peptidase-IV expression on human luteinizing granulosa cells. J Clin Endocrinol Metab. 1994;79:1007–11. doi: 10.1210/jcem.79.4.7962267. [DOI] [PubMed] [Google Scholar]
- 22.Stefanovic V, Ardaillou N, Vlahovic P, et al. Interferon-γ induces dipeptidylpeptidase IV expression in human glomerular epithelial cells. Immunol. 1993;80:465–70. [PMC free article] [PubMed] [Google Scholar]
- 23.Krown SF. Interferons and interferon inducers in cancer treatment. Semin Oncol. 1986;13:207–17. [PubMed] [Google Scholar]
- 24.Stefanovic V, Vlahovic P, Ardaillou N, et al. Cell surface aminopeptidase A and N activities in human glomerular epithelial cells. Kidney Int. 1992;41:1571–80. doi: 10.1038/ki.1992.227. [DOI] [PubMed] [Google Scholar]
- 25.Stefanovic V, Vlahovic P, Ardaillou N, et al. Receptor-mediated induction of aminopeptidase A (APA) of human glomerular epithelial cells (HGEC) by glucocorticoids. FEBS Letters. 1991;294:171–4. doi: 10.1016/0014-5793(91)80661-l. [DOI] [PubMed] [Google Scholar]
- 26.Wayman GA, Impey S, Wu Z, et al. Synergistic activation of the type I adenylyl cyclase by Ca2+ and Gs-coupled receptors in vivo. J Biol Chem. 1994;269:25400–5. [PubMed] [Google Scholar]
- 27.Gilman AG. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–49. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- 28.Cassel D, Pfeuffer T. Mechanism of cholera toxin action: covalent modification of the guanyl nucleotide-binding protein of the adenylate cyclase system. Proc Natl Acad Sci USA. 1978;75:2669–73. doi: 10.1073/pnas.75.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samuelsson B, Goldyne M, Granstrom E, et al. Prostaglandins and thromboxanes. Annu Rev Biochem. 1978;47:997–1029. doi: 10.1146/annurev.bi.47.070178.005025. [DOI] [PubMed] [Google Scholar]
- 30.Baricault L, Fransen JAM, Garcia M, et al. Rapid sequestration of DPPIV/CD26 and other cell surface proteins in an autophagic-like compartment in Caco-2 cells treated with forskolin. J Cell Sci. 1995;108:2109–21. doi: 10.1242/jcs.108.5.2109. [DOI] [PubMed] [Google Scholar]
- 31.Tieku S, Hooper NM. Inhibition of aminopeptidases N, A and W. A re-evaluation of the actions of bestatin and inhibitors of angiotensin converting enzyme. Biochem Pharmacol. 1992;44:1725–30. doi: 10.1016/0006-2952(92)90065-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathe G. Bestatin, an aminopeptidase inhibitor with a multiple-pharmacological function. Biomed Pharmacother. 1991;45:49–54. doi: 10.1016/0753-3322(91)90122-a. [DOI] [PubMed] [Google Scholar]
- 33.Yoneda J, Saiki I, Fujii H, et al. Inhibition of tumor invasion and extracellular matrix degradation by ubenimex (bestatin) Clin Exp Metastasis. 1992;10:49–59. doi: 10.1007/BF00163576. [DOI] [PubMed] [Google Scholar]
- 34.Xu Y, Wellner D, Scheinberg DA. Substance P and bradykinin are natural inhibitors of CD13/aminopeptidase N. Biochem Biophys Res Commun. 1995;208:664–74. doi: 10.1006/bbrc.1995.1390. [DOI] [PubMed] [Google Scholar]
- 35.Kotani T, Aratake Y, Ogata Y, et al. Dipeptidyl aminopeptidase IV as a marker for thyroid cancer. Cancer Letters. 1991;57:203–8. doi: 10.1016/0304-3835(91)90158-e. [DOI] [PubMed] [Google Scholar]
- 36.Piazza GA, Callanan HM, Mowery J, et al. Evidence for a role of dipeptidyl peptidase IV in fibronectin-mediated interactions of hepatocytes with extracellular matrix. Biochem J. 1989;262:327–34. doi: 10.1042/bj2620327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson RC, Zhu D, Augustin-Voss HG, et al. Lung endothelial dipeptidyl peptidase IV is an adhesion molecule for lung-metastatic rat breast and prostate carcinoma cells. J Cell Biol. 1993;121:1423–32. doi: 10.1083/jcb.121.6.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Menrad A, Speicher D, Wacker J, et al. Biochemical and functional characterization of aminopeptidase N expressed by human melanoma cells. Cancer Res. 1993;53:1450–5. [PubMed] [Google Scholar]
- 39.Saiki I, Fujii H, Yoneda J, et al. Role of aminopeptidase N (CD13) in tumor-cell invasion and extracellular matrix degradation. Int J Cancer. 1993;54:137–43. doi: 10.1002/ijc.2910540122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ino K, Goto S, Okamoto T, et al. Expression of aminopeptidase N on human choriocarcinoma cells and cell growth suppression by the inhibition of aminopeptidase N activity. Jpn J Cancer Res. 1994;85:927–33. doi: 10.1111/j.1349-7006.1994.tb02970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morrison ME, Vijayasaradhi S, Engelstein D, et al. A marker for neoplastic progression of human melanocytes is a cell surface ectopeptidase. J Exp Med. 1993;177:1135–43. doi: 10.1084/jem.177.4.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shipp MA, Tarr GE, Chen CY, et al. CD10/neutral endopeptidase 24.11 hydrolyzes bombesin-like peptides and regulates the growth of small cell carcinomas of the lung. Proc Natl Acad Sci USA. 1991;88:10662–6. doi: 10.1073/pnas.88.23.10662. [DOI] [PMC free article] [PubMed] [Google Scholar]